Beyond Participation: Evaluating the Role of Patients in Designing Oncology Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Eligibility Assessment
2.3. Data Abstraction
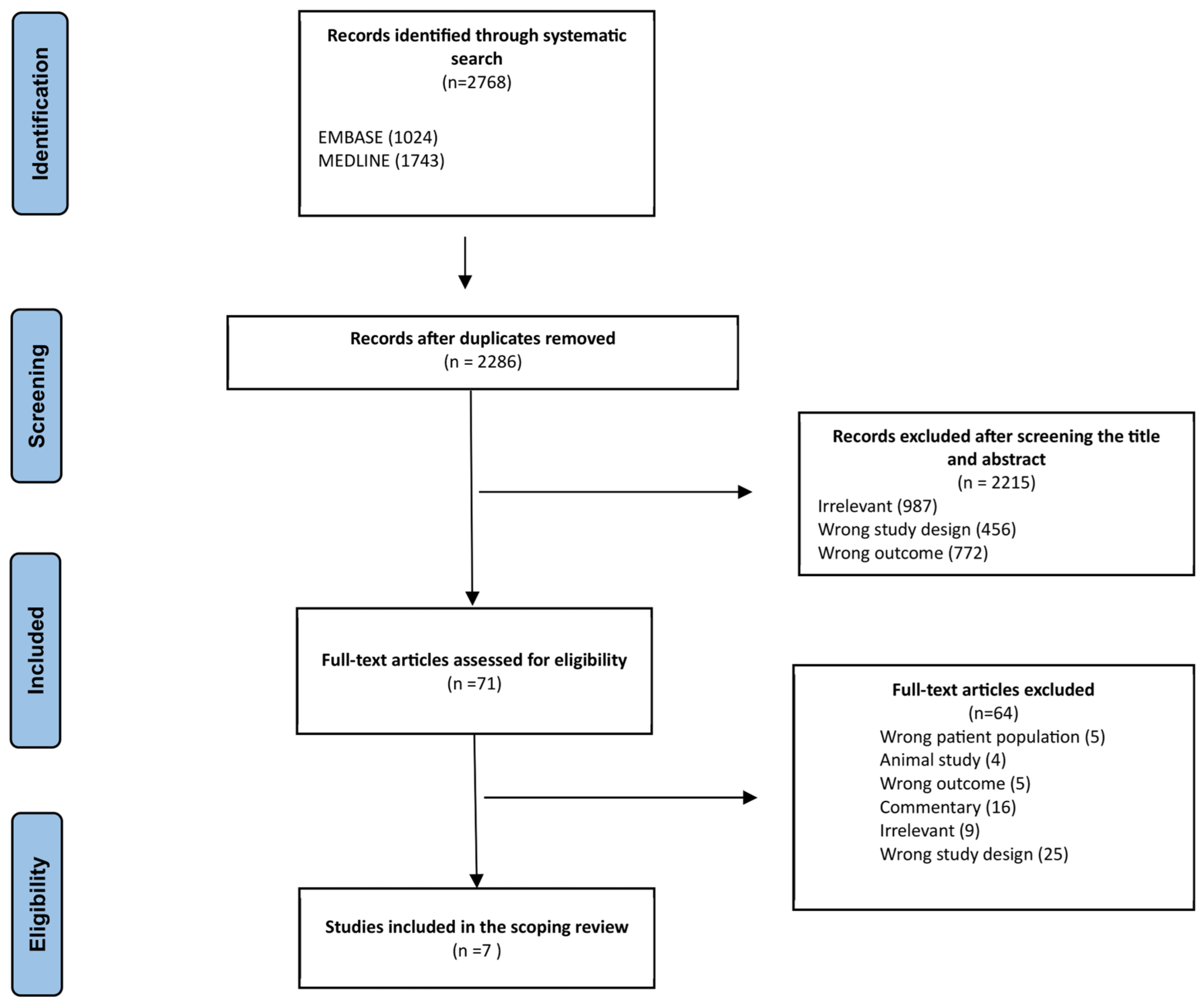
3. Results
3.1. Identified Studies
3.2. Study Characteristics
| Authors | Study Design | Country | Cancer Type | Stage | Status of Patient Stakeholder | Number of Patients | Number of Other Stakeholders | Stage of Research in Which Patients Were Engaged | Training Provided or Previous Experience | Recruitment Source | Recruitment Strategy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Barger et al., 2019 [26] | Pragmatic Clinical Trial | United States | Breast, Colorectal, or Non-Small Cell Lung | 0, I, IA, IB, IIA, IIB, IIC, IIIA, IIIB, IIIC, IV, IVA, IVB | Active Patient, Survivor, Caregiver | 10 | 11 | Planning phase, study design, interpretation, and dissemination of results. | Yes | 6 from the SWOG Patient Advocate Committee | NA |
| Forbes et al., 2010 [28] | Pragmatic Clinical Trial | UK | Breast | NA | Survivor, Other | 15 | 54 | Study design | No | Cancer charities: (1) Macmillan (2) Breast Cancer Care (3) Asian Women’s Breast Cancer Support Group | (1) Website (2) Flyers and newsletter (3) Approached women in public areas |
| Hoeg et al., 2019 [27] | Pragmatic Clinical Trial | Denmark | Breast | NA | Survivor | 7 | NA | Study design | No | New Zealand University Hospital, Department of Oncology | Hospital record screening by two nurses followed by approaching patients in person |
| Marsden et al., 2004 [29] | Pragmatic Clinical Trial | UK | Breast | I and II | Active Patient, Survivor | 83 | 7 | Study design | No | (1) Consumers’ Advisory Group for Clinical Trials (CAG-CT) (2) Participants of the pilot HRT study (3) The Lynda Jackson Macmillan Centre at Mount Vernon Hospital (4) CAG-CT (5) Clinicians involved in the undertaking of the previously described pilot HRT study | (1) Invitations to participate via newsletters (2) Telephone |
| Nicholas et al., 2021 [30] | Pragmatic Clinical Trial | UK | Esophageal | cT1-4a and/or cN+, cM0 | Active Patient, Survivor, and Caregiver | 21 | NA | Study design | No | (1) Manchester University NHS Trust patient engagement teams (2) Wales Cancer Research Centre (3) Treating clinicians who identified potential patients (4) Existing patient networks, such as esophageal cancer support groups and other cancer support centers | (1) Sent out adverts via social media networks to regular patient contributors. (2) Disseminated adverts through social media networks and regular patient contributors. (3) Sent personal invitations directly to individual patients. (4) Advertised meetings within these support groups and centers. |
| Smith et al., 2022 [31] | Pragmatic Observational Trial | United States | High-grade Non-Muscle-Invasive Bladder Cancer | Ta, T1, CIS, T2, T3, T4 | Active Patient, Caregiver | 286 | NA | The engagement plan guided PPI throughout the stages of the study, including study design, conduct, analysis, and dissemination. | No | BCAN PSN (Bladder Cancer Patient Survey Network) | Not Specified |
