Point-of-Care Orthopedic Oncology Device Development
Abstract
:1. Introduction
- Superior mechanical performance tailored to the anatomical area free from stress-shielding phenomena leading to osteolysis.
- Superior biological performance that promotes bone ingrowth to achieve osseointegration.
- Optimal aesthetic outcomes with perfect fit.
- Optimal postoperative function with fast recovery and fewer complications and risks.
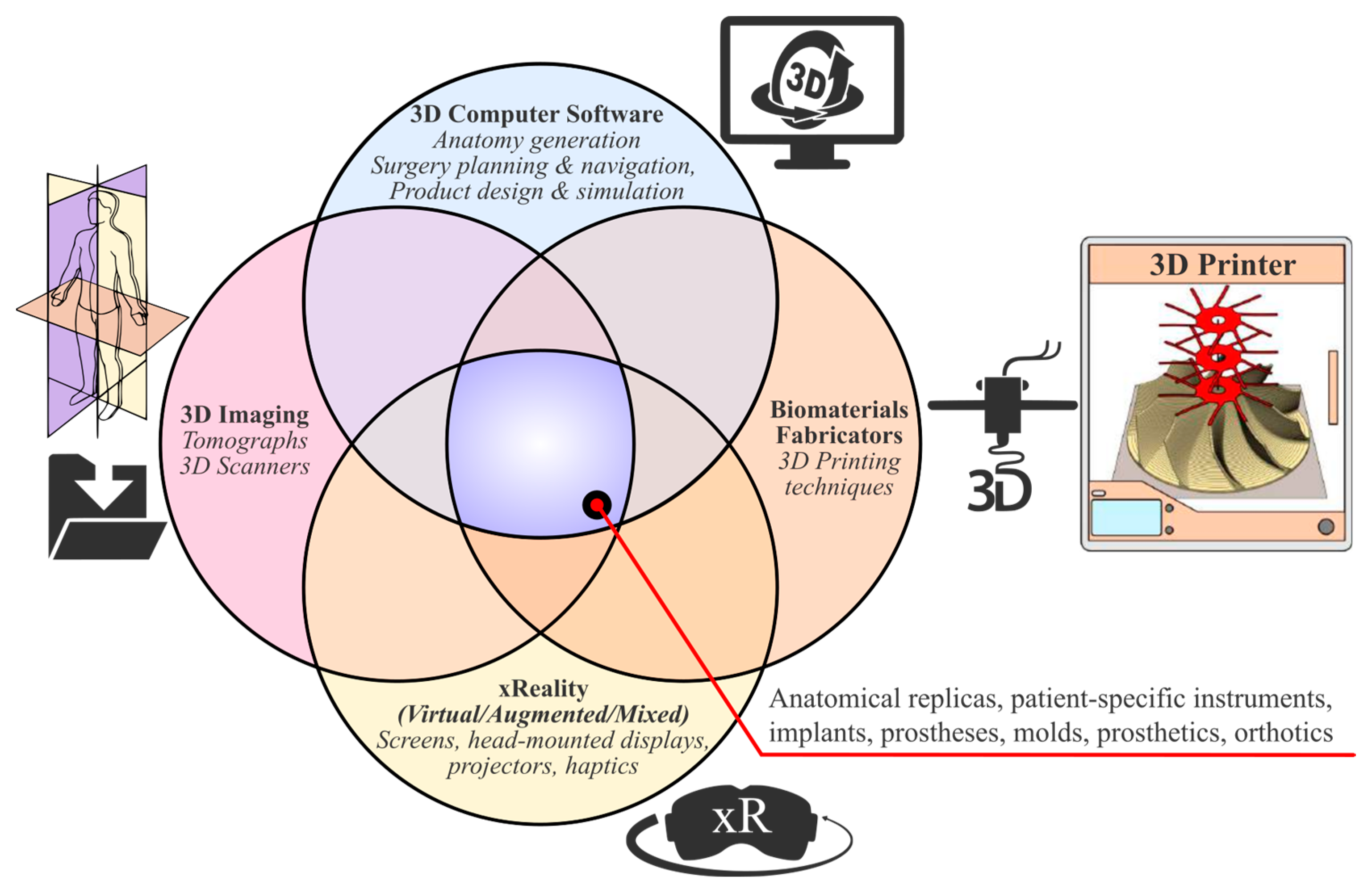
1.1. Enabling 3D Technologies
- 3D medical imaging: Tomographs (internal and external forms) and 3D scanners (external shapes) are used to capture high-fidelity anatomical image data for 3D visualization.
- 3D computer software: These are used to (a) process medical image data; (b) plan pre-operatively and navigate intra-operatively; and (c) design, simulate, and optimize medical devices.
- xReality: These are virtual (VR), augmented (AR), and mixed (MR) devices used for (semi)immersive experiences to support pre-surgical planning, simulation, and surgical navigation. With VR (headsets), the user has the feeling of being part of an artificial world (virtual presence) that is different from actual physical reality, while with AR and MR (headsets, monitors, projectors), the user has a semi-immersive experience in which computer-generated content (text, images, animations) is superimposed over the user’s actual environment.
- 3D printing: This refers to (bio)material fabrication techniques for realizing physical medical devices from digital 3D models created with computer software.
1.2. Three-Dimensional Printing of Individualized Medical Devices
- Physical replicas aid in better anatomic anticipation and physical surgical simulations for pre-operative planning, communication, teaching, and training. Particularly useful is the utilization of multi-material 3D printing where hard and soft structures are fabricated to mimic biological tissues [23,24].
- Patient-specific solutions help
- To improve intra-operative navigation and surgical (biomechanics and functions) and aesthetic results.
- To decrease operating time, surgical risks and errors, blood loss, radiation exposure/fluoroscopy shots, and postoperative complications and infections.
- To lower cost and development times for one-off unique parts compared with traditional manufacturing methods.
- To create better coordination and communication between multi-disciplinary and inter-epistemic teams without disrupting their workflows.
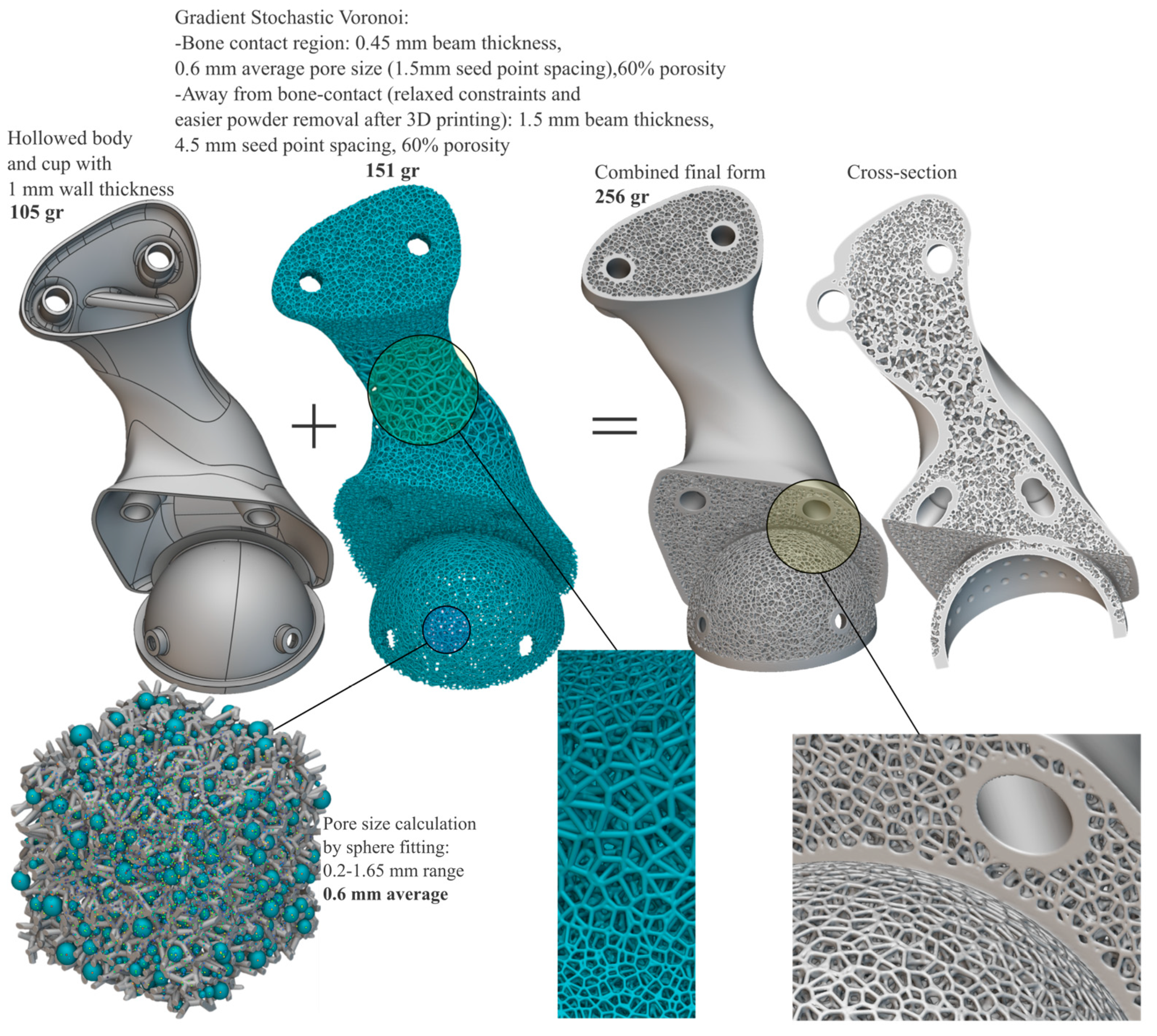
- Biomimetic weight, surface, and topology optimized structures with favorable inter-connected porous geometries can be implemented to fabricate scaffolds for tissue engineering and bone grafts [25] or for artificial bone endo-prostheses for increased osseointegration and bone-matching mechanical properties [26,27]. Thus, properties are topologically and selectively tuned along the monolithic part, per se.
