Review on Lymph Node Metastases, Sentinel Lymph Node Biopsy, and Lymphadenectomy in Sarcoma
Abstract
1. Introduction
2. Lymph Node Metastasis Frequency in Selected Sarcomas
2.1. General Statement
2.2. Clear Cell Sarcoma
2.3. Angiosarcoma
2.4. Rhabdomyosarcoma
2.5. Epithelioid Sarcoma
3. Diagnostic Assessment of Lymph Node Metastases in Sarcoma
4. SLNB
4.1. Technical Overview
4.2. Significance in STS
5. LND
5.1. Technical Overview
5.2. Significance in STS
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gatta, G.; van der Zwan, J.M.; Casali, P.G.; Siesling, S.; Dei Tos, A.P.; Kunkler, I.; Otter, R.; Licitra, L.; Mallone, S.; Tavilla, A.; et al. Rare cancers are not so rare: The rare cancer burden in Europe. Eur. J. Cancer 2011, 47, 2493–2511. [Google Scholar] [CrossRef] [PubMed]
- Hui, J.Y.C. Epidemiology and Etiology of Sarcomas. Surg. Clin. N. Am. 2016, 96, 901–914. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Toro, J.R.; Travis, L.B.; Wu, H.J.; Zhu, K.; Fletcher, C.D.M.; Devesa, S.S. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: An analysis of 26,758 cases. Int. J. Cancer 2006, 119, 2922–2930. [Google Scholar] [CrossRef] [PubMed]
- Zahm, S.H.; Fraumeni, J.F. The epidemiology of soft tissue sarcoma. Semin. Oncol. 1997, 24, 504–514. [Google Scholar] [PubMed]
- Sbaraglia, M.; Bellan, E.; Dei Tos, A.P. The 2020 WHO Classification of Soft Tissue Tumours: News and perspectives. Pathologica 2020, 113, 70–84. [Google Scholar] [CrossRef]
- Biau, D.J.; Ferguson, P.C.; Chung, P.; Griffin, A.M.; Catton, C.N.; O’Sullivan, B.; Wunder, J.S. Local recurrence of localized soft tissue sarcoma. Cancer 2012, 118, 5867–5877. [Google Scholar] [CrossRef]
- Brennan, M.F.; Antonescu, C.R.; Moraco, N.; Singer, S. Lessons Learned From the Study of 10,000 Patients With Soft Tissue Sarcoma. Ann. Surg. 2014, 260, 416–422. [Google Scholar] [CrossRef]
- Fan, Z.; Chi, C.; Tong, Y.; Huang, Z.; Song, Y.; You, S. Score for the Risk and Overall Survival of Lung Metastasis in Patients First Diagnosed With Soft Tissue Sarcoma: A Novel Nomogram-Based Risk Assessment System. Technol. Cancer Res. Treat. 2022, 21, 153303382110662. [Google Scholar] [CrossRef]
- Gamboa, A.C.; Gronchi, A.; Cardona, K. Soft-tissue sarcoma in adults: An update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J. Clin. 2020, 70, 200–229. [Google Scholar] [CrossRef]
- Halsted, W.S.I. The Results of Radical Operations for the Cure of Carcinoma of the Breast. Ann. Surg. 1907, 46, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B. Laboratory and clinical research in breast cancer—A personal adventure: The David A. Karnofsky memorial lecture. Cancer Res. 1980, 40, 3863–3874. [Google Scholar] [PubMed]
- Kawada, K.; Taketo, M.M. Significance and Mechanism of Lymph Node Metastasis in Cancer Progression. Cancer Res. 2011, 71, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Reintgen, D.; Cruse, C.W.; Wells, K.; Berman, C.; Fenske, N.; Glass, F.; Schroer, K.; Heller, R.; Ross, M.; Lyman, G.; et al. The Orderly Progression of Melanoma Nodal Metastases. Ann. Surg. 1994, 220, 759–767. [Google Scholar] [CrossRef]
- Kalnins, I.K.; Leonard, A.G.; Sako, K.; Razack, M.S.; Shedd, D.P. Correlation between prognosis and degree of lymph node involvement in carcinoma of the oral cavity. Am. J. Surg. 1977, 134, 450–454. [Google Scholar] [CrossRef]
- Perez, M.; Hansen, C.P.; Burdio, F.; Pellino, G.; Pisanu, A.; Salvia, R.; Di Martino, M.; Abu Hilal, M.; Aldrighetti, L.; Ielpo, B. Lymph Node Ratio Nomogram-Based Prognostic Model for Resected Distal Cholangiocarcinoma. J. Am. Coll. Surg. 2022, 235, 703–712. [Google Scholar] [CrossRef]
- Cusimano, M.C.; Vicus, D.; Pulman, K.; Maganti, M.; Bernardini, M.Q.; Bouchard-Fortier, G.; Laframboise, S.; May, T.; Hogen, L.F.; Covens, A.L.; et al. Assessment of Sentinel Lymph Node Biopsy vs. Lymphadenectomy for Intermediate- and High-Grade Endometrial Cancer Staging. JAMA Surg. 2021, 156, 157. [Google Scholar] [CrossRef]
- Lowes, S.; Leaver, A.; Cox, K.; Satchithananda, K.; Cosgrove, D.; Lim, A. Evolving imaging techniques for staging axillary lymph nodes in breast cancer. Clin. Radiol. 2018, 73, 396–409. [Google Scholar] [CrossRef]
- Crystal, J.; Faries, M.B. Sentinel Lymph Node Biopsy. Surg. Oncol. Clin. N. Am. 2020, 29, 401–414. [Google Scholar] [CrossRef]
- Masoud, S.J.; Perone, J.A.; Farrow, N.E.; Mosca, P.J.; Tyler, D.S.; Beasley, G.M. Sentinel Lymph Node Biopsy and Completion Lymph Node Dissection for Melanoma. Curr. Treat. Options Oncol. 2018, 19, 55. [Google Scholar] [CrossRef]
