Exploring the Frequency and Risk Factors of Hyperprogressive Disease in Patients with Advanced Melanoma Treated with Immune Checkpoint Inhibitors
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. ICI Type and HPD
3.3. Variables Associated with HPD
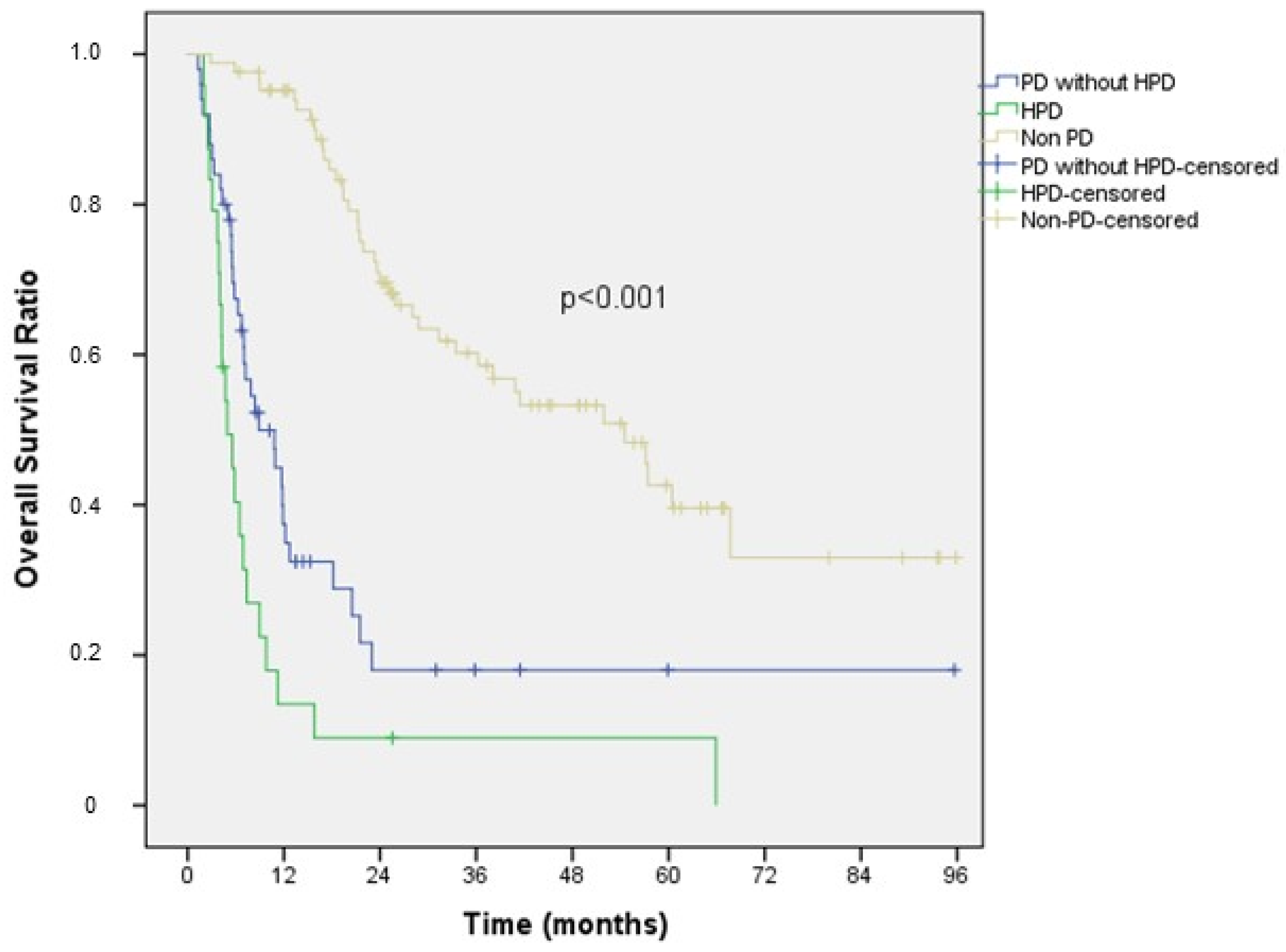
3.4. Survival Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes with Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients with Advanced Melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, K.W.; Won, S.E.; Yoon, S.; Chae, Y.K.; Tirumani, S.H.; Ramaiya, N.H. Definition, Incidence, and Challenges for Assessment of Hyperprogressive Disease during Cancer Treatment with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2021, 4, e211136. [Google Scholar] [CrossRef] [PubMed]
- Schuiveling, M.; Tonk, E.H.J.; Verheijden, R.J.; Suijkerbuijk, K.P.M. Hyperprogressive Disease Rarely Occurs during Checkpoint Inhibitor Treatment for Advanced Melanoma. Cancer Immunol. Immunother. 2021, 70, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- Forschner, A.; Hilke, F.J.; Bonzheim, I.; Gschwind, A.; Demidov, G.; Amaral, T.; Ossowski, S.; Riess, O.; Schroeder, C.; Martus, P.; et al. MDM2, MDM4 and EGFR Amplifications and Hyperprogression in Metastatic Acral and Mucosal Melanoma. Cancers 2020, 12, 540. [Google Scholar] [CrossRef]
- Champiat, S.; Dercle, L.; Ammari, S.; Massard, C.; Hollebecque, A.; Postel-Vinay, S.; Chaput, N.; Eggermont, A.; Marabelle, A.; Soria, J.C.; et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin. Cancer Res. 2017, 23, 1920–1928. [Google Scholar] [CrossRef]
- Russo, G.L.; Moro, M.; Sommariva, M.; Cancila, V.; Boeri, M.; Centonze, G.; Ferro, S.; Ganzinelli, M.; Gasparini, P.; Huber, V.; et al. Antibody-Fc/FcR Interaction on Macrophages as a Mechanism for Hyperprogressive Disease in Non-Small Cell Lung Cancer Subsequent to PD-1/PD-L1 Blockade. Clin. Cancer Res. 2019, 25, 989–999. [Google Scholar] [CrossRef]
- Understanding Hyperprogression in Cancer. Cancer Discov. 2019, 9, 821. [CrossRef]
- Ferrara, R.; Besse, B.; Mezquita, L.; Texier, M.; Lahmar, J.; Audigier-Valette, C.; Tessonnier, L.; Mazieres, J.; Zalcman, G.; Brosseau, S.; et al. Hyperprogressive Disease in Patients with Advanced Non–Small Cell Lung Cancer Treated with PD-1/PD-L1 Inhibitors or with Single-Agent Chemotherapy. JAMA Oncol. 2018, 4, 1543–1552. [Google Scholar] [CrossRef]