- Novel medical devices are accelerated and design and/or timely manufacturing at the PoC is empowered.
1.3. xReality Applications
1.4. Scope
2. Materials and Methods
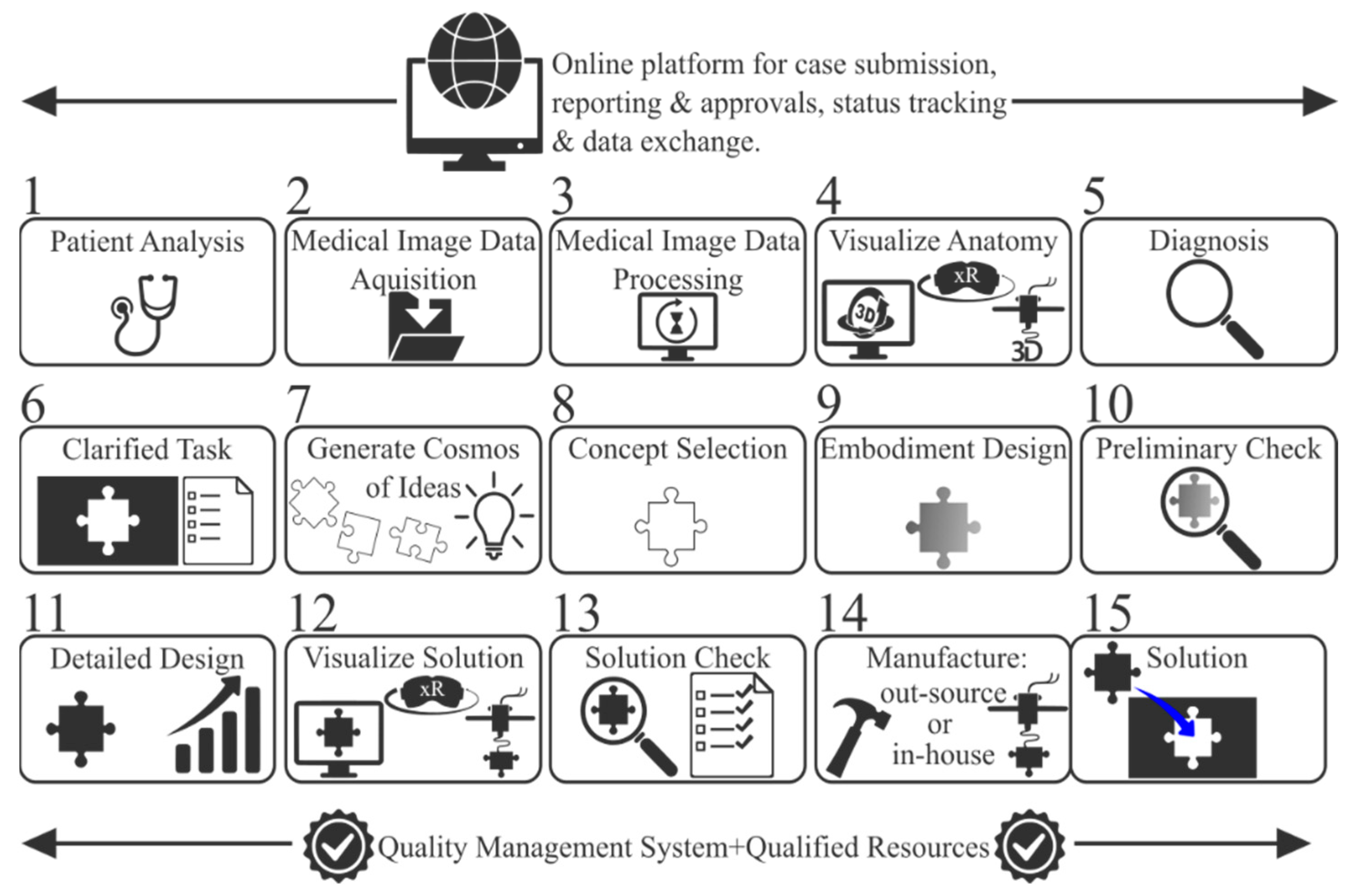
2.1. Step 1—Patient Analysis
2.2. Step 2—Medical Image Acquisition
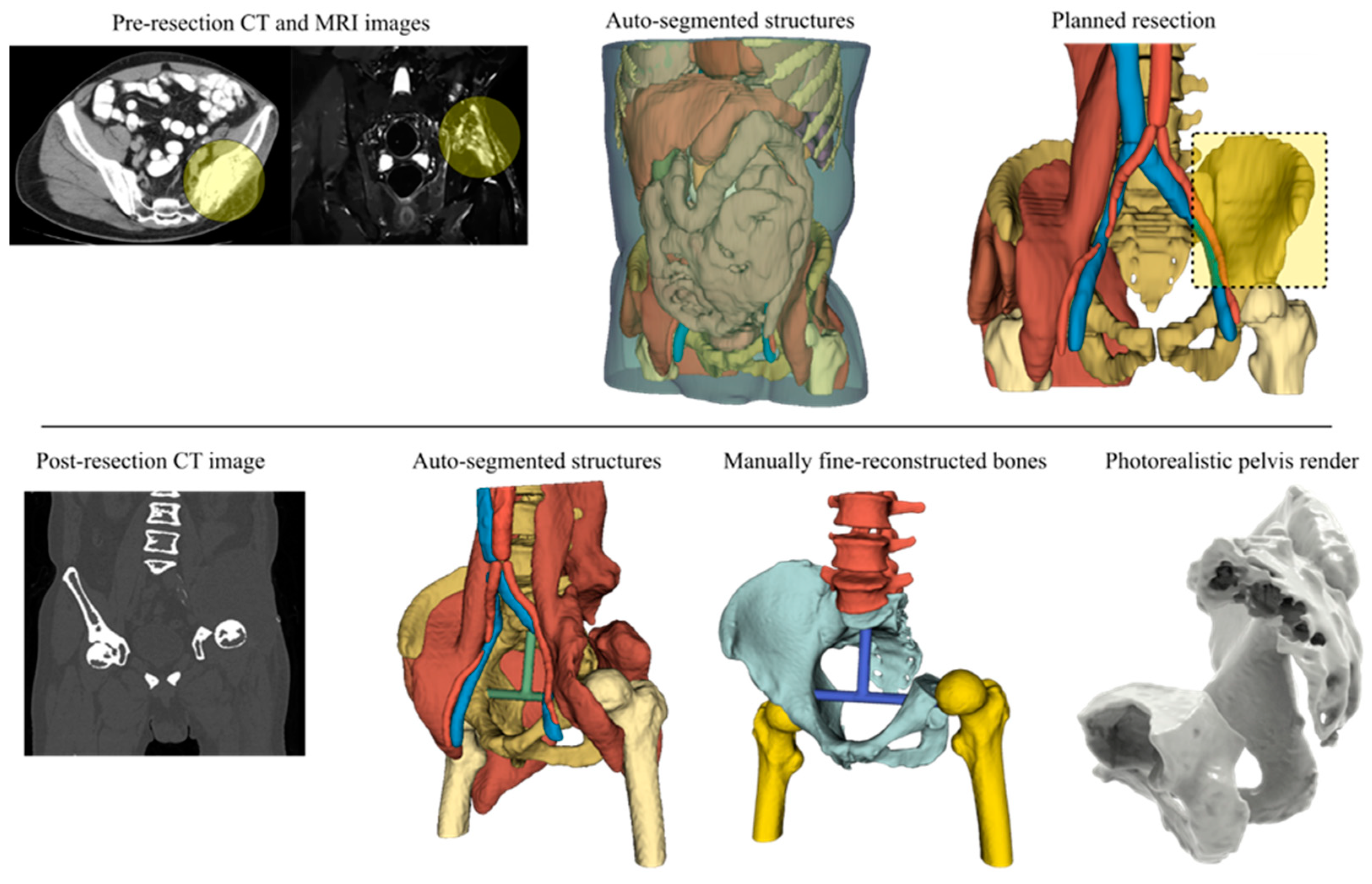
2.3. Steps 3 and 4—Image Data Processing and Anatomy Visualization