- Falk Delgado, A.; Zommorodi, S.; Falk Delgado, A. Sentinel Lymph Node Biopsy and Complete Lymph Node Dissection for Melanoma. Curr. Oncol. Rep. 2019, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Giammarile, F.; Vidal-Sicart, S.; Paez, D.; Pellet, O.; Enrique, E.-L.; Mikhail-Lette, M.; Morozova, O.; Maria Camila, N.M.; Diana Ivonne, R.S.; Delgado Bolton, R.C.; et al. Sentinel Lymph Node Methods in Breast Cancer. Semin. Nucl. Med. 2022, 52, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Gore, R.M.; Miller, F.H. Stomach Cancer. In Oncologic Imaging; Elsevier: Amsterdam, The Netherlands, 2002; pp. 391–418. [Google Scholar] [CrossRef]
- Harrison, B. Update on sentinel node pathology in breast cancer. Semin. Diagn. Pathol. 2022, 39, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Cascinelli, N.; Morabito, A.; Santinami, M.; MacKie, R.M.; Belli, F. Immediate or delayed dissection of regional nodes in patients with melanoma of the trunk: A randomised trial. Lancet 1998, 351, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.M. Management of patients with intermediate-thickness melanoma. Annu. Rev. Med. 1996, 47, 211–217. [Google Scholar] [CrossRef] [PubMed]
- von Mehren, M.; Randall, R.L.; Benjamin, R.S.; Boles, S.; Bui, M.M.; Ganjoo, K.N.; George, S.; Gonzalez, R.J.; Heslin, M.J.; Kane, J.M.; et al. Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 536–563. [Google Scholar] [CrossRef] [PubMed]
- Basile, G.; Mattei, J.C.; Alshaygy, I.; Griffin, A.M.; Catton, C.N.; Chung, P.W.; Shultz, D.B.; Razak, A.R.A.; Demicco, E.G.; Ferguson, P.C.; et al. Curability of patients with lymph node metastases from extremity soft-tissue sarcoma. Cancer 2020, 126, 5098–5108. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.; Coit, D.G.; Woodruff, J.M.; Brennan, M.F. Lymph Node Metastasis From Soft Tissue Sarcoma in Adults Analysis of Data From a Prospective Database of 1772 Sarcoma Patients. Ann. Surg. 1993, 217, 72–77. [Google Scholar] [CrossRef]
- Gandhi, J.; Mehta, S.; Patel, T.; Gami, A.; Shah, M.; Jetly, D. Metastasis of soft tissue sarcomas in lymph node: A cytomorphological study. Diagn. Cytopathol. 2017, 45, 784–788. [Google Scholar] [CrossRef]
- Johannesmeyer, D.; Smith, V.; Cole, D.J.; Esnaola, N.F.; Camp, E.R. The impact of lymph node disease in extremity soft-tissue sarcomas: A population-based analysis. Am. J. Surg. 2013, 206, 289–295. [Google Scholar] [CrossRef]
- Keung, E.Z.; Chiang, Y.-J.; Voss, R.K.; Cormier, J.N.; Torres, K.E.; Hunt, K.K.; Feig, B.W.; Roland, C.L. Defining the incidence and clinical significance of lymph node metastasis in soft tissue sarcoma. Eur. J. Surg. Oncol. 2018, 44, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, H.; Zhang, C.; Liao, Z.; Li, T.; Yang, T.; Zhang, G.; Yang, J. Pan-Soft Tissue Sarcoma Analysis of the Incidence, Survival, and Metastasis: A Population-Based Study Focusing on Distant Metastasis and Lymph Node Metastasis. Front. Oncol. 2022, 12, 890040. [Google Scholar] [CrossRef] [PubMed]
- Mazeron, J.-J.; Suit, H.D. Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer 1987, 60, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, C.; Matsumoto, S.; Shimoji, T.; Ae, K.; Okawa, A. Lymphadenectomy and Histologic Subtype Affect Overall Survival of Soft Tissue Sarcoma Patients With Nodal Metastases. Clin. Orthop. Relat. Res. 2013, 471, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Serban, B.; Cretu, B.; Cursaru, A.; Nitipir, C.; Orlov-Slavu, C.; Cirstoiu, C. Local recurrence management of extremity soft tissue sarcoma. EFORT Open Rev. 2023, 8, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Emori, M.; Tsuchie, H.; Nagasawa, H.; Sonoda, T.; Tsukamoto, A.; Shimizu, J.; Murahashi, Y.; Mizushima, E.; Takada, K.; Murase, K.; et al. Early Lymph Node Metastasis May Predict Poor Prognosis in Soft Tissue Sarcoma. Int. J. Surg. Oncol. 2019, 2019, 6708474. [Google Scholar] [CrossRef] [PubMed]
- Riad, S.; Griffin, A.M.; Liberman, B.; Blackstein, M.E.; Catton, C.N.; Kandel, R.A.; O’Sullivan, B.; White, L.M.; Bell, R.S.; Ferguson, P.C.; et al. Lymph Node Metastasis in Soft Tissue Sarcoma in an Extremity. Clin. Orthop. Relat. Res. 2004, 426, 129–134. [Google Scholar] [CrossRef]