| Solomon et al., 2017 [32] | Pragmatic Clinical Trial | United States | Lung, Head and Neck, Sarcoma, Prostate, Ovarian, Colorectal, Melanoma, Glioblastoma | Advanced Cancer | Active Patient, and Caregiver | 12 | 15 | Study design | No | Four study centers (an academic, a municipal, and a community hospital in NYC and a rural hospital in Connecticut) | Identified by site principal investigators (PIs) or their organizational leadership |
3.3. Challenges to Patient Engagement and Reported Benefits
| Authors | Engagement Method (Frequency) | Engagement Frequency | Benefits of Patient Involvement | Specific Ways in Which Patient Input Influenced the Trial Design | Reported Challenges or Barriers to Patient Involvement | Outcomes of the Trial Influenced by Patient Involvement | Whether and How the Effectiveness of Patient Engagement Was Evaluated | Compensation for Patients |
|---|---|---|---|---|---|---|---|---|
| Barger et al., 2019 [26] |
|
|
|
|
|
|
|
|
| Forbes et al., 2010 [28] |
|
|
|
|
|
|
|
|
| Hoeg et al., 2019 [27] |
|
|
|
|
|
|
|
|
| Marsden et al., 2004 [29] |
|
|
|
|
|
|
|
|
| Nicholas et al., 2021 [30] |
|
|
|
|
|
|
|
|
| Smith et al., 2022 [31] |
|
|
|
|
|
|
|
|
| Solomon et al., 2017 [32] |
|
|
|
|
|
|
|
|
3.4. Patient-Centric Trial Framework
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Spreafico, A.; Hansen, A.R.; Razak, A.R.A.; Bedard, P.L.; Siu, L.L. The Future of Clinical Trial Design in Oncology. Cancer Discov. 2021, 11, 822–837. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, C.; Goldacre, B.; Mahtani, K.R. Why clinical trial outcomes fail to translate into benefits for patients. Trials 2017, 18, 122. [Google Scholar] [CrossRef] [PubMed]
- Khozin, S.; Blumenthal, G.M.; Pazdur, R. Real-world Data for Clinical Evidence Generation in Oncology. Clin. Med. Russ. J. 2017, 109, djx187. [Google Scholar] [CrossRef]
- Siepmann, T.; Spieth, P.M.; Kubasch, A.S.; Penzlin, A.I.; Illigens, B.M.-W.; Barlinn, K. Randomized controlled trials—A matter of design. Neuropsychiatr. Dis. Treat. 2016, 2016, 1341–1349. [Google Scholar] [CrossRef]
- Andermann, A.; Collaboration, C. Taking action on the social determinants of health in clinical practice: A framework for health professionals. CMAJ 2016, 188, E474–E483. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Prat, F.; Steensma, D.P.; Kurzrock, R.; Stewart, D.J.; Sekeres, M.A.; Leveque, J. Cancer research in the United States: A critical review of current status and proposal for alternative models. Cancer 2018, 124, 2881–2889. [Google Scholar] [CrossRef]
- Fogel, D.B. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review. Contemp. Clin. Trials Commun. 2018, 11, 156–164. [Google Scholar] [CrossRef]
- Epstein, R.M.; Street, R.L. The Values and Value of Patient-Centered Care. Ann. Fam. Med. 2011, 9, 100–103. [Google Scholar] [CrossRef]
- Narbutas, Š.; York, K.; Stein, B.D.; Magsanoc-Alikpala, K.; Majima, Y.; Kalo, Z.; Almasi, T.; Inotai, A. Overview on Patient Centricity in Cancer Care. Front. Pharmacol. 2017, 8, 698. [Google Scholar] [CrossRef]