- Kamada, T.; Togashi, Y.; Tay, C.; Ha, D.; Sasaki, A.; Nakamura, Y.; Sato, E.; Fukuoka, S.; Tada, Y.; Tanaka, A.; et al. PD-1+ Regulatory T Cells Amplified by PD-1 Blockade Promote Hyperprogression of Cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 9999–10008. [Google Scholar] [CrossRef]
- Angelicola, S.; Ruzzi, F.; Landuzzi, L.; Scalambra, L.; Gelsomino, F.; Ardizzoni, A.; Nanni, P.; Lollini, P.L.; Palladini, A. IFN-γ and CD38 in Hyperprogressive Cancer Development. Cancers 2021, 13, 309. [Google Scholar] [CrossRef]
- Kato, S.; Goodman, A.; Walavalkar, V.; Barkauskas, D.A.; Sharabi, A.; Kurzrock, R. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin. Cancer Res. 2017, 23, 4242–4250. [Google Scholar] [CrossRef] [PubMed]
- Farag, C.M.; Antar, R.; Akosman, S.; Ng, M.; Whalen, M.J.; Farag, C.M.; Antar, R.; Akosman, S.; Ng, M.; Whalen, M.J. What Is Hemoglobin, Albumin, Lymphocyte, Platelet (HALP) Score? A Comprehensive Literature Review of HALP’s Prognostic Ability in Different Cancer Types. Oncotarget 2023, 14, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Kuang, T.; Qiu, Z.; Wang, K.; Zhang, L.; Dong, K.; Wang, W. Pan-Immune Inflammation Value as a Prognostic Biomarker for Cancer Patients Treated with Immune Checkpoint Inhibitors. Front. Immunol. 2024, 15, 1326083. [Google Scholar] [CrossRef] [PubMed]
- Anpalakhan, S.; Signori, A.; Cortellini, A.; Verzoni, E.; Giusti, R.; Aprile, G.; Ermacora, P.; Catino, A.; Pipitone, S.; Di Napoli, M.; et al. Using Peripheral Immune-Inflammatory Blood Markers in Tumors Treated with Immune Checkpoint Inhibitors: An INVIDIa-2 Study Sub-Analysis. iScience 2023, 26, 107970. [Google Scholar] [CrossRef] [PubMed]
- Bigot, F.; Castanon, E.; Baldini, C.; Hollebecque, A.; Carmona, A.; Postel-Vinay, S.; Angevin, E.; Armand, J.P.; Ribrag, V.; Aspeslagh, S.; et al. Prospective Validation of a Prognostic Score for Patients in Immunotherapy Phase I Trials: The Gustave Roussy Immune Score (GRIm-Score). Eur. J. Cancer 2017, 84, 212–218. [Google Scholar] [CrossRef]
- Sen, S.; Hess, K.; Hong, D.S.; Naing, A.; Piha-Paul, S.; Janku, F.; Fu, S.; Subbiah, I.M.; Liu, H.; Khanji, R.; et al. Development of a Prognostic Scoring System for Patients with Advanced Cancer Enrolled in Immune Checkpoint Inhibitor Phase 1 Clinical Trials. Br. J. Cancer 2018, 118, 763–769. [Google Scholar] [CrossRef]
- Fucà, G.; Guarini, V.; Antoniotti, C.; Morano, F.; Moretto, R.; Corallo, S.; Marmorino, F.; Lonardi, S.; Rimassa, L.; Sartore-Bianchi, A.; et al. The Pan-Immune-Inflammation Value Is a New Prognostic Biomarker in Metastatic Colorectal Cancer: Results from a Pooled-Analysis of the Valentino and TRIBE First-Line Trials. Br. J. Cancer 2020, 123, 403–409. [Google Scholar] [CrossRef]
