A Meta-Analysis to Assess the Efficacy of HER2-Targeted Treatment Regimens in HER2-Positive Metastatic Colorectal Cancer (mCRC)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
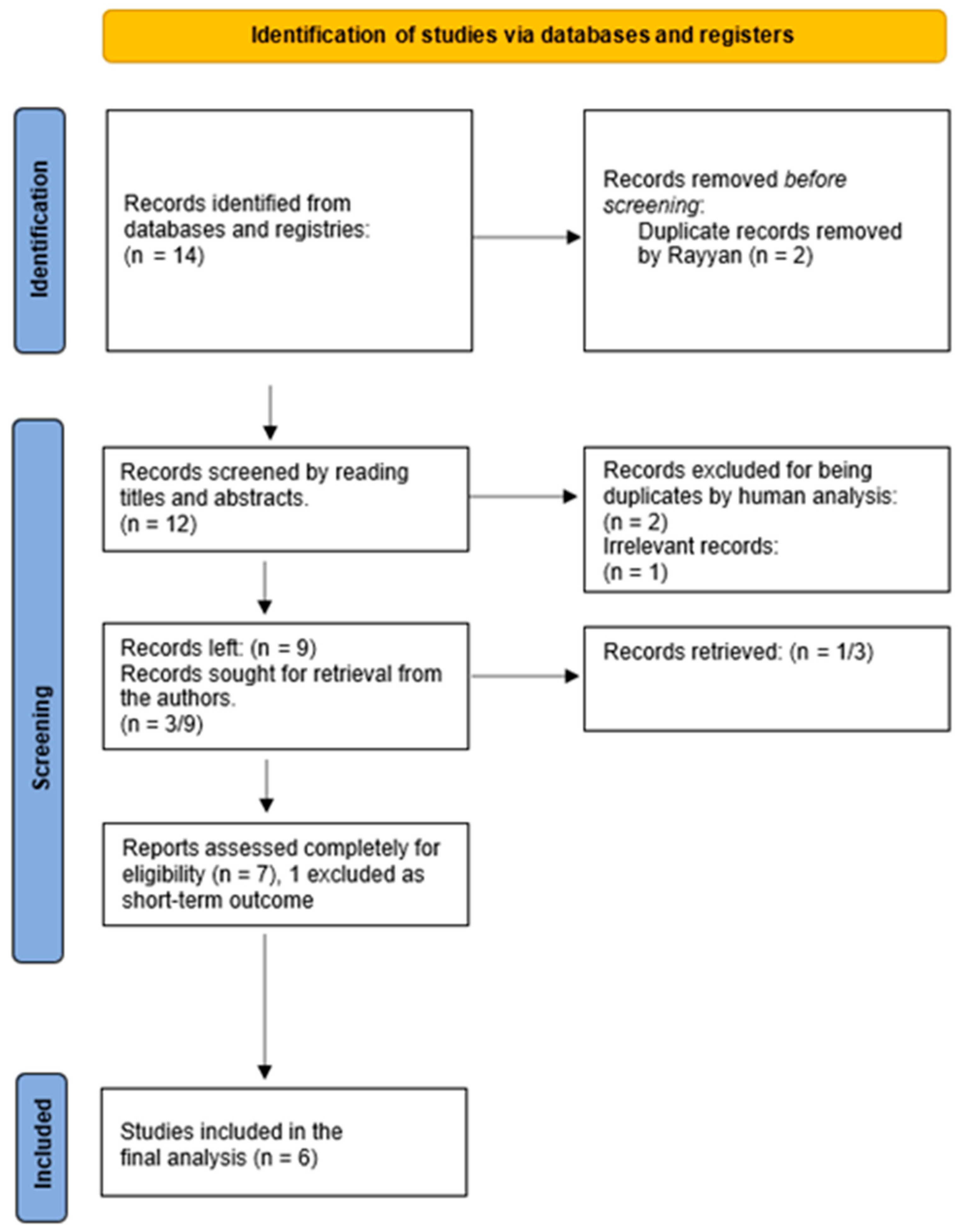
2.4. Study Selection
2.5. Data Collection Process
2.6. Primary Outcome
2.7. Secondary Outcome
2.8. Quality Assessment
2.9. Data Synthesis
- Calculate the weighted average of ORR: calculate the weighted average of ORR by weighing the ORR estimates from each study by their sample size using the formula:
- 2.