- Bombard, Y.; Baker, G.R.; Orlando, E.; Fancott, C.; Bhatia, P.; Casalino, S.; Onate, K.; Denis, J.-L.; Pomey, M.-P. Engaging patients to improve quality of care: A systematic review. Implement. Sci. 2018, 13, 98. [Google Scholar] [CrossRef]
- Yeoman, G.; Furlong, P.; Seres, M.; Binder, H.; Chung, H.; Garzya, V.; Jones, R.R. Defining patient centricity with patients for patients and caregivers: A collaborative endeavour. BMJ Innov. 2017, 3, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, T.; Hinton, L.; Finlay, T.; Macfarlane, A.; Fahy, N.; Clyde, B.; Chant, A. Frameworks for supporting patient and public involvement in research: Systematic review and co-design pilot. Health Expect. 2019, 22, 785–801. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N. Patient centric approach for clinical trials: Current trend and new opportunities. Perspect. Clin. Res. 2015, 6, 134–138. [Google Scholar] [CrossRef]
- Faulkner, S.D.; Somers, F.; Boudes, M.; Nafria, B.; Robinson, P. Using Patient Perspectives to Inform Better Clinical Trial Design and Conduct: Current Trends and Future Directions. Pharm. Med. 2023, 37, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Biankin, A.V.; Piantadosi, S.; Hollingsworth, S.J. Patient-centric trials for therapeutic development in precision oncology. Nature 2015, 526, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Mangin, D.; Stephen, G.; Bismah, V.; Risdon, C. Making patient values visible in healthcare: A systematic review of tools to assess patient treatment priorities and preferences in the context of multimorbidity. BMJ Open 2016, 6, e010903. [Google Scholar] [CrossRef] [PubMed]
- Ersek, J.L.; Nadler, E.; Freeman-Daily, J.; Mazharuddin, S.; Kim, E.S. Clinical Pathways and the Patient Perspective in the Pursuit of Value-Based Oncology Care. Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 597–606. [Google Scholar] [CrossRef]
- Murphy, A.; Bere, N.; Vamvakas, S.; Mavris, M. The Added Value of Patient Engagement in Early Dialogue at EMA: Scientific Advice as a Case Study. Front. Med. 2022, 8, 811855. [Google Scholar] [CrossRef]
- Trudeau, M.; Hoskins, P.; Reiman, T.; Chambers, A.; Mai, H.; Wheatley-Price, P. Clinician Participation in Cadth’s Pan-Canadian Oncology Drug Review: Contribution and Impact on Cancer Drug Funding Recommendations. Curr. Oncol. 2017, 24, 71–74. [Google Scholar] [CrossRef][Green Version]
- Frank, L.; Basch, E.; Selby, J.V. The PCORI Perspective on Patient-Centered Outcomes Research. JAMA 2014, 312, 1513–1514. [Google Scholar] [CrossRef]
- Unger, J.M.; Cook, E.; Tai, E.; Bleyer, A. The Role of Clinical Trial Participation in Cancer Research: Barriers, Evidence, and Strategies. Am. Soc. Clin. Oncol. Educ. Book 2016, 36, 185–198. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic Review or Scoping Review? Guidance for Authors When Choosing between a Systematic or Scoping Review Approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Hupe, M. EndNote X9. J. Electron. Resour. Med. Libr. 2019, 16, 117–119. [Google Scholar] [CrossRef]