- Qi, Q.; Zhuang, L.; Shen, Y.; Geng, Y.; Yu, S.; Chen, H.; Liu, L.; Meng, Z.; Wang, P.; Chen, Z. A Novel Systemic Inflammation Response Index (SIRI) for Predicting the Survival of Patients with Pancreatic Cancer after Chemotherapy. Cancer 2016, 122, 2158–2167. [Google Scholar] [CrossRef]
- Chen, X.L.; Xue, L.; Wang, W.; Chen, H.N.; Zhang, W.H.; Liu, K.; Chen, X.Z.; Yang, K.; Zhang, B.; Chen, Z.X.; et al. Prognostic Significance of the Combination of Preoperative Hemoglobin, Albumin, Lymphocyte and Platelet in Patients with Gastric Carcinoma: A Retrospective Cohort Study. Oncotarget 2015, 6, 41370–41382. [Google Scholar] [CrossRef]
- Hu, B.; Yang, X.R.; Xu, Y.; Sun, Y.F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.M.; Qiu, S.J.; Zhou, J.; et al. Systemic Immune-Inflammation Index Predicts Prognosis of Patients after Curative Resection for Hepatocellular Carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef]
- Arkenau, H.T.; Barriuso, J.; Olmos, D.; Ang, J.E.; De Bono, J.; Judson, I.; Kaye, S. Prospective Validation of a Prognostic Score to Improve Patient Selection for Oncology Phase I Trials. J. Clin. Oncol. 2009, 27, 2692–2696. [Google Scholar] [CrossRef] [PubMed]
- Kartolo, A.; Holstead, R.; Khalid, S.; Emack, J.; Hopman, W.; Robinson, A.; Baetz, T. Serum Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Prognosticating Immunotherapy Efficacy. Immunotherapy 2020, 12, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Bian, J.; Zhang, J.; Zhang, T.; Lu, X. Hyperprogressive Disease in Patients Suffering from Solid Malignancies Treated by Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 843707. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, J.; Bu, F.; Zhang, H.; Fei, K.; Zhang, P. Clinical Characteristics of Hyperprogressive Disease in NSCLC after Treatment with Immune Checkpoint Inhibitor: A Systematic Review and Meta-Analysis. BMC Cancer 2020, 20, 707. [Google Scholar] [CrossRef] [PubMed]
- Fournier, M.; Mortier, L.; Dereure, O.; Dalac, S.; Oriano, B.; Dalle, S.; Lebbé, C. Hyperprogression in Advanced Melanoma Is Not Restricted to Immunotherapy. Eur. J. Cancer 2023, 193, 113289. [Google Scholar] [CrossRef] [PubMed]