- Sobin, L.H.; Fleming, I.D. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer 1997, 80, 1803–1804. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA A Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Lochner, J.; Menge, F.; Vassos, N.; Hohenberger, P.; Kasper, B. Prognosis of Patients with Metastatic Soft Tissue Sarcoma: Advances in Recent Years. Oncol. Res. Treat. 2020, 43, 613–619. [Google Scholar] [CrossRef]
- Ashamalla, M.; Guirguis, A.; Mokhtar, B.E.; Ashamalla, H. Clinical Presentation and Patterns of Care in SCARE Soft Tissue Sarcoma. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, E749. [Google Scholar] [CrossRef]
- Jacobs, A.J.; Morris, C.D.; Levin, A.S. Synovial Sarcoma Is Not Associated With a Higher Risk of Lymph Node Metastasis Compared With Other Soft Tissue Sarcomas. Clin. Orthop. Relat. Res. 2018, 476, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Behranwala, K.A.; A’Hern, R.; Omar, A.-M.; Thomas, J.M. Prognosis of Lymph Node Metastasis in Soft Tissue Sarcoma. Ann. Surg. Oncol. 2004, 11, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Daigeler, A.; Kuhnen, C.; Moritz, R.; Stricker, I.; Goertz, O.; Tilkorn, D.; Steinstraesser, L.; Steinau, H.U.; Lehnhardt, M. Lymph node metastases in soft tissue sarcomas—A single center analysis of 1,597 patients. Langenbeck’s Arch. Surg. 2009, 394, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Sherman, K.L.; Kinnier, C.V.; Farina, D.A.; Wayne, J.D.; Laskin, W.B.; Agulnik, M.; Attar, S.; Hayes, J.P.; Peabody, T.; Bilimoria, K.Y. Examination of national lymph node evaluation practices for adult extremity soft tissue sarcoma. J. Surg. Oncol. 2014, 110, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Gusho, C.A.; Fice, M.P.; O’Donoghue, C.M.; Gitelis, S.; Blank, A.T. A Population-based Analysis of Lymph Node Metastasis in Extremity Soft Tissue Sarcoma: An Update. J. Surg. Res. 2021, 262, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Sambri, A.; Bianchi, G.; Cevolani, L.; Donati, D.; Abudu, A. Can radical margins improve prognosis in primary and localized epithelioid sarcoma of the extremities? J. Surg. Oncol. 2018, 117, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Sambri, A.; Bianchi, G.; Righi, A.; Ferrari, C.; Donati, D. Surgical margins do not affect prognosis in high grade myxofibrosarcoma. Eur. J. Surg. Oncol. 2016, 42, 1042–1048. [Google Scholar] [CrossRef]
- Wakeman, K.M.; Zhang, Q.S.; Bandhlish, A.; Cranmer, L.D.; Ricciotti, R.W.; Mantilla, J.G. Fédération Nationale Des Centres de Lutte Contre Le Cancer (FNCLCC) Grading, Margin Status and Tumor Location Associate With Survival Outcomes in Malignant Peripheral Nerve Sheath Tumors. Am. J. Clin. Oncol. 2022, 45, 28–35. [Google Scholar] [CrossRef]
- Hasegawa, T.; Yamamoto, S.; Yokoyama, R.; Umeda, T.; Matsuno, Y.; Hirohashi, S. Prognostic significance of grading and staging systems using MIB-1 score in adult patients with soft tissue sarcoma of the extremities and trunk. Cancer 2002, 95, 843–851. [Google Scholar] [CrossRef]
- Ruka, W.; Emrich, L.J.; Driscoll, D.L.; Karakousis, C.P. Prognostic significance of lymph node metastasis and bone, major vessel, or nerve involvement in adults with high-grade soft tissue sarcomas. Cancer 1988, 62, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Stojadinovic, A.; Leung, D.H.Y.; Allen, P.; Lewis, J.J.; Jaques, D.P.; Brennan, M.F. Primary Adult Soft Tissue Sarcoma: Time-Dependent Influence of Prognostic Variables. J. Clin. Oncol. 2002, 20, 4344–4352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hu, J.; Liu, Z.; Wu, H.; Cheng, H.; Li, C. Prognostic nomogram in patients with epithelioid sarcoma: A SEER-based study. Cancer Med. 2023, 12, 3079–3088. [Google Scholar] [CrossRef] [PubMed]
- Italiano, A.; Mathoulin-Pelissier, S.; Cesne, A.L.; Terrier, P.; Bonvalot, S.; Collin, F.; Michels, J.-J.; Blay, J.-Y.; Coindre, J.-M.; Bui, B. Trends in survival for patients with metastatic soft-tissue sarcoma. Cancer 2011, 117, 1049–1054. [Google Scholar] [CrossRef]
- Jami, S.A.; Mobarak, S.A.; Jiandang, S.; Xi, Z.; Tanvir, M.M.S.; Monilal, S.S. Clinical and strategic outcomes of metastatic synovial sarcoma on limb. Int. J. Health Sci. 2020, 14, 38–43. [Google Scholar]
