Systematic Review of Calcineurin Inhibitors and Incidence of Skin Malignancies after Kidney Transplantation in Adult Patients: A Study of 309,551 Cases
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Terms
2.2. Eligibility Assessment
2.3. Data Extraction
2.4. Outcomes of Interest
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
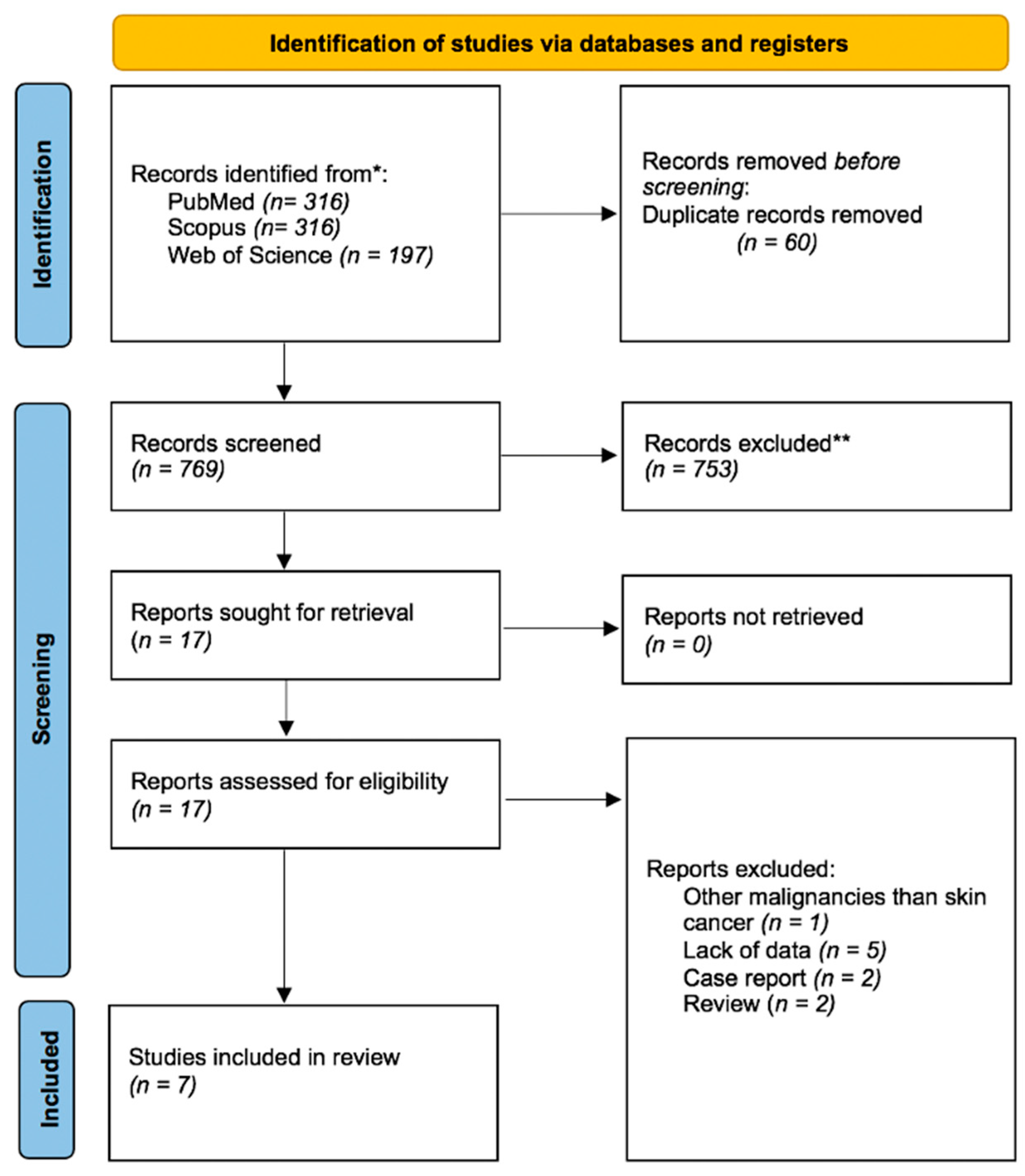
3.1. Article Selection
3.2. Article Characteristics
3.3. Patient Characteristics
3.4. Melanoma Risk
3.5. Non-Melanoma Skin Cancer Risk
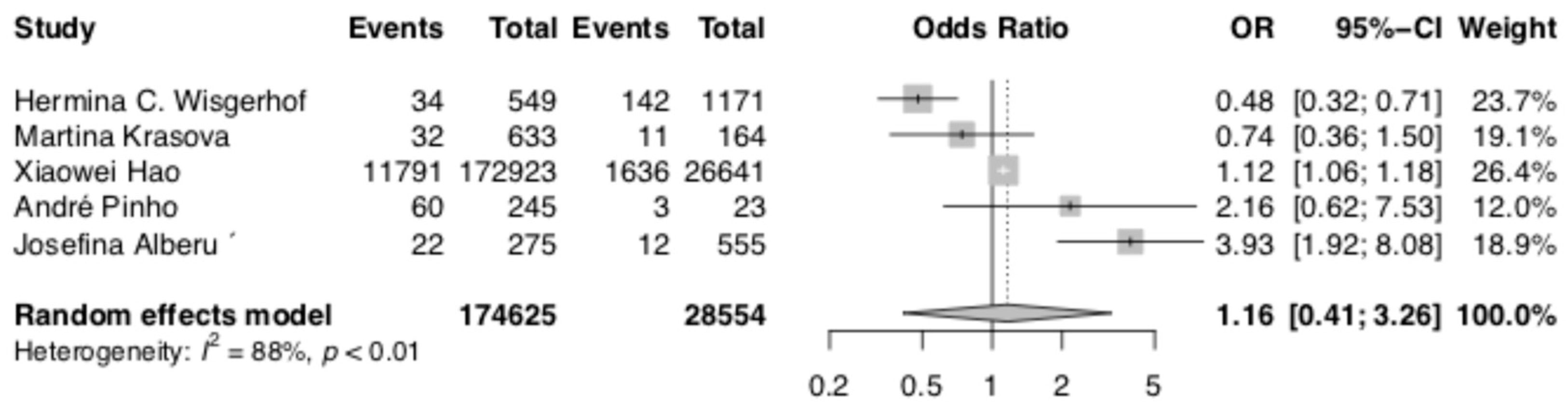
3.6. Total Skin Cancer Risk
3.7. Quality Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Haberal, M.; Boyvat, F.; Akdur, A.; Kırnap, M.; Özçelik, Ü.; Yarbuğ Karakayalı, F. Surgical complications after kidney transplantation. Exp. Clin. Transpl. 2016, 14, 587–595. [Google Scholar]
- Silva, S.B.; Caulliraux, H.M.; Araújo, C.A.; Rocha, E. Cost comparison of kidney transplant versus dialysis in Brazil. Cad. Saude Publica 2016, 32, S0102-311X2016000605005. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.G.; Harden, P.; Chapman, J. The World Kidney Day Steering Committee 2012. The global role of kidney transplantation. J. Nephropathol. 2012, 1, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.Y.; Tang, S.C.W. Personalized immunosuppression after kidney transplantation. Nephrology 2022, 27, 475–483. [Google Scholar] [CrossRef]
- Legendre, C.; Zuber, J.; Anglicheau, D.; Le Quintrec, M.; Martinez, F.; Mamzer-Bruneel, M.F.; Thervet, E. Immunosuppression en transplantation rénale [Immunosuppression in kidney transplantation]. Ann. Urol. (Paris) 2007, 41, 276–284. [Google Scholar] [CrossRef]
- Parlakpinar, H.; Gunata, M. Transplantation and immunosuppression: A review of novel transplant-related immunosuppressant drugs. Immunopharmacol. Immunotoxicol. 2021, 43, 651–665. [Google Scholar] [CrossRef]
- Tönshoff, B. Immunosuppressants in organ transplantation. Handb. Exp. Pharmacol. 2020, 261, 441–469. [Google Scholar] [CrossRef]
- Robbins, H.A.; Clarke, C.A.; Arron, S.T.; Tatalovich, Z.; Kahn, A.R.; Hernandez, B.Y.; Paddock, L.; Yanik, E.L.; Lynch, C.F.; Kasiske, B.L.; et al. Melanoma risk and survival among organ transplant recipients. J. Investig. Dermatol. 2015, 135, 2657–2665. [Google Scholar] [CrossRef]
- Clarke, C.A.; Robbins, H.A.; Tatalovich, Z.; Lynch, C.F.; Pawlish, K.S.; Finch, J.L.; Hernandez, B.Y.; Fraumeni, J.F., Jr.; Madeleine, M.M.; Engels, E.A. Risk of Merkel cell carcinoma after solid organ transplantation. J. Natl. Cancer Inst. 2015, 107, dju382. [Google Scholar] [CrossRef]