2.4. Steps 5 and 6—Rehabilitation Strategy and Task Clarification
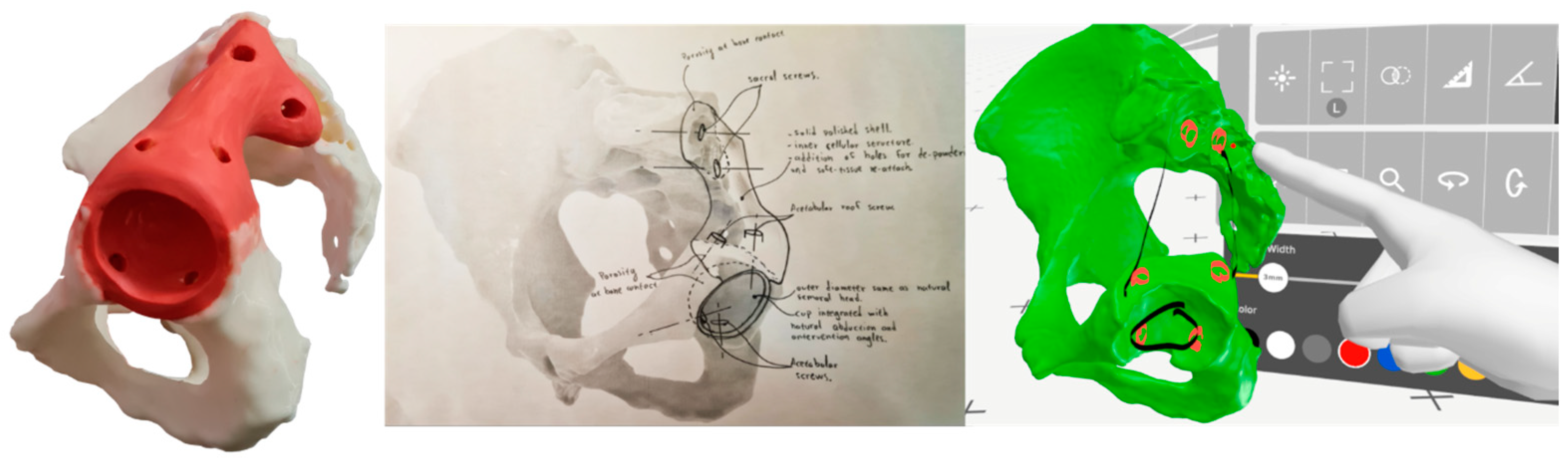
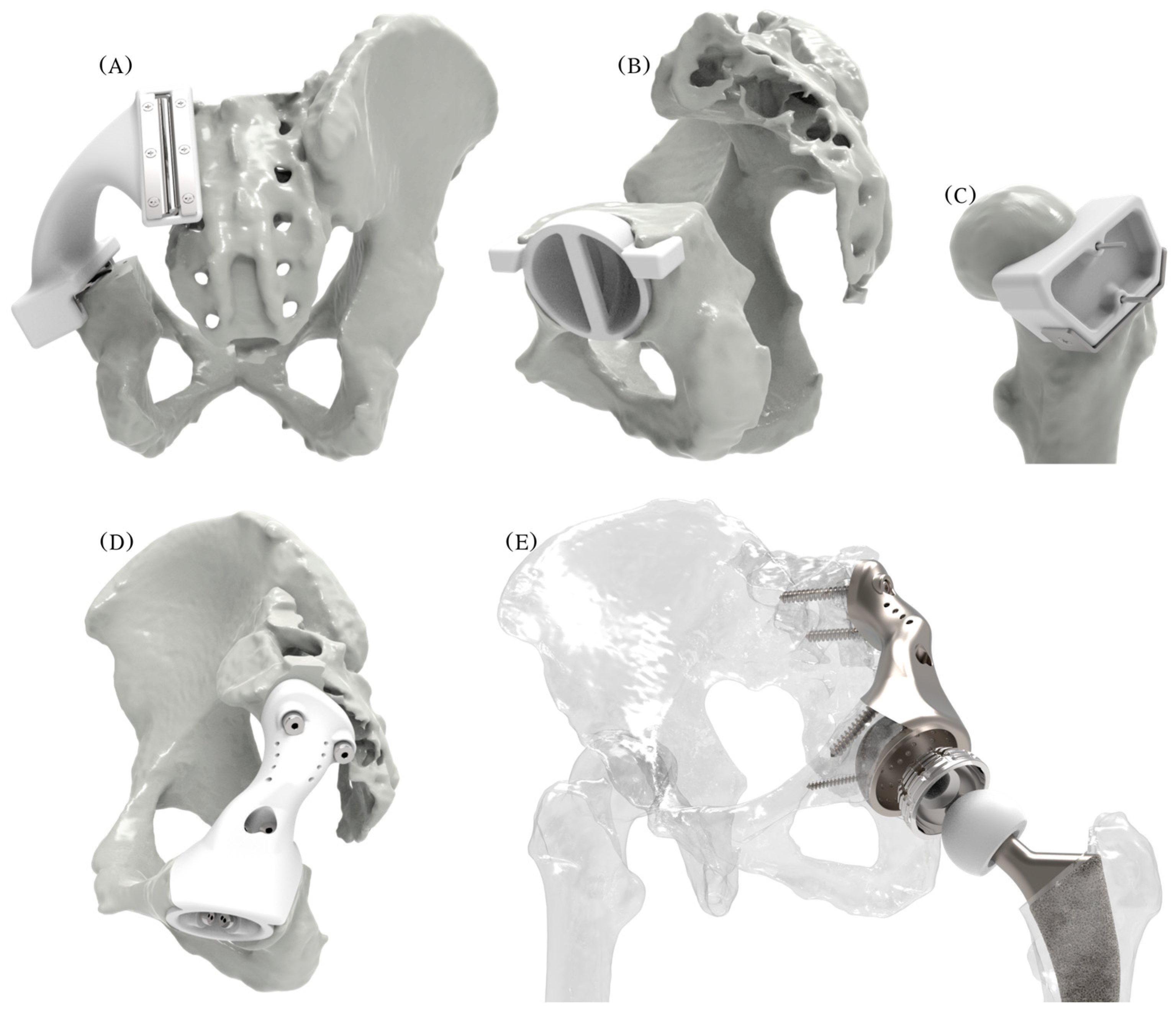
2.5. Steps 7 and 8—Conceptual Design and Evaluation
2.6. Steps 9, 10, and 11—Embodiment and Detailed Design
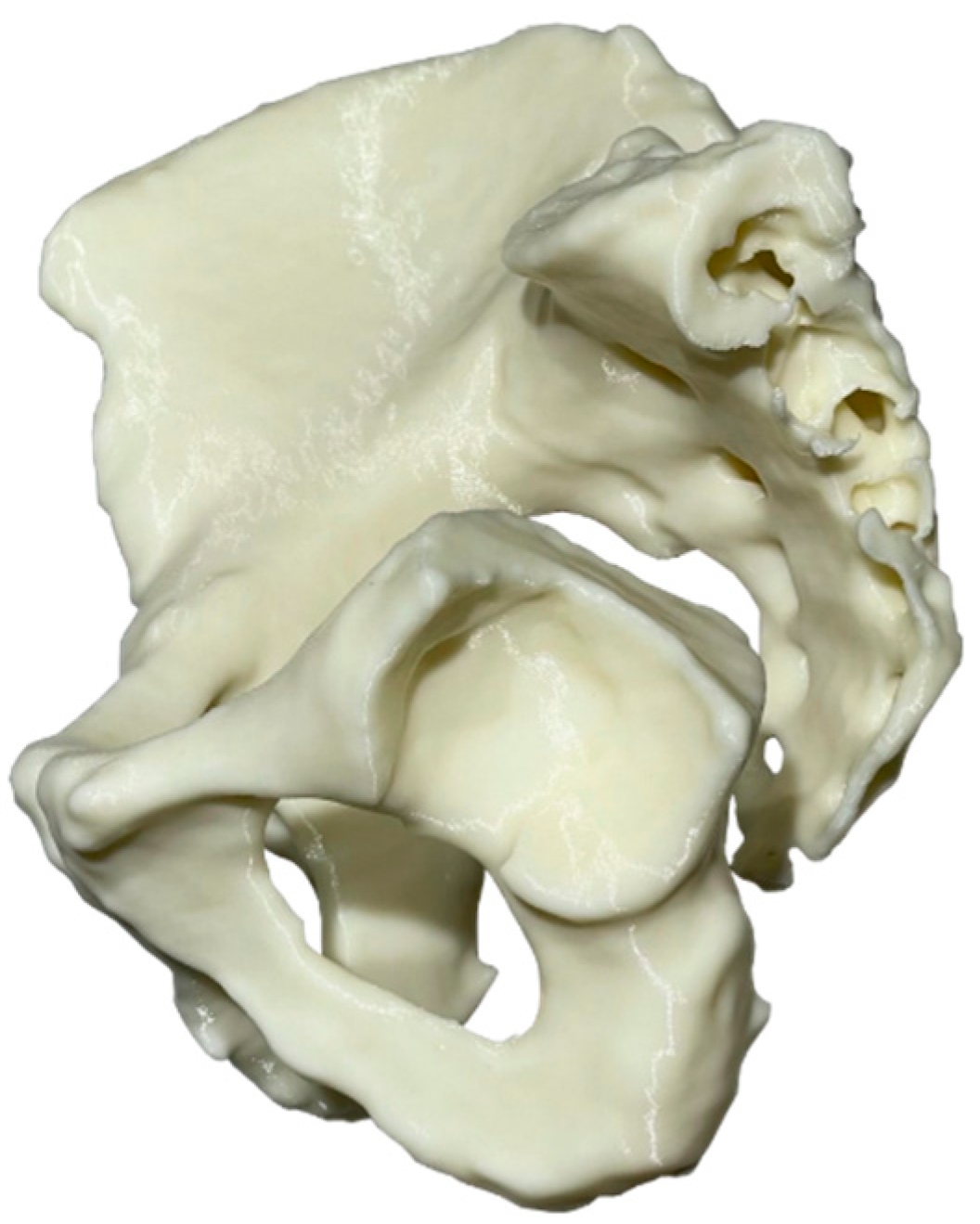
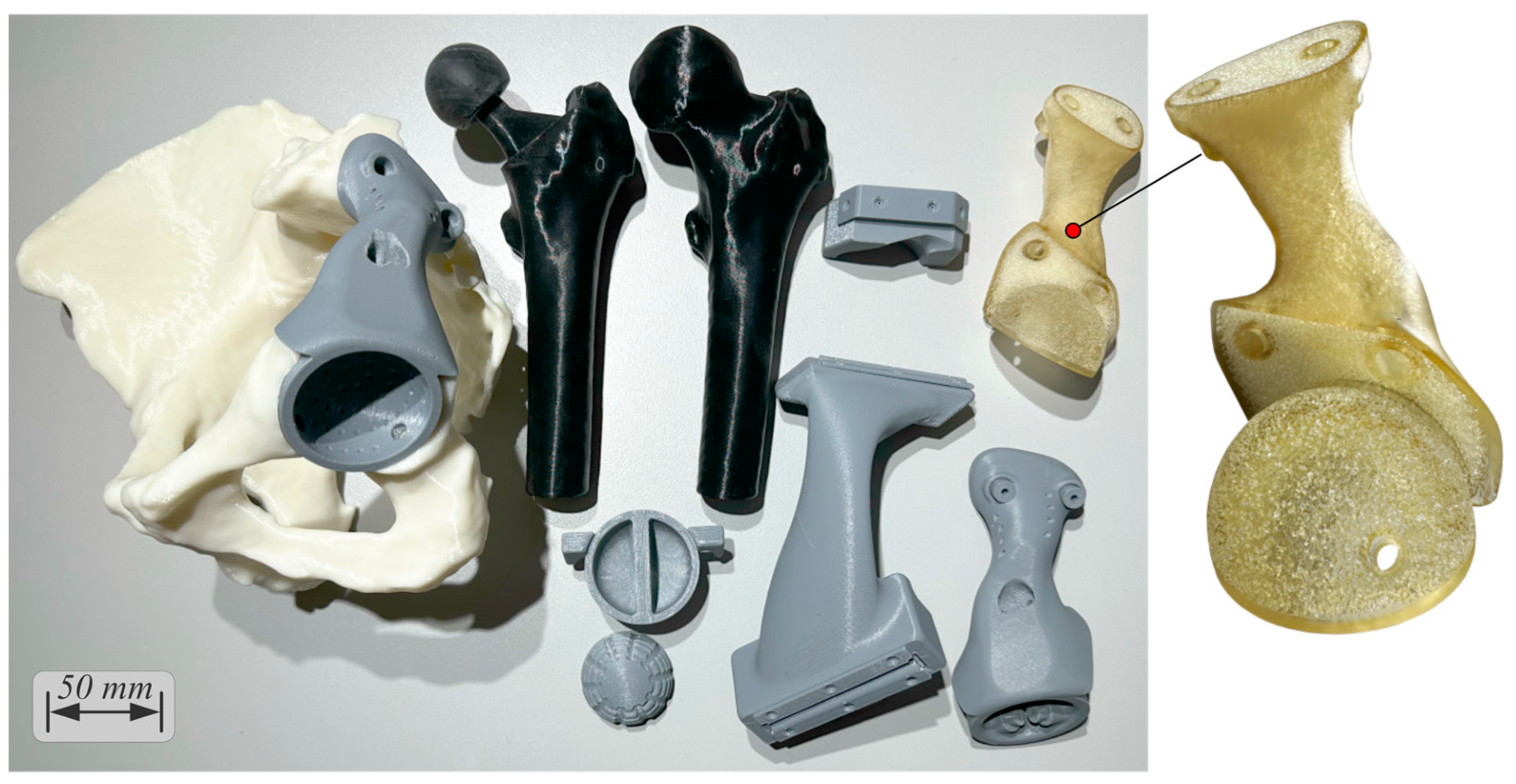
2.7. Step 12—Prototyping
2.8. Steps 13, 14, and 15—Final Meeting, Manufacturing, and Implantation
- The cutting jig and drilling jig-trial endo-prosthesis instrument with all metal inserts, the reaming check tool, and the femoral head cutting jig were ordered to be 3D-printed out of surgical-grade PA12 with a suitable powder-bed fusion technology (laser sintering). The total cost was EUR 380 for 6 working days and delivery.