- Fisher, S.B.; Chiang, Y.-J.; Feig, B.W.; Cormier, J.N.; Hunt, K.K.; Torres, K.E.; Roland, C.L. Comparative Performance of the 7th and 8th Editions of the American Joint Committee on Cancer Staging Systems for Soft Tissue Sarcoma of the Trunk and Extremities. Ann. Surg. Oncol. 2018, 25, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, P.C.; Deheshi, B.M.; Chung, P.; Catton, C.N.; O’Sullivan, B.; Gupta, A.; Griffin, A.M.; Wunder, J.S. Soft tissue sarcoma presenting with metastatic disease. Cancer 2011, 117, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Li, A.B.; Jiang, B.-J.; Wang, H.-H.; Yang, Y.-S.; Zhang, X.-B.; Lan, G.-H.; Shu, W.-B. Prognostic Factors for Survival in Patients with Clear Cell Sarcoma: An Analysis of the Surveillance, Epidemiology, and End Results (SEER) Database. Med. Sci. Monit. 2019, 25, 6950–6956. [Google Scholar] [CrossRef]
- Witt, R.G.; Voss, R.K.; Chiang, Y.-J.; Nguyen, S.; Scally, C.P.; Lin, P.P.; Torres, K.E.; Moon, B.S.; Satcher, R.L.; Hunt, K.K.; et al. Practice Pattern Variability in the Management of Regional Lymph Node Metastasis in Extremity and Trunk Soft Tissue Sarcoma: A Survey of the Society of Surgical Oncology and Musculoskeletal Tumor Society Membership. Ann. Surg. Oncol. 2023, 30, 3668–3676. [Google Scholar] [CrossRef]
- Bianchi, G.; Charoenlap, C.; Cocchi, S.; Rani, N.; Campagnoni, S.; Righi, A.; Frisoni, T.; Donati, D.M. Clear cell sarcoma of soft tissue: A retrospective review and analysis of 31 cases treated at Istituto Ortopedico Rizzoli. Eur. J. Surg. Oncol. 2014, 40, 505–510. [Google Scholar] [CrossRef]
- Wang, L.; Lao, I.W.; Yu, L.; Wang, J. Clinicopathological features and prognostic factors in angiosarcoma: A retrospective analysis of 200 patients from a single Chinese medical institute. Oncol. Lett. 2017, 14, 5370–5378. [Google Scholar] [CrossRef]
- Buehler, D.; Rice, S.R.; Moody, J.S.; Rush, P.; Hafez, G.-R.; Attia, S.; Longley, B.J.; Kozak, K.R. Angiosarcoma Outcomes and Prognostic Factors. Am. J. Clin. Oncol. 2014, 37, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Bae, J.; Choi, S.; Jang, K.-T.; Yu, J.; Hong, J.Y.; Lim, S.Y.; Jeong, H.-S. Regional Lymph Node Metastasis of Scalp Angiosarcoma: A Detailed Clinical Observation Study of 40 Cases. Ann. Surg. Oncol. 2020, 27, 3018–3027. [Google Scholar] [CrossRef] [PubMed]
- Alaggio, R.; Zhang, L.; Sung, Y.-S.; Huang, S.-C.; Chen, C.-L.; Bisogno, G.; Zin, A.; Agaram, N.P.; LaQuaglia, M.P.; Wexler, L.H.; et al. A Molecular Study of Pediatric Spindle and Sclerosing Rhabdomyosarcoma. Am. J. Surg. Pathol. 2016, 40, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, R.; Fuchs, J.; Rodeberg, D. Rhabdomyosarcoma. Semin. Pediatr. Surg. 2016, 25, 276–283. [Google Scholar] [CrossRef]
- Bompas, E.; Campion, L.; Italiano, A.; Le Cesne, A.; Chevreau, C.; Isambert, N.; Toulmonde, M.; Mir, O.; Ray-Coquard, I.; Piperno-Neumann, S.; et al. Outcome of 449 adult patients with rhabdomyosarcoma: An observational ambispective nationwide study. Cancer Med. 2018, 7, 4023–4035. [Google Scholar] [CrossRef]
- Carli, M.; Colombatti, R.; Oberlin, O.; Bisogno, G.; Treuner, J.; Koscielniak, E.; Tridello, G.; Garaventa, A.; Pinkerton, R.; Stevens, M. European Intergroup Studies (MMT4-89 and MMT4-91) on Childhood Metastatic Rhabdomyosarcoma: Final Results and Analysis of Prognostic Factors. J. Clin. Oncol. 2004, 22, 4787–4794. [Google Scholar] [CrossRef]
- Terwisscha van Scheltinga, C.E.J.; Wijnen, M.H.W.A.; Martelli, H.; Guerin, F.; Rogers, T.; Craigie, R.J.; Burrieza, G.G.; Dall’Igna, P.; De Corti, F.; Smeulders, N.; et al. In transit metastases in children, adolescents and young adults with localized rhabdomyosarcoma of the distal extremities: Analysis of the EpSSG RMS 2005 study. Eur. J. Surg. Oncol. 2022, 48, 1536–1542. [Google Scholar] [CrossRef]
- Brady, A.-C.; Rao, K.A.; Lane, R.; Garvin, L.; Sola, J.E.; Perez, E.A. Increased lymph node ratio predicts poor survival in pediatric rhabdomyosarcoma. J. Pediatr. Surg. 2020, 55, 369–375. [Google Scholar] [CrossRef]
- Wardin, H.d.T.d.; Xu, B.; Dermawan, J.K.; Smith, M.H.; Wolden, S.L.; Antonescu, C.R.; Wexler, L.H. Extremity Rhabdomyosarcoma—An Integrated Clinicopathologic and Genomic Study to Improve Risk Stratification. JCO Precis. Oncol. 2023, 7, e2200705. [Google Scholar] [CrossRef]
- Terwisscha van Scheltinga, S.E.J.; Wijnen, M.H.W.A.; Martelli, H.; Rogers, T.; Mandeville, H.; Gaze, M.N.; McHugh, K.; Corradini, N.; Orbach, D.; Jenney, M.; et al. Local staging and treatment in extremity rhabdomyosarcoma. A report from the EpSSG-RMS2005 study. Cancer Med. 2020, 9, 7580–7589. [Google Scholar] [CrossRef] [PubMed]