- Euvrard, S.; Kanitakis, J.; Claudy, A. Skin cancers after organ transplantation. N. Engl. J. Med. 2003, 348, 1681–1691. [Google Scholar] [CrossRef]
- Aguiar, B.; Santos Amorim, T.; Romãozinho, C.; Santos, L.; Macário, F.; Alves, R.; Campos, M.; Mota, A. Malignancy in kidney transplantation: A 25-year single-center experience in Portugal. Transplant. Proc. 2015, 47, 976–980. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Mantel, N.; Haenszel, W. Statistical Aspects of the Analysis of Data from Retrospective Studies of Disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar]
- Robins, J.; Breslow, N.; Greenland, S. Estimators of the Mantel-Haenszel variance consistent in both sparse data and large-strata limiting models. Biometrics 1986, 42, 311–323. [Google Scholar] [CrossRef]
- Greenland, S.; Robins, J.M. Estimation of a common effect parameter from sparse follow-up data. Biometrics 1985, 41, 55–68. [Google Scholar] [CrossRef]
- Fleiss, J.L. The statistical basis of meta-analysis. Stat. Methods Med. Res. 1993, 2, 121–145. [Google Scholar] [CrossRef]
- Knapp, G.; Hartung, J. Improved tests for a random effects meta-regression with a single covariate. Stat. Med. 2003, 22, 2693–2710. [Google Scholar] [CrossRef] [PubMed]
- Thorlund, K.; Imberger, G.; Johnston, B.C.; Walsh, M.; Awad, T.; Thabane, L.; Gluud, C.; Devereaux, P.J.; Wetterslev, J. Evolution of heterogeneity (I2) estimates and their 95% confidence intervals in large meta-analyses. PLoS ONE 2012, 7, e39471. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Lai, W.; Xia, X.; Xu, J.; Wu, Y.; Lv, C.; Meng, Q.; Lv, K.; Huang, S.; Luo, Z.; et al. Skin cancer outcomes and risk factors in renal transplant recipients: Analysis of organ procurement and transplantation network data from 2000 to 2021. Front. Oncol. 2022, 12, 1017498. [Google Scholar] [CrossRef] [PubMed]
- Ascha, M.; Ascha, M.S.; Tanenbaum, J.; Bordeaux, J.S. Risk Factors for melanoma in renal transplant recipients. JAMA Dermatol. 2017, 153, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Krásová, M.; Sečníková, Z.; Göpfertová, D.; Hercogová, J.; Viklický, O.; Jůzlová, K.; Jiráková, A.; Šmerhovský, Z. Immunosuppressive therapy in the posttransplant period and skin cancer. Dermatol. Ther. 2016, 29, 433–436. [Google Scholar] [CrossRef]