- Matos, I.; Martin-Liberal, J.; García-Ruiz, A.; Hierro, C.; De Olza, M.O.; Viaplana, C.; Azaro, A.; Vieito, M.; Braña, I.; Mur, G.; et al. Capturing Hyperprogressive Disease with Immune-Checkpoint Inhibitors Using RECIST 1. 1 Criteria. Clin. Cancer Res. 2020, 26, 1846–1855. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Q.; Wu, S.; Xie, X. Investigation on Potential Biomarkers of Hyperprogressive Disease (HPD) Triggered by Immune Checkpoint Inhibitors (ICIs). Clin. Transl. Oncol. 2021, 23, 1782–1793. [Google Scholar] [CrossRef]
- Li, L.X.; Cappuzzo, F.; Matos, I.; Socinski, M.A.; Hopkins, A.M.; Sorich, M.J. Low Risk of Hyperprogression with First-Line Chemoimmunotherapy for Advanced Non-Small Cell Lung Cancer: Pooled Analysis of 7 Clinical Trials. Oncologist 2023, 28, e205–e211. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Hellmann, M.D.; Hamid, O.; Tsai, K.K.; Loo, K.L.; Gubens, M.A.; Rosenblum, M.; Harview, C.L.; Taube, J.M.; Handley, N.; et al. Liver Metastasis and Treatment Outcome with Anti-PD-1 Monoclonal Antibody in Patients with Melanoma and NSCLC. Cancer Immunol. Res. 2017, 5, 417–424. [Google Scholar] [CrossRef]
- Grisaru-Tal, S.; Rothenberg, M.E.; Munitz, A. Eosinophil-Lymphocyte Interactions in the Tumor Microenvironment and Cancer Immunotherapy. Nat. Immunol. 2022, 23, 1309–1316. [Google Scholar] [CrossRef]
- Jacquelot, N.; Seillet, C.; Wang, M.; Pizzolla, A.; Liao, Y.; Hediyeh-zadeh, S.; Grisaru-Tal, S.; Louis, C.; Huang, Q.; Schreuder, J.; et al. Blockade of the Co-Inhibitory Molecule PD-1 Unleashes ILC2-Dependent Antitumor Immunity in Melanoma. Nat. Immunol. 2021, 22, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Carretero, R.; Sektioglu, I.M.; Garbi, N.; Salgado, O.C.; Beckhove, P.; Hämmerling, G.J. Eosinophils Orchestrate Cancer Rejection by Normalizing Tumor Vessels and Enhancing Infiltration of CD8(+) T Cells. Nat. Immunol. 2015, 16, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.; Canzoniero, J.V.; Rosner, S.; Zhang, G.; White, J.R.; Belcaid, Z.; Cherry, C.; Balan, A.; Pereira, G.; Curry, A.; et al. Peripheral Blood Immune Cell Dynamics Reflect Antitumor Immune Responses and Predict Clinical Response to Immunotherapy. J. Immunother. Cancer 2022, 10, e004688. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Xu, Q.Q.; Yu, X.; Pan, R.; Chen, Y. Dipeptidyl Peptidase 4 Inhibitors and Their Potential Immune Modulatory Functions. Pharmacol. Ther. 2020, 209, 107503. [Google Scholar] [CrossRef] [PubMed]
- Hollande, C.; Boussier, J.; Ziai, J.; Nozawa, T.; Bondet, V.; Phung, W.; Lu, B.; Duffy, D.; Paradis, V.; Mallet, V.; et al. Inhibition of the Dipeptidyl Peptidase DPP4 (CD26) Reveals IL-33-Dependent Eosinophil-Mediated Control of Tumor Growth. Nat. Immunol. 2019, 20, 257–264. [Google Scholar] [CrossRef]