- Gallego, S.; Bernabeu, D.; Garrido-Pontnou, M.; Guillen, G.; Hindi, N.; Juan-Ribelles, A.; Márquez, C.; Mata, C.; Orcajo, J.; Ramírez, G.; et al. GEIS-SEHOP clinical practice guidelines for the treatment of rhabdomyosarcoma. Clin. Transl. Oncol. 2021, 23, 2460–2473. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.N.; Seitz, G.; Fuchs, J.; Martelli, H.; Dasgupta, R.; Routh, J.C.; Hawkins, D.S.; Koscielniak, E.; Bisogno, G.; Rodeberg, D.A. Surgical management of paratesticular rhabdomyosarcoma: A consensus opinion from the Children’s Oncology Group, European paediatric Soft tissue sarcoma Study Group, and the Cooperative Weichteilsarkom Studiengruppe. Pediatr. Blood Cancer 2021, 68, e28938. [Google Scholar] [CrossRef] [PubMed]
- Mandell, L.; Ghavimi, F.; LaQuaglia, M.; Exelby, P. Prognostic significance of regional lymph node involvement in childhood extremity rhabdomyosarcoma. Med. Pediatr. Oncol. 1990, 18, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Rodeberg, D.A.; Garcia-Henriquez, N.; Lyden, E.R.; Davicioni, E.; Parham, D.M.; Skapek, S.X.; Hayes-Jordan, A.A.; Donaldson, S.S.; Brown, K.L.; Triche, T.J.; et al. Prognostic significance and tumor biology of regional lymph node disease in patients with rhabdomyosarcoma: A report from the Children’s Oncology Group. J. Clin. Oncol. 2011, 29, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Chbani, L.; Guillou, L.; Terrier, P.; Decouvelaere, A.V.; Grégoire, F.; Terrier-Lacombe, M.J.; Ranchère, D.; Robin, Y.M.; Collin, F.; Fréneaux, P.; et al. Epithelioid Sarcoma. Am. J. Clin. Pathol. 2009, 131, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Yoo, K.H.; Kim, M.H.; Chon, H.J.; Lee, S.I.; Lee, H.J.; Koh, S.; Lee, H.Y.; Lee, H.R.; Kim, K.S.; et al. Different subtypes of epithelioid sarcoma and their clinical implication: Long-term multi-institutional experience with a rare sarcoma. APMIS 2017, 125, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Prat, J.; Woodruff, J.M.; Marcove, R.C. Epithelioid sarcoma. An analysis of 22 cases indicating the prognostic significance of vascular invasion and regional lymph node metastasis. Cancer 1978, 41, 1472–1487. [Google Scholar] [CrossRef]
- de Visscher, S.A.H.J.; van Ginkel, R.J.; Wobbes, T.; Veth, R.P.H.; ten Heuvel, S.E.; Suurmeijer, A.J.H.; Hoekstra, H.J. Epithelioid sarcoma: Still an only surgically curable disease. Cancer 2006, 107, 606–612. [Google Scholar] [CrossRef]
- Callister, M.D.; Ballo, M.T.; Pisters, P.W.T.; Patel, S.R.; Feig, B.W.; Pollock, R.E.; Benjamin, R.S.; Zagars, G.K. Epithelioid sarcoma: Results of conservative surgery and radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 384–391. [Google Scholar] [CrossRef]
- Zhang, S.; Jing, C.; Liu, H.; Zhao, Z.; Zhang, X.; Liu, T.; Xu, S.; Xu, L.; Yu, S. Epithelioid sarcoma: A single-institutional retrospective cohort study of 36 cases. J. Orthop. Surg. 2021, 29, 230949902110293. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, P.; Koseła-paterczyk, H.; Kozak, K.; Ługowska, I.; Fijuth, J.; Jeziorski, A.; Ryś, J.; Spałek, M.; Borkowska, A.; Wągrodzki, M.; et al. Postępowanie diagnostyczno-terapeutyczne u chorych na mięsaki tkanek miękkich u dorosłych—Zalecenia ekspertów. Onkol. Prakt. Klin. Edu. 2023, 9, 149–180. [Google Scholar]
- Rutkowski, P.; Świtaj, T.; Koseła-paterczyk, H.; Kotrych, D.; Goryń, T.; Mazurkiewicz, T.; Fijuth, J.; Grzesiakowska, U.; Borkowska, A.; Spałek, M.; et al. Postępowanie diagnostyczno-terapeutyczne u chorych na mięsaki kości—Zalecenia ekspertów. Onkol. Prakt. Klin. Edu. 2023, 9, 181–200. [Google Scholar]
- Ganeshalingam, S.; Koh, D.-M. Nodal staging. Cancer Imaging 2009, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Sambri, A.; Bianchi, G.; Longhi, A.; Righi, A.; Donati, D.M.; Nanni, C.; Fanti, S.; Errani, C. The role of 18F-FDG PET/CT in soft tissue sarcoma. Nucl. Med. Commun. 2019, 40, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Keung, E.Z.; Krause, K.J.; Maxwell, J.; Morris, C.D.; Crago, A.M.; Houdek, M.T.; Kane, J.; Lewis, V.; Callegaro, D.; Miller, B.; et al. Sentinel Lymph Node Biopsy for Extremity and Truncal Soft Tissue Sarcomas: A Systematic Review of the Literature. Ann. Surg. Oncol. 2023, 30, 958–967. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Healey, J.H.; Athanasian, E.A. Pathologically Benign Lymph Nodes Can Mimic Malignancy on Imaging in Patients With Angiomatoid Fibrous Histiocytoma. Clin. Orthop. Relat. Res. 2017, 475, 2274–2279. [Google Scholar] [CrossRef][Green Version]
- Siegel, N.M.; Lozano-Calderón, S.A.; El Abiad, J.M.; Morris, C.D.; Levin, A.S. Lymphadenopathy in Fungating Extremity Soft-Tissue Sarcoma: Metastasis or Reactive? Ann. Surg. Oncol. 2021, 28, 4695–4705. [Google Scholar] [CrossRef]