- Bouwes Bavinck, J.N.; Hardie, D.R.; Green, A.; Cutmore, S.; MacNaught, A.; O’Sullivan, B.; Siskind, V.; Van Der Woude, F.J.; Hardie, I.R. The risk of skin cancer in renal transplant recipients in Queensland, Australia. A follow-up study. Transplantation 1996, 61, 715–721. [Google Scholar] [CrossRef]
- Alberú, J.; Pascoe, M.D.; Campistol, J.M.; Schena, F.P.; Rial Mdel, C.; Polinsky, M.; Neylan, J.F.; Korth-Bradley, J.; Goldberg-Alberts, R.; Maller, E.S. Sirolimus CONVERT Trial Study Group. Lower malignancy rates in renal allograft recipients converted to sirolimus-based, calcineurin inhibitor-free immunotherapy: 24-month results from the CONVERT trial. Transplantation 2011, 92, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Pinho, A.; Gouveia, M.; Cardoso, J.C.; Xavier, M.M.; Vieira, R.; Alves, R. Non-melanoma skin cancer in Portuguese kidney transplant recipients—Incidence and risk factors. An. Bras. Dermatol. 2016, 91, 455–462. [Google Scholar] [CrossRef]
- Wisgerhof, H.C.; Wolterbeek, R.; de Fijter, J.W.; Willemze, R.; Bouwes Bavinck, J.N. Kidney transplant recipients with cutaneous squamous cell carcinoma have an increased risk of internal malignancy. J. Investig. Dermatol. 2012, 132, 2176–2183. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.J.; Mark, P.B.; Patel, R.K.; Stevens, K.K.; Palmer, N. Renal association clinical practice guideline in post-operative care in the kidney transplant recipient. BMC Nephrol. 2017, 18, 174. [Google Scholar] [CrossRef]
- Smith, A.; Niu, W.; Desai, A. The effect of conversion from a calcineurin inhibitor to sirolimus on skin cancer reduction in post-renal transplantation patients. Cureus 2017, 9, e1564. [Google Scholar] [CrossRef]
- Wisgerhof, H.C.; Edelbroek, J.R.; de Fijter, J.W.; Haasnoot, G.W.; Claas, F.H.; Willemze, R.; Bavinck, J.N. Subsequent squamous- and basal cell carcinomas in kidney-transplant recipients after the first skin cancer: Cumulative incidence and risk factors. Transplantation 2010, 89, 1231–1238. [Google Scholar] [CrossRef]
- Burke, M.T.; Isbel, N.; Barraclough, K.A.; Jung, J.W.; Wells, J.W.; Staatz, C.E. Genetics and nonmelanoma skin cancer in kidney transplant recipients. Pharmacogenomics 2015, 16, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, H.M.; Fryer, A.A.; Reece, S.; Smith, A.G.; Harden, P.N. Clinical risk factors associated with nonmelanoma skin cancer in renal transplant recipients. Am. J. Kidney Dis. 2000, 36, 167–176. [Google Scholar] [CrossRef]
- Sheil, A.G. Cancer in renal allograft recipients in Australia and New Zealand. Transplant. Proc. 1977, 9, 1133–1136. [Google Scholar]