- Calculate the standard error of the weighted average of ORR: calculate the standard error of the weighted average of ORR using the formula:where wi is the weight assigned to each study (i.e., the study’s sample size divided by the total sample size), ORRi is the ORR estimate for each study, and ni is each study’s sample size.
- 3.
- Calculate the 95% confidence interval: calculate the 95% confidence interval for the weighted average of ORR using the formula:ORR is the weighted average of ORR, and SE is the standard error of the weighted average of ORR.CI = ORR ± (1.96 × SE)
- 4.
- The pooled ORR provides an overall estimate of the treatment effect in single-arm studies and can be used to inform clinical decision making and guide further research. Nonetheless, it is essential to emphasize that the pooled ORR is only as valid as each study’s individual ORR estimates and may be subject to confounders or biases.
3. Results
3.1. Study Characteristics
3.2. Result of Synthesis
3.3. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [PubMed]
- Thanikachalam, K.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef]
- Cheng, L.; Eng, C.; Nieman, L.Z.; Kapadia, A.S.; Du, X.L. Trends in colorectal cancer incidence by anatomic site and disease stage in the United States from 1976 to 2005. Am. J. Clin. Oncol. 2011, 34, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [PubMed]
- Bailey, C.E.; Hu, C.Y.; You, Y.N.; Bednarski, B.K.; Rodriguez-Bigas, M.A.; Skibber, J.M.; Cantor, S.B.; Chang, G.J. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 2015, 150, 17–22. [Google Scholar] [CrossRef]
- Heinimann, K. Hereditary Colorectal Cancer: Clinics, Diagnostics and Management. Ther. Umschau. Rev. Ther. 2018, 75, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Johdi, N.A.; Sukor, N.F. Colorectal Cancer Immunotherapy: Options and Strategies. Front. Immunol. 2020, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Schmoll, H.J.; Van Cutsem, E.; Stein, A.; Valentini, V.; Glimelius, B.; Haustermans, K.; Nordlinger, B.; Van de Velde, C.J.; Balmana, J.; Regula, J.; et al. ESMO consensus guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann. Oncol. 2012, 23, 2479–2516. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Nordlinger, B.; Arnold, D. Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25 (Suppl. 3), iii1–iii9. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Arnold, D.; Taniguchi, H.; Pentheroudakis, G.; Yamazaki, K.; Xu, R.-H.; Kim, T.; Ismail, F.; Tan, I.; Yeh, K.-H.; et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: A JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann. Oncol. 2018, 29, 44–70. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; van Krieken, J.H.; Aderka, D.; Aguilar, E.A.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; Van De Velde, C.J.H.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, P.; Ahmed, S. Current status of immunotherapy in metastatic colorectal cancer. Int. J. Color. Dis. 2019, 34, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Guarini, C.; Grassi, T.; Pezzicoli, G.; Porta, C. Beyond RAS and BRAF: HER2, a New Actionable Oncotarget in Advanced Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 6813. [Google Scholar] [CrossRef]