- Zuo, B.; Li, T.; Liu, X.; Wang, S.; Cheng, J.; Liu, X.; Cui, W.; Shi, H.; Ling, C. Dipeptidyl Peptidase 4 Inhibitor Reduces Tumor-Associated Macrophages and Enhances Anti-PD-L1-Mediated Tumor Suppression in Non-Small Cell Lung Cancer. Clin. Transl. Oncol. 2023, 25, 3188–3202. [Google Scholar] [CrossRef]
- Economopoulou, P.; Anastasiou, M.; Papaxoinis, G.; Spathas, N.; Spathis, A.; Oikonomopoulos, N.; Kotsantis, I.; Tsavaris, O.; Gkotzamanidou, M.; Gavrielatou, N.; et al. Patterns of Response to Immune Checkpoint Inhibitors in Association with Genomic and Clinical Features in Patients with Head and Neck Squamous Cell Carcinoma (HNSCC). Cancers 2021, 13, 286. [Google Scholar] [CrossRef]
- Kim, C.G.; Kim, K.H.; Pyo, K.H.; Xin, C.F.; Hong, M.H.; Ahn, B.C.; Kim, Y.; Choi, S.J.; Yoon, H.I.; Lee, J.G.; et al. Hyperprogressive Disease during PD-1/PD-L1 Blockade in Patients with Non-Small-Cell Lung Cancer. Ann. Oncol. 2019, 30, 1104–1113. [Google Scholar] [CrossRef]
- Tunali, I.; Gray, J.E.; Qi, J.; Abdalah, M.; Jeong, D.K.; Guvenis, A.; Gillies, R.J.; Schabath, M.B. Novel Clinical and Radiomic Predictors of Rapid Disease Progression Phenotypes among Lung Cancer Patients Treated with Immunotherapy: An Early Report. Lung Cancer 2019, 129, 75–79. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, K.H.; Kang, J.; Borcoman, E.; Saada-Bouzid, E.; Kronbichler, A.; Hong, S.H.; de Rezende, L.F.M.; Ogino, S.; Keum, N.; et al. Hyperprogressive Disease during Anti-PD-1 (PDCD1)/PD-L1 (CD274) Therapy: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 1699. [Google Scholar] [CrossRef]
| Variable | HPD (n = 24) | Non-HPD (n = 134) | p-Value |
|---|---|---|---|
| Median age (year, range) | 53.3 (37.1–75.1) | 58.2 (21.6–80.4) | 0.300 |
| Age (y), n (%) | 0.052 | ||
| ≥65 | 3 (12.5) | 43 (32.1) | |
| <65 | 21 (87.5) | 91 (67.9) | |
| Sex, n (%) | 0.120 | ||
| Female | 13 (54.2) | 50 (37.3) | |
| Male | 11 (45.8) | 84 (62.7) | |
| BMI, mean (kg/m2, range) | 23.4 (14.9–35.6) | 25.2 (16.4–43.0) | 0.707 |
| Histologic subtype, n (%) | 0.740 | ||
| Acral | 4 (16.7) | 24 (17.9) | |
| Nonacral cutenous | 8 (33.3) | 50 (37.3) | |
| Mucosal | 3 (12.5) | 8 (6.0) | |
| Uveal | 2 (8.3) | 6 (4.5) | |
| Unknown | 7 (29.2) | 46 (34.3) | |
| BRAF status, n (%) | 0.843 | ||
| Mutant | 9 (37.5) | 44 (32.8) | |
| Wild | 15 (62.5) | 89 (66.4) | |
| Unknown | 0 (0.0) | 1 (0.7) | |
| Metastatic sites, n (%) | |||
| Liver | 12 (50.0) | 27 (20.1) | 0.002 |
| Lung | 15 (62.5) | 63 (47.0) | 0.162 |