- Torabi, M.; Aquino, S.L.; Harisinghani, M.G. Current concepts in lymph node imaging. J. Nucl. Med. 2004, 45, 1509–1518. [Google Scholar]
- Noworolski, S.M.; Fischbein, N.J.; Kaplan, M.J.; Lu, Y.; Nelson, S.J.; Carvajal, L.; Henry, R.G. Challenges in dynamic contrast-enhanced MRI imaging of cervical lymph nodes to detect metastatic disease. J. Magn. Reson. Imaging 2003, 17, 455–462. [Google Scholar] [CrossRef]
- Brown, G.; Richards, C.J.; Bourne, M.W.; Newcombe, R.G.; Radcliffe, A.G.; Dallimore, N.S.; Williams, G.T. Morphologic Predictors of Lymph Node Status in Rectal Cancer with Use of High-Spatial-Resolution MR Imaging with Histopathologic Comparison. Radiology 2003, 227, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, G. Preoperative Staging of Rectal Cancer Using Magnetic Resonance Imaging with External Phase-Arrayed Coils. Arch. Surg. 2002, 137, 447. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Zhang, Y.; Wei, M.; Yang, X.; Wang, Z. Magnetic Resonance Imaging Evaluation of the Accuracy of Various Lymph Node Staging Criteria in Rectal Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 709070. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, G.; Sakurai, T.; Oura, S.; Suzuma, T.; Tamaki, T.; Umemura, T.; Kokawa, Y.; Yang, Q. Evaluation of axillary lymph node status in breast cancer with MRI. Breast Cancer 1999, 6, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-M.; Wang, C.-P.; Chen, C.-N.; Lin, C.-Y.; Li, T.-Y.; Chou, C.-H.; Hsu, Y.-C.; Kuo, P.-Y.; Yang, T.-L.; Lou, P.-J.; et al. The application of ultrasound in detecting lymph nodal recurrence in the treated neck of head and neck cancer patients. Sci. Rep. 2017, 7, 3958. [Google Scholar] [CrossRef] [PubMed]
- Fuglø, H.M.; Jørgensen, S.M.; Loft, A.; Hovgaard, D.; Petersen, M.M. The diagnostic and prognostic value of 18F-FDG PET/CT in the initial assessment of high-grade bone and soft tissue sarcoma. A retrospective study of 89 patients. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, U.; Hosono, A.; Makimoto, A.; Sakurada, A.; Terauchi, T.; Arai, Y.; Imai, Y.; Kim, E.E. Accuracy of 18F Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Staging of Pediatric Sarcomas. J. Pediatr. Hematol./Oncol. 2007, 29, 608–612. [Google Scholar] [CrossRef]
- Tateishi, U.; Yamaguchi, U.; Seki, K.; Terauchi, T.; Arai, Y.; Kim, E.E. Bone and Soft-Tissue Sarcoma: Preoperative Staging with Fluorine 18 Fluorodeoxyglucose PET/CT and Conventional Imaging. Radiology 2007, 245, 839–847. [Google Scholar] [CrossRef]
- Kassem, T.W.; Abdelaziz, O.; Emad-Eldin, S. Diagnostic value of 18F-FDG-PET/CT for the follow-up and restaging of soft tissue sarcomas in adults. Diagn. Interv. Imaging 2017, 98, 693–698. [Google Scholar] [CrossRef]
- Rodríguez-Alfonso, B.; Mucientes Rasilla, J.; Mitjavila Casanovas, M.; Cardona Arboniés, J.; Cubedo, R. 18F-FDG-PET-TC en sarcomas de partes blandas: ¿cuándo? Rev. Española Med. Nucl. Imagen Mol. 2014, 33, 43–49. [Google Scholar] [CrossRef]
- Macpherson, R.E.; Pratap, S.; Tyrrell, H.; Khonsari, M.; Wilson, S.; Gibbons, M.; Whitwell, D.; Giele, H.; Critchley, P.; Cogswell, L.; et al. Retrospective audit of 957 consecutive 18F-FDG PET–CT scans compared to CT and MRI in 493 patients with different histological subtypes of bone and soft tissue sarcoma. Clin. Sarcoma Res. 2018, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, U.; Yamaguchi, U.; Maeda, T.; Seki, K.; Terauchi, T.; Kawai, A.; Arai, Y.; Moriyama, N.; Kakizoe, T. Staging performance of carbon-11 choline positron emission tomography/computed tomography in patients with bone and soft tissue sarcoma: Comparison with conventional imaging. Cancer Sci. 2006, 97, 1125–1128. [Google Scholar] [CrossRef] [PubMed]
- Nowecki, Z.I.; Rutkowski, P.; Nasierowska-Guttmejer, A.; Ruka, W. Sentinel lymph node biopsy in melanoma patients with clinically negative regional lymph nodes--one institution’s experience. Melanoma Res. 2003, 13, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Zhang, X.; Cui, M.; Wang, J. Sentinel Lymph Node Mapping in Endometrial Cancer: A Comprehensive Review. Front. Oncol. 2021, 11, 701758. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.R.; Lo, S.N.; Nosrati, M.; Stretch, J.R.; Spillane, A.J.; Saw, R.P.M.; Shannon, K.F.; Nieweg, O.E.; Ch’ng, S.; Kim, K.B.; et al. Improving Selection for Sentinel Lymph Node Biopsy Among Patients With Melanoma. JAMA Netw. Open 2023, 6, e236356. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Jost, L.; Sleijfer, S.; Verweij, J.; Blay, J.Y. Soft tissue sarcomas: ESMO Clinical Recommendations for diagnosis, treatment and follow-up. Ann. Oncol. 2009, 20, iv132–iv136. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef]