- Cremolini, C.; Morano, F.; Moretto, R.; Berenato, R.; Tamborini, E.; Perrone, F.; Rossini, D.; Gloghini, A.; Busico, A.; Zucchelli, G.; et al. Negative hyper-selection of metastatic colorectal cancer patients for anti-EGFR monoclonal antibodies: The PRESSING case-control study. Ann. Oncol. 2017, 28, 3009–3014. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Marsoni, S.; Siena, S. Human Epidermal Growth Factor Receptor 2 as a Molecular Biomarker for Metastatic Colorectal Cancer. JAMA Oncol. 2018, 4, 19–20. [Google Scholar] [CrossRef]
- Djaballah, S.A.; Daniel, F.; Milani, A.; Ricagno, G.; Lonardi, S. HER2 in Colorectal Cancer: The Long and Winding Road From Negative Predictive Factor to Positive Actionable Target. Am. Soc. Clin. Oncol. 2022, 42, 219–232. [Google Scholar] [CrossRef]
- Greally, M.; Kelly, C.M.; Cercek, A. HER2: An emerging target in colorectal cancer. Curr. Probl. Cancer 2018, 42, 560–571. [Google Scholar] [CrossRef]
- Passardi, A.; Canale, M.; Valgiusti, M.; Ulivi, P. Immune Checkpoints as a Target for Colorectal Cancer Treatment. Int. J. Mol. Sci. 2017, 18, 1324. [Google Scholar] [CrossRef]
- Ross, J.S.; Fakih, M.; Ali, S.M.; Elvin, J.A.; Schrock, A.B.; Suh, J.; Vergilio, J.-A.; Ramkissoon, S.; Severson, E.; Daniel, S.; et al. Targeting HER2 in colorectal cancer: The landscape of amplification and short variant mutations in ERBB2 and ERBB3. Cancer 2018, 124, 1358–1373. [Google Scholar] [CrossRef]
- Kavuri, S.M.; Jain, N.; Galimi, F.; Cottino, F.; Leto, S.M.; Migliardi, G.; Searleman, A.C.; Shen, W.; Monsey, J.; Trusolino, L.; et al. HER2 activating mutations are targets for colorectal cancer treatment. Cancer Discov. 2015, 5, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Cortés, J.; Kim, S.B.; Im, S.A.; Hegg, R.; Im, Y.H.; Roman, L.; Pedrini, J.L.; Pienkowski, T.; Knott, A.; et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N. Engl. J. Med. 2012, 366, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, K.L.; Burstein, H.J.; Storniolo, A.M.; Rugo, H.; Sledge, G.; Koehler, M.; Ellis, C.; Casey, M.; Vukelja, S.; Bischoff, J.; et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J. Clin. Oncol. 2010, 28, 1124–1130. [Google Scholar] [CrossRef]
- Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Training. (n.d.). Available online: https://training.cochrane.org/handbook/current (accessed on 9 September 2022).
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 1 January 2022).
- Tsurutani, J.; Iwata, H.; Krop, I.; Jänne, P.A.; Doi, T.; Takahashi, S.; Park, H.; Redfern, C.; Tamura, K.; Wise-Draper, T.M.; et al. Targeting HER2 with Trastuzumab Deruxtecan: A Dose-Expansion, Phase I Study in Multiple Advanced Solid Tumors. Cancer Discov. 2020, 10, 688–701, Erratum in Cancer Discov. 2020, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Lonardi, S.; Martino, C.; Fenocchio, E.; Tosi, F.; Ghezzi, S.; Leone, F.; Bergamo, F.; Zagonel, V.; Ciardiello, F.; et al. Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: The phase II HERACLES-B trial. ESMO Open 2020, 5, e000911. [Google Scholar] [CrossRef] [PubMed]