- The endo-prosthesis was ordered to be 3D-printed out of surgical-grade Ti-6Al-4V with a suitable powder-bed fusion technology (laser melting), followed by glass bead blasting for satin surface finish. The total cost was EUR 1800 for 6 working days and delivery.
3. Results and Discussion
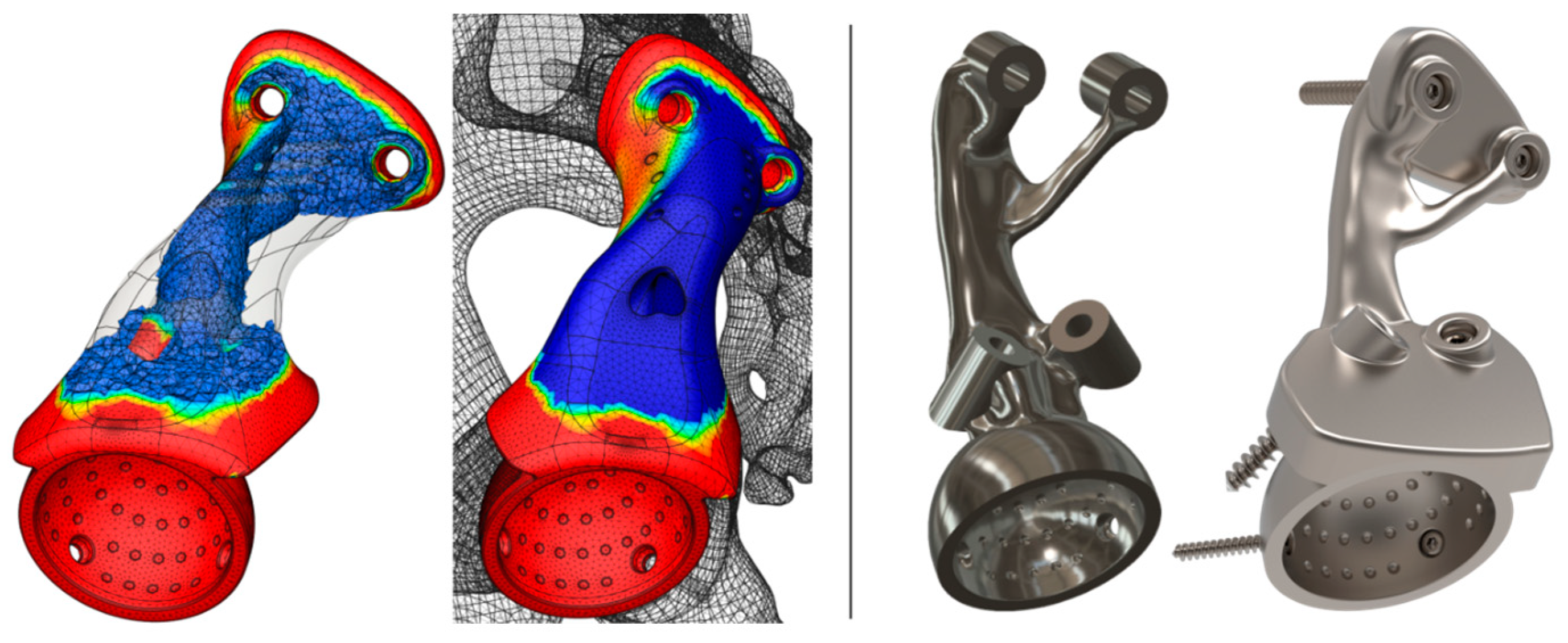
- Extra-mechanical simulations considering anisotropic and inhomogeneous bone tissue properties [93,94] and surrounding soft tissue; the threads of bone screws and their tightening reaction forces; and dynamic fatigue analyses should be executed by considering more loading scenarios such as direct impacts caused by sideways falls.
- Regarding the mechanical performance of the spongy architectures, alternative functionally graded structures could be explored and simulated while also investigating local stress concentrations, contact phenomena, and deformation levels on the nodes and beams to ensure that these can withstand the exerted loads without failure. Ideally, cellular structures should be generated by simulation-driven multi-objective and multi-scale tools that account for spatial variations in parameters across parts, per se.
- Research on coating substances that can be applied to the porous surface for bacterial-fighting and osseointegration-boosting activities.
- More case studies should enrich the portfolio, and follow-ups must be reported.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Özger, H.; Sim, F.; Puri, A.; Eralp, L. Orthopedic Surgical Oncology for Bone Tumors; Springer: Cham, Germany, 2022. [Google Scholar]
- Li, X.; Feng, Y.; Wang, C.; Li, G.; Lei, W.; Zhang, Z.; Wang, L. Evaluation of Biological Properties of Electron Beam Melted Ti6Al4V Implant with Biomimetic Coating In Vitro and In Vivo. PLoS ONE 2012, 7, e52049. [Google Scholar] [CrossRef] [PubMed]
- Regis, M.; Marin, E.; Fedrizzi, L.; Pressacco, M. Additive manufacturing of Trabecular Titanium orthopedic implants. MRS Bull. 2015, 40, 137–144. [Google Scholar] [CrossRef]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C 2016, 59, 690–701. [Google Scholar] [CrossRef] [PubMed]
- ISO/ASTM 52900:2021; Additive Manufacturing—General Principles—Fundamentals and Vocabulary. ISO: Geneva, Switzerland, 2021.
- Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M. Additive Manufacturing Technologies, 3rd ed.; Springer: Cham, Germany, 2021. [Google Scholar]
- Wohlers Associates. Wohlers Report: 3D Printing and Additive Manufacturing Global State of the Industry; Wohlers Associates: Fort Collins, CO, USA, 2021. [Google Scholar]
- ASME. Medical Additive Manufacturing/3D Printing: Year in Review 2019–2020; ASME: New York, NY, USA, 2020. [Google Scholar]
- Ejnisman, L.; Gobbato, B.; de França Camargo, A.; Zancul, E. Three-Dimensional Printing in Orthopedics: From the Basics to Surgical Applications. Curr. Rev. Musculoskelet. Med. 2021, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Charbonnier, B.; Hadida, M.; Marchat, D. Additive manufacturing pertaining to bone: Hopes, reality and future challenges for clinical applications. Acta Biomater. 2021, 121, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Salmi, M. Additive Manufacturing Processes in Medical Applications. Materials 2021, 14, 191. [Google Scholar] [CrossRef]
- Lowther, M.; Louth, S.; Davey, A.; Hussain, A.; Ginestra, P.; Carter, L.; Eisenstein, N.; Grover, L.; Cox, S. Clinical, industrial, and research perspectives on powder-bed fusion additively manufactured metal implants. Addit. Manuf. 2019, 28, 565–584. [Google Scholar] [CrossRef]
- Fogarasi, M.; Snodderly, K.L.; Di Prima, M.A. A survey of additive manufacturing trends for FDA-cleared medical devices. Nat. Rev. Bioeng. 2023, 1, 687–689. [Google Scholar] [CrossRef]
- Levesque, J.; Shah, A.; Ekhtiari, S.; Yan, J.; Thornley, P.; Williams, D. Three-dimensional printing in orthopaedic surgery: A scoping review. EFORT Open Rev. 2020, 5, 430–441. [Google Scholar] [CrossRef]
- Kermavnar, T.; Shannon, A.; O’Sullivan, K.; McCarthy, C.; Dunne, C.; O’Sullivan, L. Three-Dimensional Printing of Medical Devices Used Directly to Treat Patients: A Systematic Review. 3D Print. Addit. Manuf. 2021, 8, 366–408. [Google Scholar] [CrossRef]
- Angelini, A.; Trovarelli, G.; Berizzi, A.; Pala, E.; Breda, A.; Ruggieri, P. Three-dimension-printed custom-made prosthetic reconstructions: From revision surgery to oncologic reconstructions. Int. Orthop. 2018, 43, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Angelini, A.; Kotrych, D.; Trovarelli, G.; Szafrański, A.; Bohatyrewicz, A.; Ruggieri, P. Analysis of principles inspiring design of three-dimensional-printed custom-made prostheses in two referral centres. Int. Orthop. 2020, 44, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kang, H.; Kim, J.; Kim, H. New 3-dimensional implant application as an alternative to allograft in limb salvage surgery: A technical note on 10 cases. Acta Orthop. 2020, 91, 489–496. [Google Scholar] [CrossRef]
- Martelli, N.; Serrano, C.; van den Brink, H.; Pineau, J.; Prognon, P.; Borget, I.; El Batti, S. Advantages and disadvantages of 3-dimensional printing in surgery: A systematic review. Surgery 2016, 159, 1485–1500. [Google Scholar] [CrossRef]
- Langridge, B.; Momin, S.; Coumbe, B.; Woin, E.; Griffin, M.; Butler, P. Systematic Review of the Use of 3-Dimensional Printing in Surgical Teaching and Assessment. J. Surg. Educ. 2018, 75, 209–221. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A. Additive manufacturing applications in orthopaedics: A review. J. Clin. Orthop. Trauma 2018, 9, 202–206. [Google Scholar] [CrossRef]
- Kumar, L.; Haleem, A.; Javaid, M. Impact of three-dimensional printing in orthopedics. J. Glob. Health 2021, 5, 178–182. [Google Scholar] [CrossRef]
- Goh, G.D.; Sing, S.L.; Lim, Y.; Thong, J.; Peh, Z.; Mogali, S.R.; Yeong, W.Y. Machine learning for 3D printed multi-materials tissue-mimicking anatomical models. Mater. Des. 2021, 211, 110125. [Google Scholar] [CrossRef]
- Bezek, L.B.; Cauchi, M.P.; De Vita, R.; Foerst, J.R.; Williams, C.B. 3D printing tissue-mimicking materials for realistic transseptal puncture models. J. Mech. Behav. Biomed. Mater. 2020, 110, 103971. [Google Scholar] [CrossRef]
- Brachet, A.; Bełżek, A.; Furtak, D.; Geworgjan, Z.; Tulej, D.; Kulczycka, K.; Karpiński, R.; Maciejewski, M.; Baj, J. Application of 3D Printing in Bone Grafts. Cells 2023, 12, 859. [Google Scholar] [CrossRef]
- Benedetti, M.; du Plessis, A.; Ritchie, R.; Dallago, M.; Razavi, S.; Berto, F. Architected cellular materials: A review on their mechanical properties towards fatigue-tolerant design and fabrication. Mater. Sci. Eng. Rep. 2021, 144, 100606. [Google Scholar] [CrossRef]
- McGregor, M.; Patel, S.; McLachlin, S.; Vlasea, M. Architectural bone parameters and the relationship to titanium lattice design for powder-bed fusion additive manufacturing. Addit. Manuf. 2021, 47, 102273. [Google Scholar] [CrossRef]
- Frizziero, L.; Santi, G.; Liverani, A.; Giuseppetti, V.; Trisolino, G.; Maredi, E.; Stilli, S. Paediatric Orthopaedic Surgery with 3D Printing: Improvements and Cost Reduction. Symmetry 2019, 11, 1317. [Google Scholar] [CrossRef]
- Pajot, T.; Benichou, L.; Moreau, E.; Tallon, V.; Meningaud, J.; Khonsari, R.; Ketoff, S. Implementation of a digital chain for the design and manufacture of implant-based surgical guides in a hospital setting. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Teo, A.; Ng, D.; Lee, P.; O’Neill, G. Point-of-Care 3D Printing: A Feasibility Study of Using 3D Printing for Orthopaedic Trauma. Injury 2021, 52, 3286–3292. [Google Scholar] [CrossRef] [PubMed]
- Popescu, D.; Marinescu, R.; Sandache, O. Upper Limbs Orthoses Production in 3D Printing Points-of-Care. In Proceedings of the 2021 International Conference on e-Health and Bioengineering (EHB), Iasi, Romania, 18–19 November 2021; pp. 1–4. [Google Scholar]
- Calvo-Haro, J.; Pascau, J.; Asencio-Pascual, J.; Calvo-Manuel, F.; Cancho-Gil, M.; Del Cañizo López, J.; Fanjul-Gómez, M.; García-Leal, R.; González-Casaurrán, G.; González-Leyte, M.; et al. Point-of-care manufacturing: A single university hospital’s initial experience. 3D Print. Med. 2021, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Guebeli, A.; Thieringer, F.; Honigmann, P. In-hospital professional production of patient-specific 3D-printed devices for hand and wrist rehabilitation. Hand Surg. Rehabil. 2021, 40, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Honigmann, P.; Sharma, N.; Schumacher, R.; Rueegg, J.; Haefeli, M.; Thieringer, F. In-Hospital 3D Printed Scaphoid Prosthesis Using Medical-Grade Polyetheretherketone (PEEK) Biomaterial. Biomed Res. Int. 2021, 2021, 1301028. [Google Scholar] [CrossRef]
- Andrés-Cano, P.; Calvo-Haro, J.; Fillat-Gomà, F.; Andrés-Cano, I.; Perez-Mañanes, R. Role of the orthopaedic surgeon in 3D printing: Current applications and legal issues for personalized medicine. Rev. Esp. Cir. Ortop. Traumatol. 2021, 65, 138–151. [Google Scholar] [CrossRef]
- Czyżewski, W.; Jachimczyk, J.; Hoffman, Z.; Szymoniuk, M.; Litak, J.; Maciejewski, M.; Kura, K.; Rola, R.; Torres, K. Low-Cost Cranioplasty-A Systematic Review of 3D Printing in Medicine. Materials 2022, 15, 4731. [Google Scholar] [CrossRef]
- Han, J.; Kang, H.; Kim, M.; Kwon, G. Mapping the intellectual structure of research on surgery with mixed reality: Bibliometric network analysis (2000–2019). J. Biomed. Inform. 2020, 109, 103516. [Google Scholar] [CrossRef] [PubMed]