| Bone | 11 (45.8) | 40 (29.9) | 0.123 |
| Brain | 4 (16.7) | 16 (11.9) | 0.741 |
| Number of metastatic sites, n (%) | <0.001 | ||
| <3 | 6 (25.0) | 86 (64.2) | |
| ≥3 | 18 (75.0) | 48 (35.8) | |
| Types of ICI, n (%) | |||
| PD-1 inhibitor | 16 (66.7) | 89 (66.4) | 0.981 |
| PD-1-CTLA-4 combination | 8 (33.3) | 45 (33.6) | |
| Previous treatments n (%) | |||
| BRAF+MEK inhibitors | 6 (25.0) | 24 (17.9) | 0.572 |
| Chemotherapy | 6 (25.0) | 20 (14.9) | 0.236 |
| Previous ICI n (%) | |||
| CTLA-4 inhibitor | 5 (20.8) | 23 (17.2) | 0.840 |
| PD-1 inhibitor | 4 (19.0) | 17 (12.7) | |
| No | 15 (62.5) | 94 (70.1) | |
| Line of treatment n (%) | |||
| 1 | 10 (41.7) | 79 (59.0) | 0.116 |
| ≥2 | 14 (58.3) | 55 (41.0) | |
| ECOG performance status n (%) | |||
| 0–1 | 18 (75.0) | 125 (93.3) | 0.013 |
| 2–4 | 6 (25.0) | 9 (6.7) | |
| LDH, n (%) | |||
| Normal | 6 (30.0) | 71 (56.3) | 0.004 |
| >ULN | 5 (25.0) | 37 (29.4) | |
| >1.5xULN | 9 (45.0) | 18 (14.3) | |
| Albumin, g/dl, n (%) | |||
| <4.0 | 5 (26.3) | 39 (31.2) | 0.667 |
| ≥4.0 | 14 (73.7) | 86 (68.8) | |
| CRP, mg/dl, n (%) | |||
| ≤0.5 | 6 (37.5) | 53 (50.5) | 0.333 |
| >0.5 | 10 (62.5) | 52 (49.5) | |
| AEC/μL, n (%) | |||
| <100 | 11 (57.9) | 37 (28.7) | 0.011 |
| ≥100 | 8 (42.1) | 92 (71.3) | |
| NLR, n (%) | |||
| ≤5.0 | 14 (73.7) | 110 (84.6) | 0.320 |
| >5.0 | 5 (26.3) | 20 (15.4) | |
| PLR, n (%) | |||
| ≤200 | 9 (47.4) | 90 (69.2) | 0.059 |
| >200 | 10 (52.6) | 40 (30.8) | |
| LMR, n (%) | |||
| >2.78 | 12 (63.2) | 64 (49.2) | 0.257 |
| ≤2.78 | 7 (36.8) | 66 (50.8) | |
| MPV/lymphocyte, n (%) | |||
| >6.0 | 13 (68.4) | 61 (46.9) | 0.080 |
| ≤6.0 | 6 (31.6) | 69 (53.1) | |
| GRIm score, n (%) | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) | 0.063 |
| MDA-ICI score, n (%) | |||
| Low risk | 6 (31.6) | 82 (64.6) | 0.002 |
| Intermediate risk | 8 (42.1) | 38 (29.9) | |
| High risk | 5 (26.3) | 7 (5.5) | |
| RMH score, n (%) | |||
| Low risk | 8 (40.0) | 96 (76.8) | 0.001 |
| High risk | 12 (60.0) | 29 (23.2) | |
| Patient | Age/Gender | Histologic Subtype | BRAF Status | Types of ICI | Liver Metastasis | Lung Metastasis | Brain Metastasis | Number of Metastatic Sites | ECOG | LDH U/L | AEC/μL | MDA-ICI Score | RMH Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | Unknown | Wild type | PD-1 inhibitor | No | Yes | No | 3 | 2 | 152 | 170 | Low risk | Low risk |
| 2 | F | Acral | Mutated | PD-1 inhibitor | No | No | Yes | 3 | 0 | 605 | 50 | Low risk | High risk |