- Babineau, J. Product Review: Covidence (Systematic Review Software). J. Can. Health Libr. Assoc. 2014, 35, 68–71. [Google Scholar] [CrossRef]
- Barger, S.; Sullivan, S.D.; Bell-Brown, A.; Bott, B.; Ciccarella, A.M.; Golenski, J.; Gorman, M.; Johnson, J.; Kreizenbeck, K.; Kurttila, F.; et al. Effective stakeholder engagement: Design and implementation of a clinical trial (SWOG S1415CD) to improve cancer care. BMC Med. Res. Methodol. 2019, 19, 119. [Google Scholar] [CrossRef] [PubMed]
- Høeg, B.L.; Tjørnhøj-Thomsen, T.; Skaarup, J.A.; Langstrup, H.; Zoffmann, V.; Saltbaek, L.; Karlsen, R.V.; Dalton, S.O.; Johansen, C.; Bidstrup, P.E. Whose perspective is it anyway? Dilemmas of patient involvement in the development of a randomized clinical trial—A qualitative study. Acta Oncol. 2019, 58, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Forbes, L.J.; Nicholls, C.M.; Linsell, L.; Graham, J.; Tompkins, C.; Ramirez, A.J. Involving users in the design of a randomised controlled trial of an intervention to promote early presentation in breast cancer: Qualitative study. BMC Med. Res. Methodol. 2010, 10, 110. [Google Scholar] [CrossRef]
- Marsden, J.; Bradburn, J.; Consumers’ Advisory Group for Clinical Trials (CAG-CT); Lynda Jackson Macmillan Centre. Patient and clinician collaboration in the design of a national randomized breast cancer trial. Health Expect. 2004, 7, 6–17. [Google Scholar] [CrossRef]
- Nicholas, O.J.; Joseph, O.; Keane, A.; Cleary, K.; Campbell, S.H.; Gwynne, S.H.; Crosby, T.; Radhakrishna, G.; Hawkins, M.A. Patient and Public Involvement Refines the Design of ProtOeus: A Proposed Phase II Trial of Proton Beam Therapy in Oesophageal Cancer. Patient Patient-Centered Outcomes Res. 2020, 14, 545–553. [Google Scholar] [CrossRef]
- Smith, A.B.; Lee, J.R.; Lawrence, S.O.; Ho, O.; Lavallee, D.C.; Chisolm, S.; MacLean, D.B.; Louwers, R.K.; Wolff, E.M.; Kessler, L.G.; et al. Patient and public involvement in the design and conduct of a large, pragmatic observational trial to investigate recurrent, high-risk non-muscle-invasive bladder cancer. Cancer 2022, 128, 103–111. [Google Scholar] [CrossRef]
- Solomon, R.; Smith, C.; Kallio, J.; Fenollosa, A.; Benerofe, B.; Jones, L.; Adelson, K.; Gonsky, J.P.; Messner, C.; Bickell, N.A. Speaking Up: How Patient and Physician Voices Shaped a Trial to Improve Goals-of-Care Discussions. Patient Patient-Centered Outcomes Res. 2017, 10, 489–501. [Google Scholar] [CrossRef]
- Nair, B. Clinical Trial Designs. Indian Dermatol. Online J. 2019, 10, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Aslam, J.; Hassam, K.; Naeem, M.A. Sequential Dengue Infection: Prevention Priorities. J. Coll. Physicians Surg. Pak. 2023, 33, 831. [Google Scholar] [PubMed]
- Thoma, A.; Farrokhyar, F.; McKnight, L.; Bhandari, M. Practical tips for surgical research: How to optimize patient recruitment. Can. J. Surg. 2010, 53, 205–210. [Google Scholar] [PubMed]