- Brady, A.-C.; Picado, O.; Tashiro, J.; Sola, J.E.; Perez, E.A. Lymph Node Sampling and Survival in Child and Adolescent Extremity Soft-Tissue Sarcoma. J. Surg. Res. 2019, 241, 205–214. [Google Scholar] [CrossRef]
- Lobeck, I.; Dupree, P.; Karns, R.; Rodeberg, D.; von Allmen, D.; Dasgupta, R. Quality assessment of lymph node sampling in rhabdomyosarcoma: A surveillance, epidemiology, and end results (SEER) program study. J. Pediatr. Surg. 2017, 52, 614–617. [Google Scholar] [CrossRef]
- Wright, S.; Armeson, K.; Hill, E.G.; Streck, C.; Leddy, L.; Cole, D.; Esnaola, N.; Camp, E.R. The role of sentinel lymph node biopsy in select sarcoma patients: A meta-analysis. Am. J. Surg. 2012, 204, 428–433. [Google Scholar] [CrossRef]
- Andreou, D.; Boldt, H.; Werner, M.; Hamann, C.; Pink, D.; Tunn, P.U. Sentinel node biopsy in soft tissue sarcoma subtypes with a high propensity for regional lymphatic spread—Results of a large prospective trial. Ann. Oncol. 2013, 24, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Parida, L.; Morrisson, G.T.; Shammas, A.; Hossain, A.K.M.M.; McCarville, M.B.; Gerstle, J.T.; Charron, M.; Rao, B.N.; Shulkin, B.L. Role of lymphoscintigraphy and sentinel lymph node biopsy in the management of pediatric melanoma and sarcoma. Pediatr. Surg. Int. 2012, 28, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L.M.; Kremer, N.; Gelfand, M.J.; Sharp, S.E.; Turpin, B.K.; Nagarajan, R.; Tiao, G.M.; Pressey, J.G.; Yin, J.; Dasgupta, R. Detection of lymph node metastases in pediatric and adolescent/young adult sarcoma: Sentinel lymph node biopsy versus fludeoxyglucose positron emission tomography imaging-A prospective trial. Cancer 2017, 123, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Jeremiasse, B.; van der Steeg, A.F.W.; Fiocco, M.; Hobbelink, M.G.G.; Merks, J.H.M.; Godzinski, J.; Shulkin, B.L.; Wijnen, M.H.W.A.; Terwisscha van Scheltinga, C.E.J. Value of the Sentinel Node Procedure in Pediatric Extremity Rhabdomyosarcoma: A Systematic Review and Retrospective Cohort Study. Ann. Surg. Oncol. 2021, 28, 9048–9059. [Google Scholar] [CrossRef] [PubMed]
- Witt, R.G.; Cope, B.; Erstad, D.J.; Chiang, Y.-J.; Nassif, E.F.; Scally, C.P.; Torres, K.E.; Hunt, K.K.; Feig, B.W.; Roland, C.L.; et al. Sentinel Lymph Node Biopsy and Formal Lymphadenectomy for Soft Tissue Sarcoma: A Single Center Experience of 86 Consecutive Cases. Ann. Surg. Oncol. 2022, 29, 7092–7100. [Google Scholar] [CrossRef] [PubMed]
- Swan, M.C.; Furniss, D.; Cassell, O.C. Surgical management of metastatic inguinal lymphadenopathy. BMJ 2004, 329, 1272–1276. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.-K.; Yu, X.-J.; Wang, Y.-G.; Lu, R.; Wang, S.-X.; Xu, H.-R.; Kang, H. Risk factors for lymph node metastasis of soft tissue sarcomas of the head, neck, and extremities, and the clinical significance of negative lymph node dissection. J. Orthop. Surg. Res. 2022, 17, 167. [Google Scholar] [CrossRef] [PubMed]
- Ecker, B.L.; Peters, M.G.; McMillan, M.T.; Sinnamon, A.J.; Zhang, P.J.; Kelz, R.R.; Roses, R.E.; Drebin, J.A.; Fraker, D.L.; Karakousis, G.C. Implications of Lymph Node Evaluation in the Management of Resectable Soft Tissue Sarcoma. Ann. Surg. Oncol. 2017, 24, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Al-Refaie, W.B.; Andtbacka, R.H.I.; Ensor, J.; Pisters, P.W.T.; Ellis, T.L.; Shrout, A.; Hunt, K.K.; Cormier, J.N.; Pollock, R.E.; Feig, B.W. Lymphadenectomy for isolated lymph node metastasis from extremity soft-tissue sarcomas. Cancer 2008, 112, 1821–1826. [Google Scholar] [CrossRef]
- Kheiran, A.; Eastley, N.C.; McCulloch, T.A.; Steele, K.H.; Tamimy, M.S.; Raurell, A.; Ashford, R.U. Surgical resection of regional lymph node metastases in soft tissue sarcoma may not result in improved long-term survival. Eur. J. Plast. Surg. 2022, 45, 561–568. [Google Scholar] [CrossRef]
- Deroose, J.P.; Eggermont, A.M.; van Geel, A.N.; Burger, J.W.; den Bakker, M.A.; de Wilt, J.H.; Verhoef, C. Long-term results of tumor necrosis factor alpha- and melphalan-based isolated limb perfusion in locally advanced extremity soft tissue sarcomas. J. Clin. Oncol. 2011, 29, 4036–4044. [Google Scholar] [CrossRef] [PubMed]
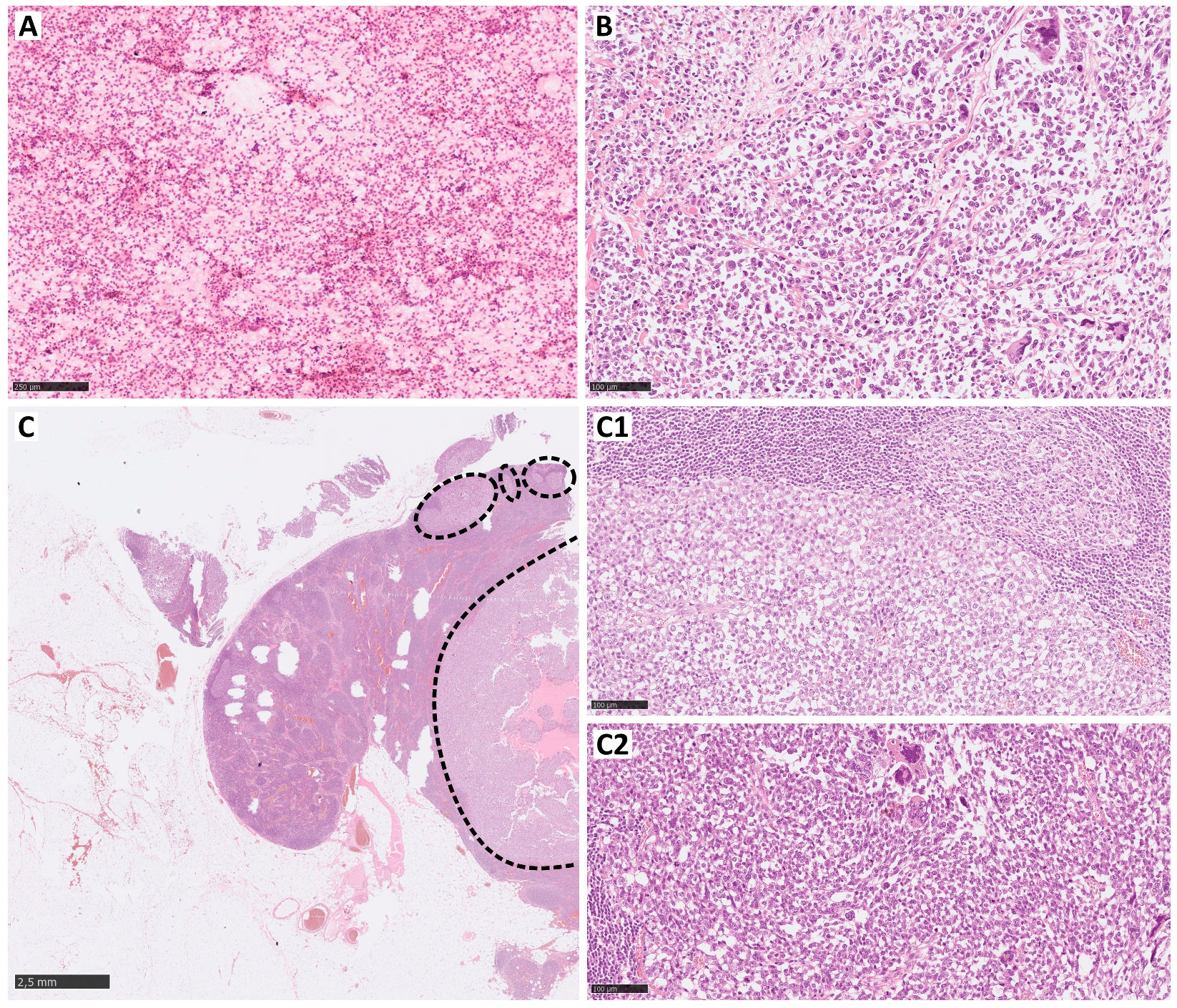
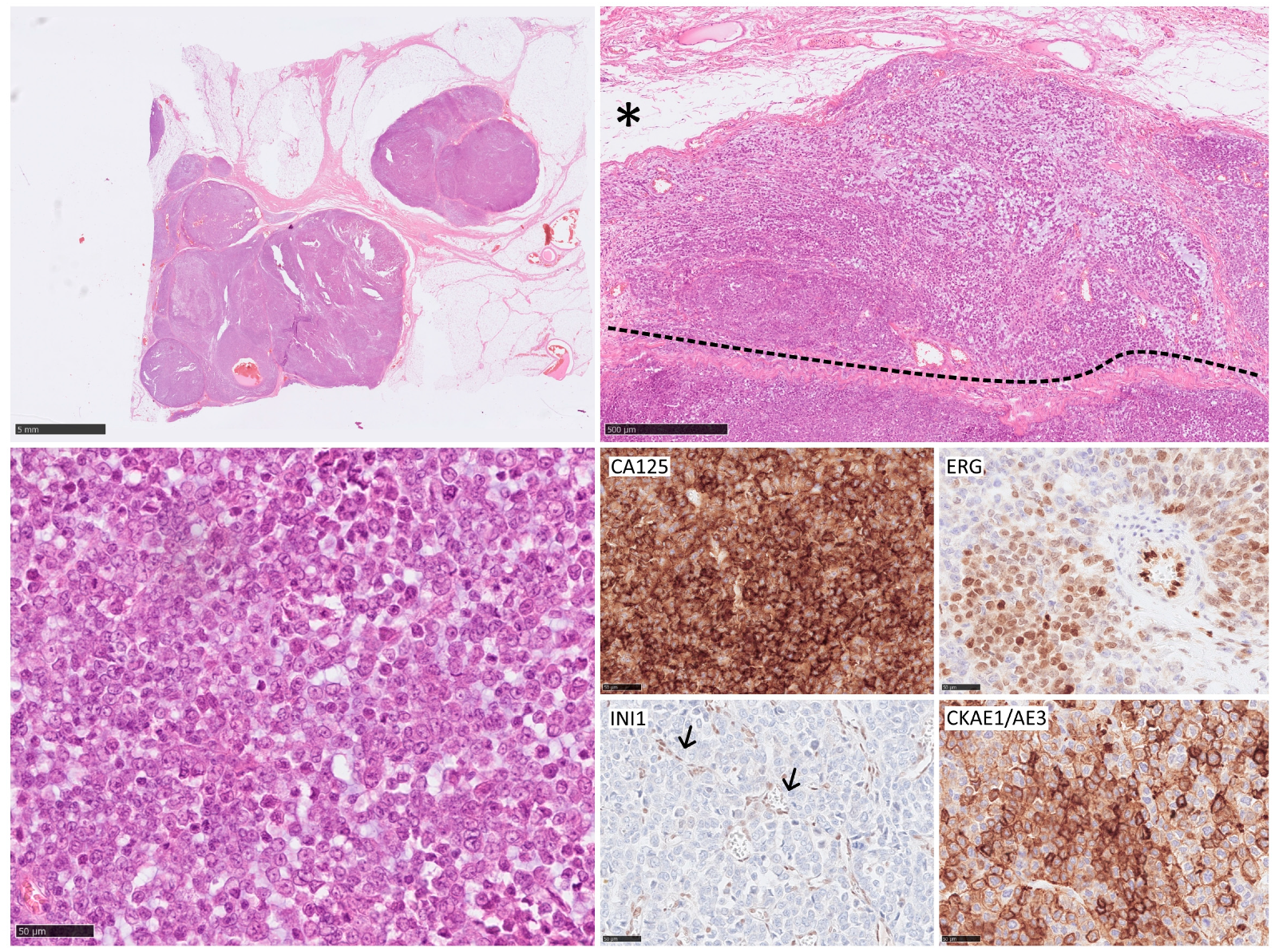