- Walbron, P.; Common, H.; Thomazeau, H.; Hosseini, K.; Peduzzi, L.; Bulaid, Y.; Sirveaux, F. Virtual reality simulator improves the acquisition of basic arthroscopy skills in first-year orthopedic surgery residents. Orthop. Traumatol. Surg. Res. 2020, 106, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Gerup, J.; Soerensen, C.; Dieckmann, P. Augmented reality and mixed reality for healthcare education beyond surgery: An integrative review. Int. J. Med. Educ. 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chau, K.; Kwok, A.; Zhu, T.; Ma, X. A systematic review of immersive technology applications for medical practice and education—Trends, application areas, recipients, teaching contents, evaluation methods, and performance. Educ. Res. Rev. 2022, 35, 100429. [Google Scholar] [CrossRef]
- Jud, L.; Fotouhi, J.; Andronic, O.; Aichmair, A.; Osgood, G.; Navab, N.; Farshad, M. Applicability of augmented reality in orthopedic surgery—A systematic review. BMC Musculoskelet. Disord. 2020, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- Casari, F.; Navab, N.; Hruby, L.; Kriechling, P.; Nakamura, R.; Tori, R.; de Lourdes dos Santos Nunes, F.; Queiroz, M.; Fürnstahl, P.; Farshad, M. Augmented Reality in Orthopedic Surgery Is Emerging from Proof of Concept Towards Clinical Studies: A Literature Review Explaining the Technology and Current State of the Art. Curr. Rev. Musculoskelet. Med. 2021, 14, 192–203. [Google Scholar] [CrossRef]
- Pratt, P.; Ives, M.; Lawton, G.; Simmons, J.; Radev, N.; Spyropoulou, L.; Amiras, D. Through the HoloLens™ looking glass: Augmented reality for extremity reconstruction surgery using 3D vascular models with perforating vessels. Eur. Radiol. Exp. 2018, 2, 2. [Google Scholar] [CrossRef]
- Al Janabi, H.; Aydin, A.; Palaneer, S.; Macchione, N.; Al-Jabir, A.; Khan, M.; Dasgupta, P.; Ahmed, K. Effectiveness of the HoloLens mixed-reality headset in minimally invasive surgery: A simulation-based feasibility study. Surg. Endosc. 2019, 34, 1143–1149. [Google Scholar] [CrossRef]
- Peh, S.; Chatterjea, A.; Pfarr, J.; Schäfer, J.; Weuster, M.; Klüter, T.; Seekamp, A.; Lippross, S. Accuracy of augmented reality surgical navigation for minimally invasive pedicle screw insertion in the thoracic and lumbar spine with a new tracking device. Spine J. 2020, 20, 629–637. [Google Scholar] [CrossRef]
- Elmi-Terander, A.; Burström, G.; Nachabé, R.; Fagerlund, M.; Ståhl, F.; Charalampidis, A.; Edström, E.; Gerdhem, P. Augmented reality navigation with intraoperative 3D imaging vs fluoroscopy-assisted free-hand surgery for spine fixation surgery: A matched-control study comparing accuracy. Sci. Rep. 2020, 10, 707. [Google Scholar] [CrossRef]
- Tanji, A.; Nagura, T.; Iwamoto, T.; Matsumura, N.; Nakamura, M.; Matsumoto, M.; Sato, K. Total elbow arthroplasty using an augmented reality–assisted surgical technique. J. Shoulder Elb. Surg. 2022, 31, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Pahl, G.; Beitz, W.; Feldhusen, J.; Grote, K. Engineering Design, 3rd ed.; Springer: London, UK, 2007. [Google Scholar]
- Floriane, L.; Frédéric, S.; Gianluca, D.; Marc, L. Enriching design with X through tailored additive manufacturing knowledge: A methodological proposal. Int. J. Interact. Des. Manuf. 2016, 11, 279–288. [Google Scholar] [CrossRef]
- Kranz, J.; Herzog, D.; Emmelmann, C. Design guidelines for laser additive manufacturing of lightweight structures in TiAl6V4. J. Laser Appl. 2015, 27, S14001. [Google Scholar] [CrossRef]
- Diegel, O.; Nordin, A.; Motte, D. A Practical Guide to Design for Additive Manufacturing; Springer: London, UK, 2019. [Google Scholar]
- British Design Council. Available online: https://www.designcouncil.org.uk/fileadmin/uploads/dc/Documents/ElevenLessons_Design_Council%2520%25282%2529.pdf (accessed on 8 December 2023).
- Biomimicry Institute. Available online: https://toolbox.biomimicry.org/methods/ (accessed on 8 December 2023).
- Enneking, W.; Dunham, W. Resection and reconstruction for primary neoplasms involving the innominate bone. J. Bone Jt. Surg. 1978, 60, 731–746. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- Cignoni, P.; Callieri, M.; Corsini, M.; Dellepiane, M.; Ganovelli, F.; Ranzuglia, G. MeshLab: An Open-Source Mesh Processing Tool. In Proceedings of the Sixth Eurographics Italian Chapter Conference, Salermo, Italy, 2–4 July 2008; pp. 129–136. [Google Scholar]
- Sharma, A.K.; Cizmic, Z.; Dennis, D.A.; Kreuzer, S.W.; Miranda, M.A.; Vigdorchik, J.M. Low dislocation rates with the use of patient-specific “Safe zones” in total hip arthroplasty. J. Orthop. 2021, 21, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Wasielewski, R.; Cooperstein, L.; Kruger, M.; Rubash, H. Acetabular anatomy and the transacetabular fixation of screws in total hip arthroplasty. J. Bone Jt. Surg. 1990, 72, 501–508. [Google Scholar] [CrossRef]
- Wu, C.; Deng, J.; Pan, J.; Li, T.; Tan, L.; Yuan, D. Anatomical conditions and patient-specific locked navigation templates for transverse sacroiliac screw placement: A retrospective study. J. Orthop. Surg. Res. 2020, 15, 260. [Google Scholar] [CrossRef]
- Hasegawa, K.; Kabata, T.; Kajino, Y.; Inoue, D.; Tsuchiya, H. Periprosthetic Occult Fractures of the Acetabulum Occur Frequently During Primary THA. Clin. Orthop. Relat. Res. 2017, 475, 484–494. [Google Scholar] [CrossRef]
- Hsu, T.; Chang, H.; Huang, L.; Zobitz, E.; Chen, P.; Lai, A.; An, N. The number of screws, bone quality, and friction coefficient affect acetabular cup stability. Med. Eng. Phys. 2007, 29, 1089–1095. [Google Scholar] [CrossRef]
- Ravera, P.; Crespo, J.; Guarnieri, A.; Braidot, A. Stress in human pelvis throughout the gait cycle: Development, evaluation, and sensitivity studies of a finite element model. In Proceedings of the IFMBE Proceedings, Toronto, ON, Canada, 7–12 June 2015; pp. 246–249. [Google Scholar]
- Ricci, P.L.; Maas, S.; Kelm, J.; Gerich, T. Finite element analysis of the pelvis including gait muscle forces: An investigation into the effect of Rami fractures on load transmission. J. Exp. Orthop. 2018, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, G.; Bender, A.; Dymke, J.; Duda, G.; Damm, P. Standardized loads acting in hip implants. PLoS ONE 2016, 11, e0155612. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, G.; Graichen, F.; Rohlmann, A. Hip joint contact forces during stumbling. Langenbeck’s Arch. Surg. 2004, 389, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Tan, Y.; Chow, C.; Tor, S.; Yeong, W. Metallic powder-bed based 3D printing of cellular scaffolds for orthopaedic implants: A state-of-the-art review on manufacturing, topological design, mechanical properties and biocompatibility. Mater. Sci. Eng. C 2017, 76, 1328–1343. [Google Scholar] [CrossRef] [PubMed]
- Barba, D.; Alabort, E.; Reed, R. Synthetic bone: Design by additive manufacturing. Acta Biomater. 2019, 97, 637–656. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.; Matlack, K.; Shea, K.; Daraio, C. Energy Absorption Properties of Periodic and Stochastic 3D Lattice Materials. Adv. Theory Simul. 2019, 2, 1900081. [Google Scholar] [CrossRef]
- Benedetti, M.; Klarin, J.; Johansson, F.; Fontanari, V.; Luchin, V.; Zappini, G.; Molinari, A. Study of the Compression Behaviour of Ti6Al4V Trabecular Structures Produced by Additive Laser Manufacturing. Materials 2019, 12, 1471. [Google Scholar] [CrossRef]
- Yang, E.; Leary, M.; Lozanovski, B.; Downing, D.; Mazur, M.; Sarker, A.; Khorasani, A.; Jones, A.; Maconachie, T.; Bateman, S.; et al. Effect of geometry on the mechanical properties of Ti-6Al-4V Gyroid structures fabricated via SLM: A numerical study. Mater. Des. 2019, 184, 108165. [Google Scholar] [CrossRef]