- Walder, B.K.; Robertson, M.R.; Jeremy, D. Skin cancer and immunosuppression. Lancet 1971, 2, 1282–1283. [Google Scholar] [CrossRef] [PubMed]
- Suchmacher, M.; Geller, M. Practical Biostatistics, Chapter 2—Determination of Association Strength between an Exposure Factor and an Event in Observational Studies; Academic Press: Cambridge, MA, USA, 2012; pp. 19–29. ISBN 9780124157941. Available online: https://www.sciencedirect.com/science/article/pii/B9780124157941000021 (accessed on 6 March 2023).
- Karczewski, M.; Stronka, M.; Karczewski, J.; Wiktorowicz, K. Skin cancer following kidney transplantation: A single-center experience. Transplant. Proc. 2011, 43, 3760–3761. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.A.; Kampp, J. Skin Cancer Epidemiology, Detection, and Management. Med. Clin. N. Am. 2015, 99, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Basal and Squamous Cell Skin Cancer Risk Factors. American Cancer Society. 2009. Available online: www.cancer.org/cancer/types/basal-and-squamous-cell-skin-cancer/causes-risks-prevention/risk-factors.html (accessed on 10 January 2023).
- Jiyad, Z.; Olsen, C.M.; Burke, M.T.; Isbel, N.M.; Green, A.C. Azathioprine and Risk of Skin Cancer in Organ Transplant Recipients: Systematic Review and Meta-Analysis. Am. J. Transplant. 2016, 16, 3490–3503. [Google Scholar] [CrossRef]
- Cordaro, A.; Dobbs, T.D.; Gibson, J.A.; Whitaker, S.; Whitaker, I.S. Skin cancer screening in organ transplant centres in the United Kingdom: A national survey. Eur. J. Dermatol. 2020, 30, 372–376. [Google Scholar] [CrossRef]
- Garrett, G.L.; Blanc, P.D.; Boscardin, J.; Lloyd, A.A.; Ahmed, R.L.; Anthony, T.; Bibee, K.; Breithaupt, A.; Cannon, J.; Chen, A.; et al. Incidence of and risk factors for skin cancer in organ transplant recipients in the United States. JAMA Dermatol. 2017, 153, 296–303. [Google Scholar] [CrossRef]
- Manickavasagar, R.; Thuraisingham, R. Post renal-transplant malignancy surveillance. Clin. Med. (Lond.) 2020, 20, 142–145. [Google Scholar] [CrossRef]
- EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: Longterm management of the transplant recipient. Nephrol. Dial. Transplant 2002, 17 (Suppl. 4), 1–67. [Google Scholar] [CrossRef]
- American Society of Transplantation. Guidelines for Post-Kidney Transplant Management in the Community Setting. AST. 2009. Available online: www.myast.org/guidelines-post-kidney-transplant-managementcommunity-setting-0 (accessed on 15 January 2023).