- Tosi, F.; Sartore-Bianchi, A.; Lonardi, S.; Amatu, A.; Leone, F.; Ghezzi, S.; Martino, C.; Bencardino, K.; Bonazzina, E.; Bergamo, F.; et al. Long-term Clinical Outcome of Trastuzumab and Lapatinib for HER2-positive Metastatic Colorectal Cancer. Clin. Color. Cancer. 2020, 19, 256–262.e2. [Google Scholar] [CrossRef]
- Siena, S.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Fakih, M.; Elez, E.; et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2021, 22, 779–789. [Google Scholar] [CrossRef]
- Thall, P.F.; Simon, R.M.; Estey, E.H. Bayesian sequential monitoring designs for single-arm clinical trials with multiple outcomes. Stat. Med. 1995, 14, 1045–1063. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Ying, J.; Yang, L.; Fang, W.; Han, W.; Hu, H.; Zhang, S.; Yuan, Y. Dual targeted therapy with pyrotinib and trastuzumab for HER2-positive advanced colorectal cancer: A phase 2 trial. Cancer Sci. 2023, 114, 1067–1074. [Google Scholar] [CrossRef]
- Strickler, J.H.; Cercek, A.; Siena, S.; André, T.; Ng, K.; Van Cutsem, E.; Wu, C.; Paulson, A.S.; Hubbard, J.M.; Coveler, A.L.; et al. MOUNTAINEER investigators. Tucatinib plus trastuzumab for chemotherapy-refractory, HER2-positive, RAS wild-type unresectable or metastatic colorectal cancer (MOUNTAINEER): A multicentre, open-label, phase 2 study. Lancet Oncol. 2023, 24, 496–508. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Van Cutsem, E.; Tabernero, J.; Siena, S.; Yoshino, T.; Nakamura, Y.; Raghav, K.P.S.; Cercek, A.; Heinemann, V.; Adelberg, D.E. MOUNTAINEER-03: Phase 3 study of tucatinib, trastuzumab, and modified FOLFOX6 as first-line treatment in HER2+ metastatic colorectal cancer. J. Clin. Oncol. 2023, 41 (Suppl. 4), TPS261. [Google Scholar] [CrossRef]
- Shitara, K.; Bang, Y.-J.; Iwasa, S.; Sugimoto, N.; Ryu, M.-H.; Sakai, D.; Chung, H.-C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Luque-Cabal, M.; García-Teijido, P.; Fernández-Pérez, Y.; Sánchez-Lorenzo, L.; Palacio-Vázquez, I. Mechanisms behind the resistance to trastuzumab in HER2-amplified breast cancer and strategies to overcome it. Clin. Med. Insights Oncol. 2016, 10 (Suppl. 1), 21–30. [Google Scholar] [CrossRef]
- Siravegna, G.; Lazzari, L.; Crisafulli, G.; Sartore-Bianchi, A.; Mussolin, B.; Cassingena, A.; Martino, C.; Lanman, R.B.; Nagy, R.J.; Fairclough, S. Radiologic and genomic evolution of individual metastases during HER2 blockade in colorectal cancer. Cancer Cell 2018, 34, 148–162. [Google Scholar] [CrossRef]
- Lee, M.S.; Loehrer, P.J.; Imanirad, I.; Cohen, S.; Ciombor, K.K.; Moore, D.T.; Carlson, C.A.; Sanoff, H.K.; McRee, A.J. Phase II study of ipilimumab, nivolumab, and panitumumab in patients with KRAS/NRAS/BRAF wild-type (WT) microsatellite stable (MSS) metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2021, 39 (Suppl. 3), 7. [Google Scholar] [CrossRef]
| Criteria | Tsurutani et al. [29] | Bianchi et al. [30] | Tosi et al. [31] | Sienna et al. [32] |
|---|---|---|---|---|
| Question objective clearly stated? | Yes | Yes | Yes | Yes |