- Aguado-Maestro, I.; De Frutos-Serna, M.; González-Nava, A.; Merino-De Santos, A.; García-Alonso, M. Are the common sterilization methods completely effective for our in-house 3D printed biomodels and surgical guides? Injury 2021, 52, 1341–1345. [Google Scholar] [CrossRef]
- Bini, S.; Schilling, P.; Patel, S.; Kalore, N.; Ast, M.; Maratt, J.; Schuett, D.; Lawrie, C.; Chung, C.; Steele, G. Digital Orthopaedics: A Glimpse into the Future in the Midst of a Pandemic. J. Arthroplast. 2020, 35, 68–73. [Google Scholar] [CrossRef]
- Vakharia, V.; Khan, S.; Marathe, K.; Giannis, T.; Webber, L.; Choi, D. Printing in a Pandemic: 3D printing solutions for healthcare during COVID-19. A Protocol for a PRISMA systematic review. Ann. 3D Print. Med. 2021, 2, 100015. [Google Scholar] [CrossRef]
- Singh, S.N.; Venkatesh, V.S.; Deoghare, A.B. A review on the role of 3D printing in the fight against COVID-19: Safety and challenges. Rapid Prototyp. J. 2021, 27, 407–420. [Google Scholar] [CrossRef]
- Dall’Ava, L.; Hothi, H.; Henckel, J.; Di Laura, A.; Tirabosco, R.; Eskelinen, A.; Skinner, J.; Hart, A. Osseointegration of retrieved 3D-printed, off-the-shelf acetabular implants. Bone Jt. Res. 2021, 10, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Li, Y.; Li, F.; Wang, X.; Zhang, K.; Liu, Z.; Tian, H. A new 3D printing porous trabecular titanium metal acetabular cup for primary total hip arthroplasty: A minimum 2-year follow-up of 92 consecutive patients. J. Orthop. Surg. Res. 2020, 15, 383. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhou, Y.; Tian, H.; Wang, J.; Liu, W.; Li, H. Minimum 7-year Follow-up of A Porous Coated Trabecular Titanium Cup Manufactured with Electron Beam Melting Technique in Primary Total Hip Arthroplasty. Orthop. Surg. 2021, 13, 817–824. [Google Scholar] [CrossRef]
- Wang, J.; Min, L.; Lu, M.; Zhang, Y.; Wang, Y.; Luo, Y.; Zhou, Y.; Duan, H.; Tu, C. What are the Complications of Three-dimensionally Printed, Custom-made, Integrative Hemipelvic Endoprostheses in Patients with Primary Malignancies Involving the Acetabulum, and What is the Function of These Patients? Clin. Orthop. Relat. Res. 2020, 478, 2487–2501. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, K.; Zhang, Y.; Wang, C.; Yang, K.; Zou, Y.; Chen, B.; Wang, J. Individual resection and reconstruction of pelvic tumor with three-dimensional printed customized hemi-pelvic prosthesis. Medicine 2019, 98, e16658. [Google Scholar] [CrossRef]
- Durand-Hill, M.; Henckel, J.; Di Laura, A.; Hart, A. Can custom 3D printed implants successfully reconstruct massive acetabular defects? A 3D-CT assessment. J. Orthop. Res. 2020, 38, 2640–2648. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, H.; Gao, C. A Systematic Review and Meta-Analysis of 3D Printing Technology for the Treatment of Acetabular Fractures. BioMed Res. Int. 2021, 2021, 5018791. [Google Scholar] [CrossRef]
- Hu, X.; Chen, Y.; Cai, W.; Cheng, M.; Yan, W.; Huang, W. Computer-Aided Design and 3D Printing of Hemipelvic Endoprosthesis for Personalized Limb-Salvage Reconstruction after Periacetabular Tumor Resection. Bioengineering 2022, 18, 400. [Google Scholar] [CrossRef]
- Xu, S.; Guo, Z.; Shen, Q.; Peng, Y.; Li, J.; Li, S.; He, P.; Jiang, Z.; Que, Y.; Cao, K.; et al. Reconstruction of Tumor-Induced Pelvic Defects with Customized, Three-Dimensional Printed Prostheses. Front. Oncol. 2022, 12, 935059. [Google Scholar] [CrossRef] [PubMed]
- Khal, A.A.; Apostu, D.; Schiau, C.; Bejinariu, N.; Pesenti, S.; Jouve, J.L. Custom-Made 3D-Printed Prosthesis after Resection of a Voluminous Giant Cell Tumour Recurrence in Pelvis. Diagnostics 2023, 13, 485. [Google Scholar] [CrossRef] [PubMed]
- Vaneker, T.; Bernard, A.; Moroni, G.; Gibson, I.; Zhang, Y. Design for Additive Manufacturing: Framework and methodology. CIRP Ann. Manuf. Technol. 2020, 69, 578–599. [Google Scholar] [CrossRef]
- Davoodi, E.; Montazerian, H.; Mirhakimi, A.; Zhianmanesh, M.; Ibhadode, O.; Shahabad, S.; Esmaeilizadeh, R.; Sarikhani, E.; Toorandaz, S.; Sarabi, S.A.; et al. Additively manufactured metallic biomaterials. Bioact. Mater. 2022, 15, 214–249. [Google Scholar] [CrossRef] [PubMed]
- Maconachie, T.; Leary, M.; Lozanovski, B.; Zhang, X.; Qian, M.; Faruque, O.; Brandt, M. SLM lattice structures: Properties, performance, applications and challenges. Mater. Des. 2019, 183, 108137. [Google Scholar] [CrossRef]
- Chen, L.; Liang, S.; Liu, Y.; Zhang, L. Additive manufacturing of metallic lattice structures: Unconstrained design, accurate fabrication, fascinated performances, and challenges. Mater. Sci. Eng. Rep. 2021, 146, 100648. [Google Scholar] [CrossRef]
- Khrapov, D.; Paveleva, A.; Kozadayeva, M.; Evsevleev, S.; Mishurova, T.; Bruno, G.; Surmenev, R.; Koptyug, A.; Surmeneva, M. Trapped powder removal from sheet-based porous structures based on triply periodic minimal surfaces fabricated by electron beam powder bed fusion. Mater. Sci. Eng. A 2023, 862, 144479. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, Z.; Demir, K.; Gu, G. Machine Learning for Advanced Additive Manufacturing. Matter 2020, 3, 1541–1556. [Google Scholar] [CrossRef]
- Park, J.; Kwon, H.; Lee, W.; Yang, I.; Park, K. Anthropometric Measurement About the Safe Zone for Transacetabular Screw Placement in Total Hip Arthroplasty in Asian Middle-Aged Women: In Vivo Three-Dimensional Model Analysis. J. Arthroplast. 2021, 36, 744–751. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, W.; Fang, X.; Tu, C.; Duan, H. Pelvic Reconstruction with a Novel Three-Dimensional-Printed, Multimodality Imaging Based Endoprosthesis Following Enneking Type I + IV Resection. Front. Oncol. 2021, 11, 629582. [Google Scholar] [CrossRef]
- Helgason, B.; Perilli, E.; Schileo, E.; Taddei, F.; Brynjólfsson, S.; Viceconti, M. Mathematical relationships between bone density and mechanical properties: A literature review. Clin. Biomech. 2008, 23, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Vivanco, F.; Burgers, A.; García-Rodríguez, S.; Crookshank, M.; Kunz, M.; MacIntyre, J.; Harrison, M.; Bryant, T.; Sellens, W.; Ploeg, L. Estimating the density of femoral head trabecular bone from hip fracture patients using computed tomography scan data. Proc. Inst. Mech. Eng. Part H J. Med. Eng. 2014, 228, 616–626. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavrodontis, I.I.; Trikoupis, I.G.; Kontogeorgakos, V.A.; Savvidou, O.D.; Papagelopoulos, P.J. Point-of-Care Orthopedic Oncology Device Development. Curr. Oncol. 2024, 31, 211-228. https://doi.org/10.3390/curroncol31010014
Mavrodontis II, Trikoupis IG, Kontogeorgakos VA, Savvidou OD, Papagelopoulos PJ. Point-of-Care Orthopedic Oncology Device Development. Current Oncology. 2024; 31(1):211-228. https://doi.org/10.3390/curroncol31010014
Chicago/Turabian StyleMavrodontis, Ioannis I., Ioannis G. Trikoupis, Vasileios A. Kontogeorgakos, Olga D. Savvidou, and Panayiotis J. Papagelopoulos. 2024. "Point-of-Care Orthopedic Oncology Device Development" Current Oncology 31, no. 1: 211-228. https://doi.org/10.3390/curroncol31010014
APA StyleMavrodontis, I. I., Trikoupis, I. G., Kontogeorgakos, V. A., Savvidou, O. D., & Papagelopoulos, P. J. (2024). Point-of-Care Orthopedic Oncology Device Development. Current Oncology, 31(1), 211-228. https://doi.org/10.3390/curroncol31010014