| 3 | F | Nonacral cutaneous | Wild type | PD-1 inhibitor | Yes | Yes | No | 5 | 2 | 365 | 20 | Intermediate risk | High risk |
| 4 | F | Nonacral cutaneous | Mutated | PD-1 inhibitor | Yes | No | No | 3 | 0 | 297 | 120 | Intermediate risk | High risk |
| 5 | M | Mucosal | Wild type | PD-1 inhibitor | No | No | No | 3 | 1 | NA | NA | NA | NA |
| 6 | M | Unknown | Mutated | PD-1 inhibitor | Yes | Yes | No | 3 | 1 | NA | NA | NA | NA |
| 7 | F | Nonacral cutaneous | Mutated | PD-1 inhibitor | No | No | Yes | 3 | 2 | 113 | 200 | Intermediate risk | Low risk |
| 8 | M | Acral | Wild type | PD-1 inhibitor | No | No | No | 2 | 0 | 515 | 10 | Intermediate risk | Low risk |
| 9 | M | Acral | Wild type | PD-1 inhibitor | No | Yes | No | 3 | 1 | 303 | 210 | Low risk | High risk |
| 10 | M | Unknown | Wild type | PD-1 inhibitor | No | Yes | No | 5 | 1 | 230 | 190 | Low risk | High risk |
| 11 | M | Nonacral cutaneous | Wild type | PD-1 inhibitor | Yes | Yes | Yes | 7 | 1 | 431 | 10 | High risk | High risk |
| 12 | M | Nonacral cutaneous | Mutated | PD-1 inhibitor | No | No | No | 3 | 2 | 1228 | 40 | High risk | High risk |
| 13 | F | Unknown | Mutated | PD-1 inhibitor | No | No | No | 1 | 0 | 120 | 50 | Intermediate risk | Low risk |
| 14 | F | Mucosal | Wild type | PD-1 inhibitor | No | Yes | No | 2 | 0 | 167 | 50 | Low risk | Low risk |
| 15 | F | Uveal | Wild type | PD-1 inhibitor | Yes | Yes | No | 3 | 0 | 3639 | 90 | High risk | High risk |
| 16 | M | Mucosal | Wild type | PD-1 inhibitor | Yes | Yes | No | 3 | 1 | NA | NA | NA | NA |
| 17 | F | Acral | Wild type | PD-1-CTLA-4 combination | Yes | No | No | 4 | 1 | 482 | 90 | Intermediate risk | High risk |
| 18 | F | Unknown | Wild type | PD-1-CTLA-4 combination | No | No | No | 2 | 0 | NA | NA | NA | NA |
| 19 | F | Nonacral cutaneous | Mutated | PD-1-CTLA-4 combination | No | Yes | Yes | 2 | 0 | 336 | 90 | Low risk | Low risk |
| 20 | M | Uveal | Wild type | PD-1-CTLA-4 combination | Yes | Yes | No | 3 | 0 | 1360 | NA | NA | High risk |
| 21 | M | Nonacral cutaneous | Mutated | PD-1-CTLA-4 combination | Yes | Yes | No | 6 | 2 | 273 | 290 | High risk | High risk |
| 22 | F | Nonacral cutaneous | Wild type | PD-1-CTLA-4 combination | Yes | Yes | No | 2 | 0 | 222 | 120 | Intermediate risk | Low risk |
| 23 | F | Unknown | Wild type | PD-1-CTLA-4 combination | Yes | Yes | No | 4 | 1 | 1384 | 80 | Intermediate risk | High risk |
| 24 | M | Unknown | Mutated | PD-1-CTLA-4 combination | Yes | Yes | No | 4 | 2 | 200 | 640 | High risk | Low risk |