| Study | Reference | Lymph Node Involvement—All Subtypes | Clear Cell Sarcoma | Angiosarcoma | Rhabdomyosarcoma | Epithelioid Sarcoma |
|---|---|---|---|---|---|---|
| Sawamura et al. | [35] | 6% (49/87) | 38% (3/8) | 20% (2/10) | 37% (7/19) | 30% (6/20) |
| Johannesmeyer et al. | [31] | 0.9% (64/7159) | 11% (9/70) | N/A * | 9.7% (11/113) | 13% (15/155) |
| Mazeron et al. | [34] | 5.9% (19/323) | N/A | (2/5) | (5/14) | 4/5 |
| Gandhi et al. | [30] | 5.8% (21/326) | (2/19) 10.5% | N/A | 31.5% (8/19) | N/A |
| Basile et al. | [28] | 4.5% (120/2689) | 27.6% | 14.0% | 17.3% | 21.9% |
| Keung et al. | [32] | 3.5% (3154/89,870) | 15.9% | 6.1% | N/A | 13.1% |
| Fong et al. | [29] | 2.6% (46/1772) | N/A | 13.5% (5/37) | 13.6% (12/88) | 16.7% (2/12) |
| Liu et al. | [33] | 6.02% (1081/17,937) | N/A | 15.43% | 26.88% | N/A |
| Riad et al. | [38] | 3.7% (39/1066) | 11.1% (2/18) | 11.1% (2/18) | 19% (4/21) | 20% (3/15) |
| Jacobs et al. | [43] | 5.3% (820/15,525) | N/A | N/A | N/A | N/A |
| Behranwala et al. | [44] | 3.4% (73/2127) | 4% (1/25) | 10.9% (5/46) | 22.2% (12/54) | 18.5% (5/27) |
| Daigeler et al. | [45] | 1.75% | 17.6% | 7.9% | 6.0% | 21.4% |
| Sherman et al. | [46] | 1.05% | 27.7% (28/290) | 32.1% (17/290) | 24.1% (14/290) | 31.8% (34/290) |
| Gusho et al. | [47] | 3.7% (547/1936) | 18.8% (24/547) | 26.7% (105/547) | 8.1% (19/547) | 14.5% (32/547) |
| Sambri et al. | [48] | N/A | N/A | N/A | N/A | 19% (15/77) |
| Study | Reference | Lymph Node Involvement—All Subtypes | Synovial Sarcoma | Leiomyosarcoma | Liposarcoma | Myxofibrosarcoma | Malignant Peripheral Nerve Sheath Tumor (MPNST) |
|---|---|---|---|---|---|---|---|
| Sawamura et al. | [35] | 6% (49/87) | N/A | N/A | N/A | N/A | N/A |
| Johannesmeyer et al. | [31] | 0.9% (64/7159) | N/A | N/A | N/A | N/A | N/A |
| Mazeron et al. | [34] | 5.9% (19/323) | 0/4 | 1/10 | N/A | N/A | N/A |
| Gandhi et al. | [30] | 5.8% (21/326) | 10.5% (2/19) | N/A | N/A | N/A | 10.5% (2/19) |
| Basile et al. | [28] | 4.5% (120/2689) | 2.7% | 3.1% | N/A | 5.2% (11/212) | 5.1% (7/138) |
| Keung et al. | [32] | 3.5% (3154/8970) | 3.3% | 3.0% | 1.6% | 1.6% | 3.8% |
| Fong et al. | [29] | 2.6% (46/1772) | N/A | N/A | N/A | N/A | N/A |
| Liu et al. | [33] | 6.02% (1081/17,937) | 5.23% | 5.08% | 3.92% (190/5105) | N/A | 6.25% (1/16) |
| Riad et al. | [38] | 3.7% (39/1066) | N/A | N/A | 2.4% (6/249) | N/A | 4.9% (2/41) |
| Jacobs et al. | [43] | 5.3% (820/15,525) | 4.2% | N/A | N/A | N/A | N/A |
| Behranwala et al. | [44] | 3.4% (73/2127) | 4.1% (7/171) | 2.7% (13/483) | 0.9% (3/340) | N/A | 4.2% (4/95) |
| Daigeler et al. | [45] | 1.75% | 0.6% | 3.6% | 0.3% | N/A | 3.2% |
| Sherman et al. | [46] | 1.05% | 6.0% (9/290) | 7.5% (14/290) | N/A | N/A | N/A |
| Gusho et al. | [47] | 3.7% (547/1936) | 3.2% (31/547) | 1.4% (22/547) | N/A | N/A | N/A |
| Sambri et al. | [49] | N/A | N/A | N/A | N/A | 3.9% (5/128) | N/A |
| Tumor Type | Positive/Total SLNB (%) | References |
|---|---|---|
| Rhabdomyosarcoma | 7.7%, 16.7%, 23%, 42.8% | [111,113,114,115] |
| Epithelioid sarcoma | 0%, 9.8%, 14.2% | [113,114,115] |
| Clear cell sarcoma | 0%, 10.8%, 14.2%, 35%, 50% | [111,112,113,114,115] |
| Angiosarcoma | 3.2% | [115] |
| Leiomyosarcoma | 1.1% | [115] |
| Synovial sarcoma | 0%, 1.5%, 4.3%, 6% | [111,112,113,115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmiel, P.; Krotewicz, M.; Szumera-Ciećkiewicz, A.; Bartnik, E.; Czarnecka, A.M.; Rutkowski, P. Review on Lymph Node Metastases, Sentinel Lymph Node Biopsy, and Lymphadenectomy in Sarcoma. Curr. Oncol. 2024, 31, 307-323. https://doi.org/10.3390/curroncol31010020
Chmiel P, Krotewicz M, Szumera-Ciećkiewicz A, Bartnik E, Czarnecka AM, Rutkowski P. Review on Lymph Node Metastases, Sentinel Lymph Node Biopsy, and Lymphadenectomy in Sarcoma. Current Oncology. 2024; 31(1):307-323. https://doi.org/10.3390/curroncol31010020
Chicago/Turabian StyleChmiel, Paulina, Maria Krotewicz, Anna Szumera-Ciećkiewicz, Ewa Bartnik, Anna M. Czarnecka, and Piotr Rutkowski. 2024. "Review on Lymph Node Metastases, Sentinel Lymph Node Biopsy, and Lymphadenectomy in Sarcoma" Current Oncology 31, no. 1: 307-323. https://doi.org/10.3390/curroncol31010020
APA StyleChmiel, P., Krotewicz, M., Szumera-Ciećkiewicz, A., Bartnik, E., Czarnecka, A. M., & Rutkowski, P. (2024). Review on Lymph Node Metastases, Sentinel Lymph Node Biopsy, and Lymphadenectomy in Sarcoma. Current Oncology, 31(1), 307-323. https://doi.org/10.3390/curroncol31010020