- Yu, Q.J.; Pun, J. Promoting Patient Engagement in Medical Informed Consent—A Qualitative Study of Chinese Doctors’ Communication Strategies. Health Commun. 2021, 38, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Gogtay, N.J.; Chaudhari, N.; Ravi, R.; Thatte, U.M. Recruitment and retention of the participants in clinical trials: Challenges and solutions. Perspect. Clin. Res. 2020, 11, 64–69. [Google Scholar] [CrossRef]
- Fleming, T.R.; DeMets, D.L.; Roe, M.T.; Wittes, J.; Calis, K.A.; Vora, A.N.; Meisel, A.; Bain, R.P.; Konstam, M.A.; Pencina, M.J.; et al. Data monitoring committees: Promoting best practices to address emerging challenges. Clin. Trials 2017, 14, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Calis, K.A.; Archdeacon, P.; Bain, R.; DeMets, D.; Donohue, M.; Elzarrad, M.K.; Forrest, A.; McEachern, J.; Pencina, M.J.; Perlmutter, J.; et al. Recommendations for data monitoring committees from the Clinical Trials Transformation Initiative. Clin. Trials 2017, 14, 342–348. [Google Scholar] [CrossRef]
- Skovlund, P.C.; Nielsen, B.K.; Thaysen, H.V.; Schmidt, H.; Finset, A.; Hansen, K.A.; Lomborg, K. The impact of patient involvement in research: A case study of the planning, conduct and dissemination of a clinical, controlled trial. Res. Involv. Engag. 2020, 6, 43. [Google Scholar] [CrossRef]
- Schroeder, K.; Bertelsen, N.; Scott, J.; Deane, K.; Dormer, L.; Nair, D.; Elliott, J.; Krug, S.; Sargeant, I.; Chapman, H.; et al. Building from Patient Experiences to Deliver Patient-Focused Healthcare Systems in Collaboration with Patients: A Call to Action. Ther. Innov. Regul. Sci. 2022, 56, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.M.; Tuzzio, L.; Cherkin, D. A Framework for Making Patient-Centered Care Front and Center. Perm. J. 2012, 16, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.G.; Fox, G.; Monfaredi, Z.; Poole, E.; Garritty, C.; Maybee, A.; Presseau, J.; Shea, B.; Fergusson, D.A. The impact of patient engagement on trials and trialists in Ontario, Canada: An interview study with IMPACT awardees. Res. Involv. Engag. 2022, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Bisson, M.; Aubrey-Bassler, K.; Chouinard, M.; Doucet, S.; Ramsden, V.R.; Dumont-Samson, O.; Howse, D.; Lambert, M.; Schwarz, C.; Luke, A.; et al. Patient engagement in health implementation research: A logic model. Health Expect. 2023, 26, 1854–1862. [Google Scholar] [CrossRef]
- Geißler, J.; Isham, E.; Hickey, G.; Ballard, C.; Corbett, A.; Lubbert, C. Patient involvement in clinical trials. Commun. Med. 2022, 2, 94. [Google Scholar] [CrossRef]
- Corneli, A.; Hanlen-Rosado, E.; McKenna, K.; Araojo, R.; Corbett, D.; Vasisht, K.; Siddiqi, B.; Johnson, T.; Clark, L.T.; Calvert, S.B. Enhancing Diversity and Inclusion in Clinical Trials. Clin. Pharmacol. Ther. 2023, 113, 489–499. [Google Scholar] [CrossRef]
- Hoos, A.; Anderson, J.; Boutin, M.; Dewulf, L.; Geissler, J.; Johnston, G.; Joos, A.; Metcalf, M.; Regnante, J.; Sargeant, I.; et al. Partnering With Patients in the Development and Lifecycle of Medicines: A Call for Action. Ther. Innov. Regul. Sci. 2015, 49, 929–939. [Google Scholar] [CrossRef]