| Model-1 | Model-2 (MDA-ICI Score) | Model-3 (RMH Score) | ||||
|---|---|---|---|---|---|---|
| Variable | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p |
| Age (y) | ||||||
| <65 | Reference | 0.168 | Reference | 0.114 | ||
| ≥65 | 0.315 (0.061–1.631) | 0.259 (0.048–1.383) | ||||
| ECOG performance status | ||||||
| 0–1 | Reference | 0.056 | Reference | 0.023 | ||
| 2–4 | 3.761 (0.966–14.640) | 4.523 (1.227–16.676) | ||||
| Number of metastatic sites | ||||||
| <3 | Reference | 0.120 | Reference | 0.035 | ||
| ≥3 | 3.007 (0.750–12.059) | 3.546 (1.093–11.507) | ||||
| Liver metastasis | ||||||
| No | Reference | 0.894 | Reference | 0.555 | ||
| Yes | 1.093 (0.298–4.012) | 1.426 (0.439–4.633) | ||||
| LDH | ||||||
| Normal | Reference | |||||
| >ULN | 1.677 (0.433–6.497) | 0.455 | ||||
| ≥1.5 ULN | 2.522 (0.602–10.556) | 0.205 | ||||
| AEC/μL | ||||||
| <100 | 2.332 (0.713–7.626) | 0.161 | 2.960 (1.029–8.511) | 0.044 | 2.461 (0.814–7.446) | 0.111 |
| ≥100 | Reference | Reference | Reference | |||
| RMH score | ||||||
| Low risk | Reference | 0.026 | ||||
| High risk | 3.675 (1.166–11.580) | |||||
| MDA-ICI score | ||||||
| Low risk | Reference | |||||
| İntermediate risk | 2.375 (0.736–7.670) | 0.148 | ||||
| High risk | 4.466 (0.947–21.061) | 0.059 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acar, C.; Yüksel, H.Ç.; Şahin, G.; Açar, F.P.; Karaca, B. Exploring the Frequency and Risk Factors of Hyperprogressive Disease in Patients with Advanced Melanoma Treated with Immune Checkpoint Inhibitors. Curr. Oncol. 2024, 31, 6343-6355. https://doi.org/10.3390/curroncol31100472
Acar C, Yüksel HÇ, Şahin G, Açar FP, Karaca B. Exploring the Frequency and Risk Factors of Hyperprogressive Disease in Patients with Advanced Melanoma Treated with Immune Checkpoint Inhibitors. Current Oncology. 2024; 31(10):6343-6355. https://doi.org/10.3390/curroncol31100472
Chicago/Turabian StyleAcar, Caner, Haydar Çağatay Yüksel, Gökhan Şahin, Fatma Pinar Açar, and Burçak Karaca. 2024. "Exploring the Frequency and Risk Factors of Hyperprogressive Disease in Patients with Advanced Melanoma Treated with Immune Checkpoint Inhibitors" Current Oncology 31, no. 10: 6343-6355. https://doi.org/10.3390/curroncol31100472
APA StyleAcar, C., Yüksel, H. Ç., Şahin, G., Açar, F. P., & Karaca, B. (2024). Exploring the Frequency and Risk Factors of Hyperprogressive Disease in Patients with Advanced Melanoma Treated with Immune Checkpoint Inhibitors. Current Oncology, 31(10), 6343-6355. https://doi.org/10.3390/curroncol31100472