| Are eligibility criteria prespecified? | Yes | Yes | Yes | Yes |
| Population in the study representative of the target population? | Yes | Yes | Yes | Yes |
| Were all eligible participants enrolled? | Yes | Yes | Yes | Yes |
| Was the sample size sufficiently large? | Yes | Yes | Yes | Yes |
| Was the intervention clearly described and delivered consistently throughout the trial? | Yes | Yes | Yes | Yes |
| Outcome measures prespecified, clearly defined, reliable, and assessed consistently? | Yes | Yes | Yes | Yes |
| Were the people assessing outcomes blinded? | Yes | Yes | N/A | Yes |
| Was lost to follow up 20% or less? | Yes | Yes | Yes | Yes |
| Were those lost to follow-up accounted for in the analysis? | Yes | Yes | Yes | Yes |
| Were statistical methods done that gave p-value? | N/A | Yes | N/A | N/A |
| Was an interrupted time series design used? | Yes | Yes | N/A | N/A |
| Was the study at a group level (e.g., the whole hospital)? | No | No | No | No |
| If yes, did the study analysis consider individual-level data to determine effects at the group level? | No | No | No | No |
| Quality | High | High | Some concern | Some concern |
| Author ID | Study Design | Intervention | Year of Publication | No. of Subjects | Prior Line of Rx | HER2 Mutation | RAS Mutation | BRAF Mutation |
|---|---|---|---|---|---|---|---|---|
| Tsurutani et al. [29] | Non-randomized phase 1 dose expansion clinical trial | Trastuzumab deruxtecan | March 2020 | 20 | 4 | 5 Kinase domain, 1 Transmembrane domain, and 0 Extracellular domain | 5 KRAS and 2 NRAS | - |
| Fu et al. [34] | Non-randomized phase 2 trial | Trastuzumab + Pyrotinib | March 2023 | 18 | 2 | 5 HER2 | 12 RAS wild-type, 5 KRAS and 1 NRAS | - |
| Bianchi et al. [30] | Single arm, multicenter, phase 2 clinical trial | Pertuzumab + Trastuzumab emtansine | January 2020 | 31 | 3 | - | - | - |
| Strickler et al. [35] | Open-label phase 2 clinical trial | Trastuzumab + Tucatinib | January 2023 | 84 | 3 | - | - | - |
| Tosi et al. [31] | Open-label Phase 2 Non-randomized | Trastuzumab + Lapatinib | January 2020 | 32 | 5 | - | 32 KRAS exon 2 (codons 12 and 13) wild-type | - |
| Siena et al. [32] | Open-label Phase 2 Non-randomized | Trastuzuma deruxtecan | June 2023 | 53 | 2 | - | 52 RAS wild-type and 1 NRAS | 53 BRAF wild-type |
| Author ID/IDs | Drug Combination | CR | PR | SD | PD |
|---|---|---|---|---|---|
| Tsurutani et al. [29] (20 patients) | Trastuzumab deruxtecan | 0% (0/20) | 15% (3/20) | 65% (13/20) | 15% (3/20) |
| Sienna et al. [32] (53 patients) | Trastuzumab deruxtecan | 2% (1/53) | 43.40% (23/53) | 37.73% (20/53) | 9% (5/53) |
| Fu et al. [34] (18 patients) | Trastuzumab + Pyrotinib | 0% (0/18) | 22.22% (4/18) | 38.89% (7/18) | No data available |
| Bianchi et al. [30] (31 patients) | Trastuzumab emtansine + Pertuzumab | 0% (0/31) | 9.68% (3/31) | 67.74% (21/31) | 22.58% (7/31) |
| Strickler et al. [35] (84 patients) | Trastuzumab + Tucatinib | 3.57% (3/84) | 34.52% (29/84) | 33.33% (28/84) | 26.19% (22/84) |
| Tosi et al. [31] (32 patients) | Trastuzumab + Lapatinib | 3.12% (1/32) | 25% (8/32) | 40.62% (13/32) | No data available |