- Bird, M.; McGillion, M.; Chambers, E.M.; Dix, J.; Fajardo, C.J.; Gilmour, M.; Levesque, K.; Lim, A.; Mierdel, S.; Ouellette, C.; et al. A generative co-design framework for healthcare innovation: Development and application of an end-user engagement framework. Res. Involv. Engag. 2021, 7, 12. [Google Scholar] [CrossRef]
- Lehane, E.; Leahy-Warren, P.; O’riordan, C.; Savage, E.; Drennan, J.; O’tuathaigh, C.; O’connor, M.; Corrigan, M.; Burke, F.; Hayes, M.; et al. Evidence-based practice education for healthcare professions: An expert view. BMJ Evid. Based Med. 2018, 24, 103–108. [Google Scholar] [CrossRef]
- Wale, J.L.; Thomas, S.; Hamerlijnck, D.; Hollander, R. Patients and public are important stakeholders in health technology assessment but the level of involvement is low—A call to action. Res. Involv. Engag. 2021, 7, 1. [Google Scholar] [CrossRef]
- Bright, K.; Mills, A.; Bradford, J.-P.; Stewart, D.J. RAPID framework for improved access to precision oncology for lethal disease: Results from a modified multi-round delphi study. Front. Health Serv. 2023, 3, 1015621. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J.; Bradford, J.-P.; Batist, G. Treatment Access, Health Economics, and the Wave of a Magic Wand. Curr. Oncol. 2022, 29, 1176–1189. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J.; Stewart, A.A.; Wheatley-Price, P.; Batist, G.; Kantarjian, H.M.; Schiller, J.; Clemons, M.; Bradford, J.; Gillespie, L.; Kurzrock, R. The importance of greater speed in drug development for advanced malignancies. Cancer Med. 2018, 7, 1824–1836. [Google Scholar] [CrossRef] [PubMed]
- Gotfrit, J.; Shin, J.J.; Mallick, R.; Stewart, D.J.; Wheatley-Price, P. Potential Life-Years Lost: The Impact of the Cancer Drug Regulatory and Funding Process in Canada. Oncologist 2019, 25, e130–e137. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J.; Batist, G.; Kantarjian, H.M.; Bradford, J.-P.; Schiller, J.H.; Kurzrock, R. The Urgent Need for Clinical Research Reform to Permit Faster, Less Expensive Access to New Therapies for Lethal Diseases. Clin. Cancer Res. 2015, 21, 4561–4568. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farah, E.; Kenney, M.; Kica, A.; Haddad, P.; Stewart, D.J.; Bradford, J.-P. Beyond Participation: Evaluating the Role of Patients in Designing Oncology Clinical Trials. Curr. Oncol. 2023, 30, 8310-8327. https://doi.org/10.3390/curroncol30090603
Farah E, Kenney M, Kica A, Haddad P, Stewart DJ, Bradford J-P. Beyond Participation: Evaluating the Role of Patients in Designing Oncology Clinical Trials. Current Oncology. 2023; 30(9):8310-8327. https://doi.org/10.3390/curroncol30090603
Chicago/Turabian StyleFarah, Eliya, Matthew Kenney, Anris Kica, Paul Haddad, David J. Stewart, and John-Peter Bradford. 2023. "Beyond Participation: Evaluating the Role of Patients in Designing Oncology Clinical Trials" Current Oncology 30, no. 9: 8310-8327. https://doi.org/10.3390/curroncol30090603
APA StyleFarah, E., Kenney, M., Kica, A., Haddad, P., Stewart, D. J., & Bradford, J.-P. (2023). Beyond Participation: Evaluating the Role of Patients in Designing Oncology Clinical Trials. Current Oncology, 30(9), 8310-8327. https://doi.org/10.3390/curroncol30090603