| Publication Year | First Author | Title | Study Design (Rct/Cohort /Case-Control) | Country | Sample (n) | Follow-Up in Years | Sex (% Women) | Mean Or Median Age Of Transplantation | Transplant Period | Sample on CNI Therapy (n) | Sample Without CNI Therapy (n) | Sample CNI-MSC (n) | Sample CNI-NMSC (n) | Sample CNI-Total Skin Cancer (n) | Sample No CNI-MSC (n) | Sample No CNI-NMSC (n) | Sample No CNI-Total Skin Cancer (n) | Median Time to Diagnosis in Years |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2022 | Xiaowei Hao [19] | Skin cancer outcomes and risk factors in renal transplant recipients: Analysis of organ procurement and transplantation network data from 2000 to 2021 | Cohort | China | 199,564 | 7.23 | 39.45 | 49.49 | 2000–2014 | 172,923 | 26,641 | 834 (0.48%) | 11,791 (6.82%) | 12,625 (7.3%) | 102 (0.38%) | 1636 (6.14%) | 1738 (6.52%) | 5.84 |
| 2017 | Mona Ascha [20] | Risk Factors for Melanoma in Renal Transplant Recipients | Cohort | USA | 105,174 | NS | 49.3 | 49.6 | 2004–2012 | 95,818 | 9356 | 435 (0.45%) | x | x | 53 (0.57%) | x | x | NS |
| 2016 | Martina Krasova [21] | Immunosuppressive therapy in the posttransplant period and skin cancer | Cohort | Czech Republic | 797 | 5.3 | 34.3 | 48.73 | 1980–2016 | 633 | 164 | x | 32 (5.06%) | x | x | 11 (6.7%) | x | 7.97 |
| 1996 | Bavinck Jan N. Bouwes [22] | The risk of skin cancer in renal transplant recipients in Queensland, Australia. A follow-up study | Cohort | Australia | 1098 | 11.7 | 62.4 | 45.9 | 1969–1994 | 519 | 403 | x | x | 98 (18.89%) | x | x | 120 (29.78%) | 4.7 |
| 2010 | Josefina Alberú [23] | Lower Malignancy Rates in Renal Allograft Recipients Converted to Sirolimus-Based, Calcineurin Inhibitor-Free Immunotherapy | RCT | International | 830 | 3.1 | 29.5 | 42.6 | NS | 275 | 555 | 3 (1.1%) | 22 (8%) | 25 (9.1%) | 0 (0%) | 12 (2.16%) | 12 (2.16%) | NS |
| 2015 | André Pinho [24] | Non-melanoma skin cancer in Portuguese kidney transplant recipients—incidence and risk factors | Case control | Portugal | 288 | 3.67 | 34 | 47 | 2004–2013 | 245 | 23 | x | 60 (24.49%) | x | x | 3 (13.04%) | x | 5.35 |
| 2012 | Hermina C. Wisgerhof [25] | Kidney Transplant Recipients with Cutaneous Squamous Cell Carcinoma Have an Increased Risk of Internal Malignancy | Cohort | Netherlands | 1800 | 11 | 38 | 43 | 1966–2006 | 549 | 1171 | x | 34 (6.19%) | x | x | 142 (12.13%) | x | 19.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulbat, A.; Richter, K.; Stefura, T.; Kołodziej-Rzepa, M.; Kisielewski, M.; Wojewoda, T.; Wysocki, W.M. Systematic Review of Calcineurin Inhibitors and Incidence of Skin Malignancies after Kidney Transplantation in Adult Patients: A Study of 309,551 Cases. Curr. Oncol. 2023, 30, 5727-5737. https://doi.org/10.3390/curroncol30060430
Kulbat A, Richter K, Stefura T, Kołodziej-Rzepa M, Kisielewski M, Wojewoda T, Wysocki WM. Systematic Review of Calcineurin Inhibitors and Incidence of Skin Malignancies after Kidney Transplantation in Adult Patients: A Study of 309,551 Cases. Current Oncology. 2023; 30(6):5727-5737. https://doi.org/10.3390/curroncol30060430
Chicago/Turabian StyleKulbat, Aleksandra, Karolina Richter, Tomasz Stefura, Marta Kołodziej-Rzepa, Michał Kisielewski, Tomasz Wojewoda, and Wojciech M. Wysocki. 2023. "Systematic Review of Calcineurin Inhibitors and Incidence of Skin Malignancies after Kidney Transplantation in Adult Patients: A Study of 309,551 Cases" Current Oncology 30, no. 6: 5727-5737. https://doi.org/10.3390/curroncol30060430
APA StyleKulbat, A., Richter, K., Stefura, T., Kołodziej-Rzepa, M., Kisielewski, M., Wojewoda, T., & Wysocki, W. M. (2023). Systematic Review of Calcineurin Inhibitors and Incidence of Skin Malignancies after Kidney Transplantation in Adult Patients: A Study of 309,551 Cases. Current Oncology, 30(6), 5727-5737. https://doi.org/10.3390/curroncol30060430