| Author | Drug Combination | ORR (95%CI) | DCR (95%CI) | PFS (Months) |
|---|---|---|---|---|
| Tsurutani et al. [29] (20 patients) | Trastuzumab deruxtecan | 15% CI 3.2–37.9 (3/20) | 80% CI 56.3–94.3 (16/20) | 4.1 (2.1–5.9) |
| Sienna et al. [32] (53 patients) | Trastuzumab deruxtecan | 45.28% CI 31.6–59.6 (24/53) | 83.01% CI 70.2–91.9 (44/53) | 6.9 (4.1 to NE) |
| Fu et al. [34] (18 patients) | Trastuzumab + Pyrotinib | 22.2% CI 6.4–47.69 (4/18) | 61.11% CI 35.8–82.7 (11/18) | 3.4 (1.8–4.3) |
| Bianchi et al. [30] (31 patients) | Trastuzumab emtansine + Pertuzumab | 9.68% (3/31) | 77.42% (24/31) | 4.1 (3.6–5.9) |
| Strickler et al. [35] (84 patients) | Trastuzumab + Tucatinib | 38.10% (32/84) | 71.43% (60/84) | 8.2 |
| Tosi et al. [31] (32 patients) | Trastuzumab + Lapatinib | 28.12% (9/32) | 68.75% (22/32) | 4.7 (3.7–6.1) |
| Cumulative weighted Meta-analysis | Pooled: a. ORR with 95% CI b. DCR with 95% CI c. PFS | a. 31.33% (95% CI 24.27–38.39) | b.74.37% (95% CI 64.57–84.17) | c. 6.2 months |
| Adverse Events | Drug Combination | ||||
|---|---|---|---|---|---|
| Trastuzumab Deruxtecan | Trastuzumab Emtansine + Pertuzumab | Trastuzumab + Tucatinib | Trastuzumab + Lapatinib | Trastuzumab + Pyrotinib | |
| Fatigue | 34.25% (25/73) | 19.35% (6/31) | 28.57% (24/84) | 59.37% (19/32) | 38.88% (7/18) |
| Nausea and Vomiting | 64.38% (47/73) | 9.68% (3/31) | 19.04% (16/84) | 46.87% (15/32) | 38.88% (7/18) |
| Diarrhea | 28.3% (15/53) * | - | 52.38% (44/84) | 84.37% (27/32) | 94.44% (17/18) |
| Dermatitis | 5% (1/20) ** | 6.45% (2/31) | 17.86% (15/84) | 78.12% (25/32) | 11.11% (2/18) |
| Hyperbilirubinemia | 6% (3/53%) * | 9.68% (3/31) | - | 3.12% (1/32) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chitkara, A.; Bakhtiar, M.; Sahin, I.H.; Hsu, D.; Zhang, J.; Anamika, F.; Mahnoor, M.; Ahmed, R.; Gholami, S.; Saeed, A. A Meta-Analysis to Assess the Efficacy of HER2-Targeted Treatment Regimens in HER2-Positive Metastatic Colorectal Cancer (mCRC). Curr. Oncol. 2023, 30, 8266-8277. https://doi.org/10.3390/curroncol30090600
Chitkara A, Bakhtiar M, Sahin IH, Hsu D, Zhang J, Anamika F, Mahnoor M, Ahmed R, Gholami S, Saeed A. A Meta-Analysis to Assess the Efficacy of HER2-Targeted Treatment Regimens in HER2-Positive Metastatic Colorectal Cancer (mCRC). Current Oncology. 2023; 30(9):8266-8277. https://doi.org/10.3390/curroncol30090600
Chicago/Turabian StyleChitkara, Akshit, Muhammad Bakhtiar, Ibrahim Halil Sahin, Dennis Hsu, Janie Zhang, FNU Anamika, Mahnoor Mahnoor, Rabeea Ahmed, Sepideh Gholami, and Anwaar Saeed. 2023. "A Meta-Analysis to Assess the Efficacy of HER2-Targeted Treatment Regimens in HER2-Positive Metastatic Colorectal Cancer (mCRC)" Current Oncology 30, no. 9: 8266-8277. https://doi.org/10.3390/curroncol30090600
APA StyleChitkara, A., Bakhtiar, M., Sahin, I. H., Hsu, D., Zhang, J., Anamika, F., Mahnoor, M., Ahmed, R., Gholami, S., & Saeed, A. (2023). A Meta-Analysis to Assess the Efficacy of HER2-Targeted Treatment Regimens in HER2-Positive Metastatic Colorectal Cancer (mCRC). Current Oncology, 30(9), 8266-8277. https://doi.org/10.3390/curroncol30090600






