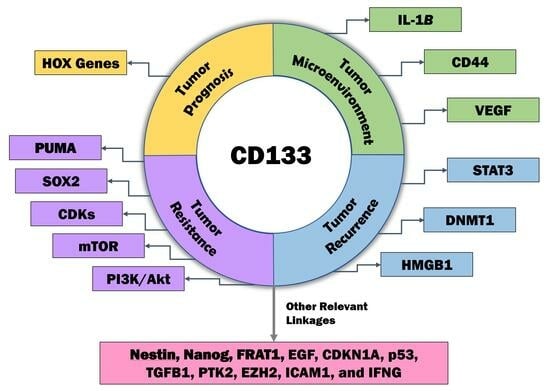
An Overview of CD133 as a Functional Unit of Prognosis and Treatment Resistance in Glioblastoma
Abstract
:1. Introduction
2. CD133 and GBM Prognosis
3. CD133 and the Tumor Microenvironment
4. CD133 and Resistance
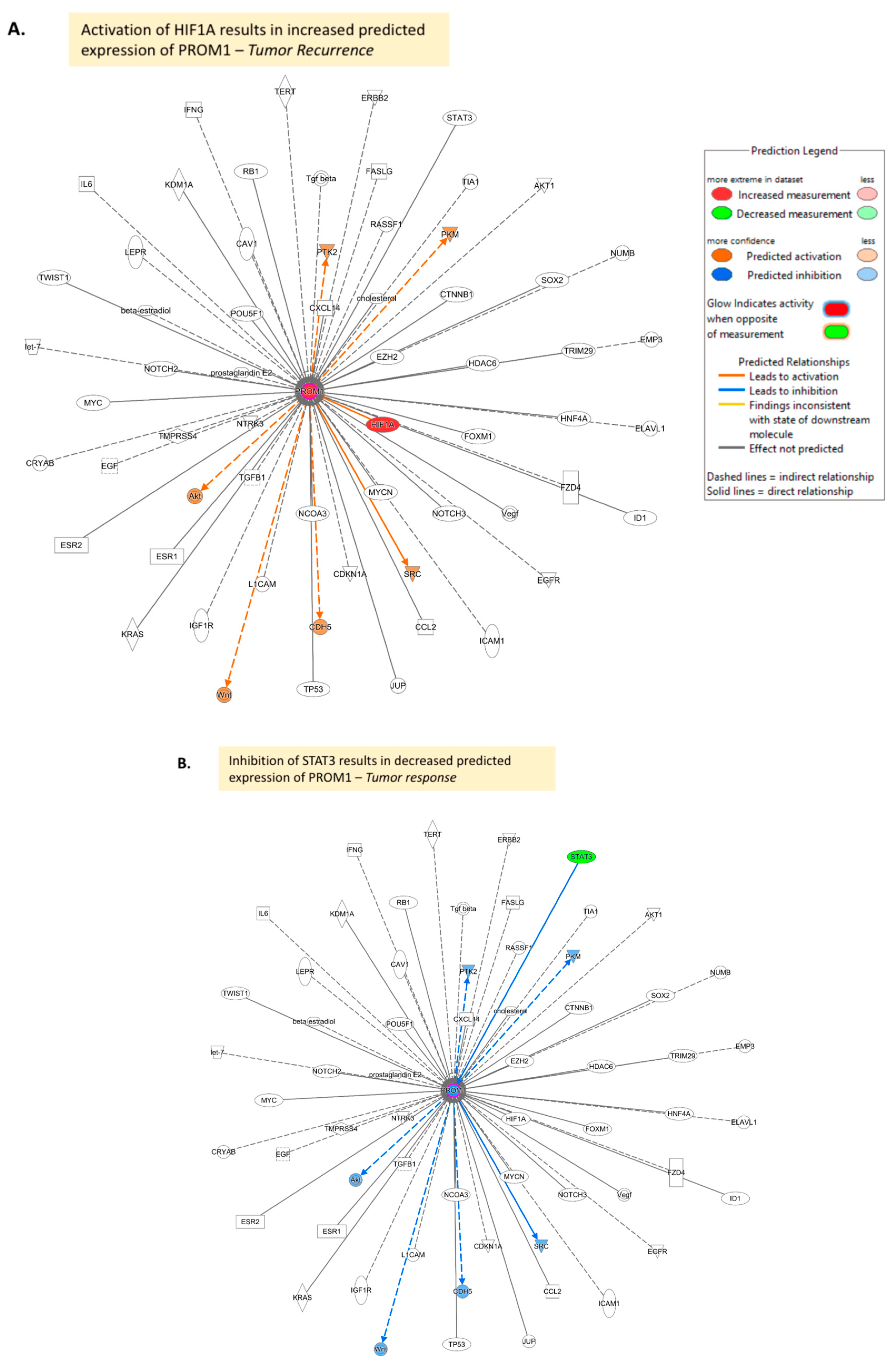
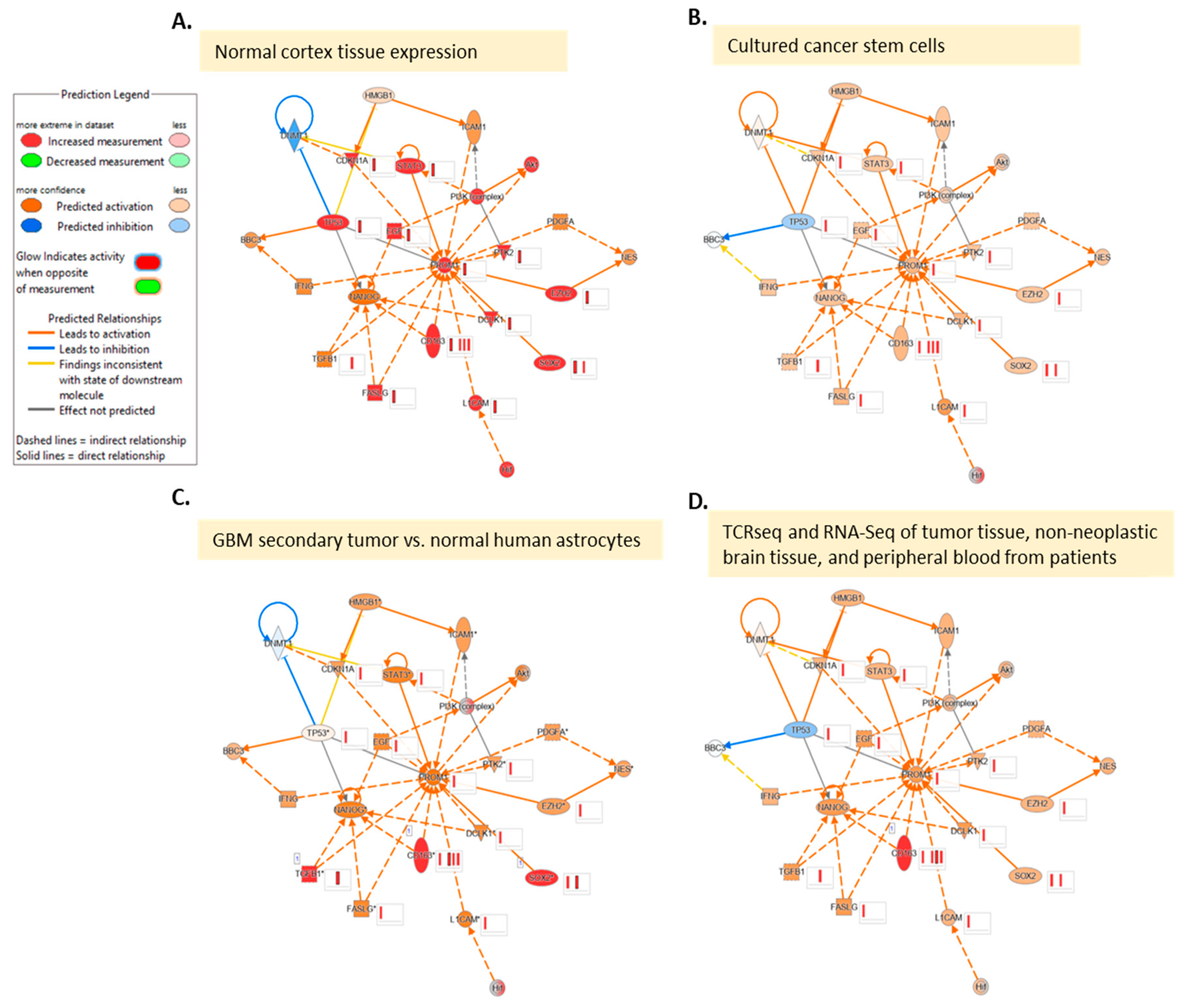
5. CD133 and Recurrence
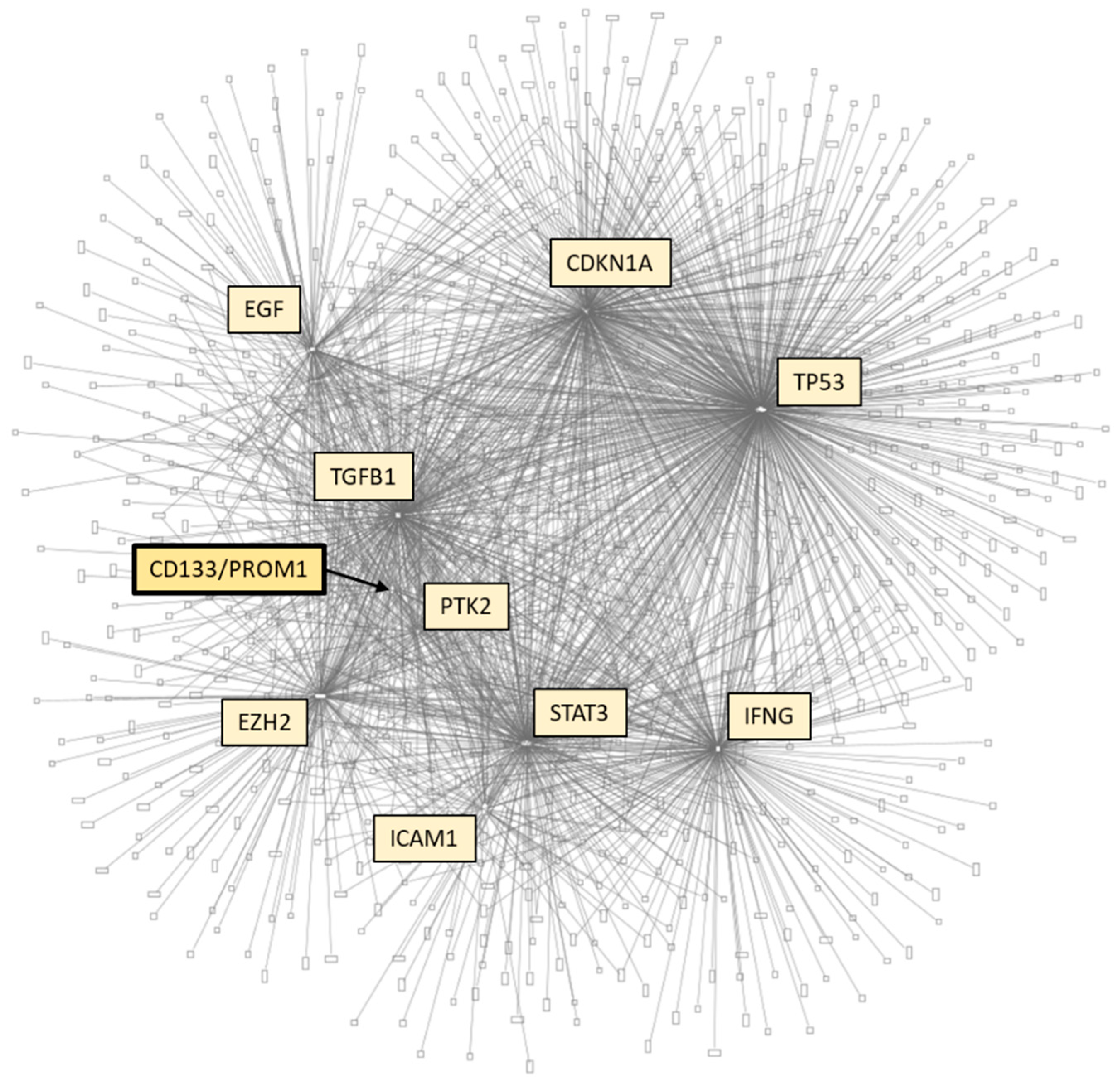
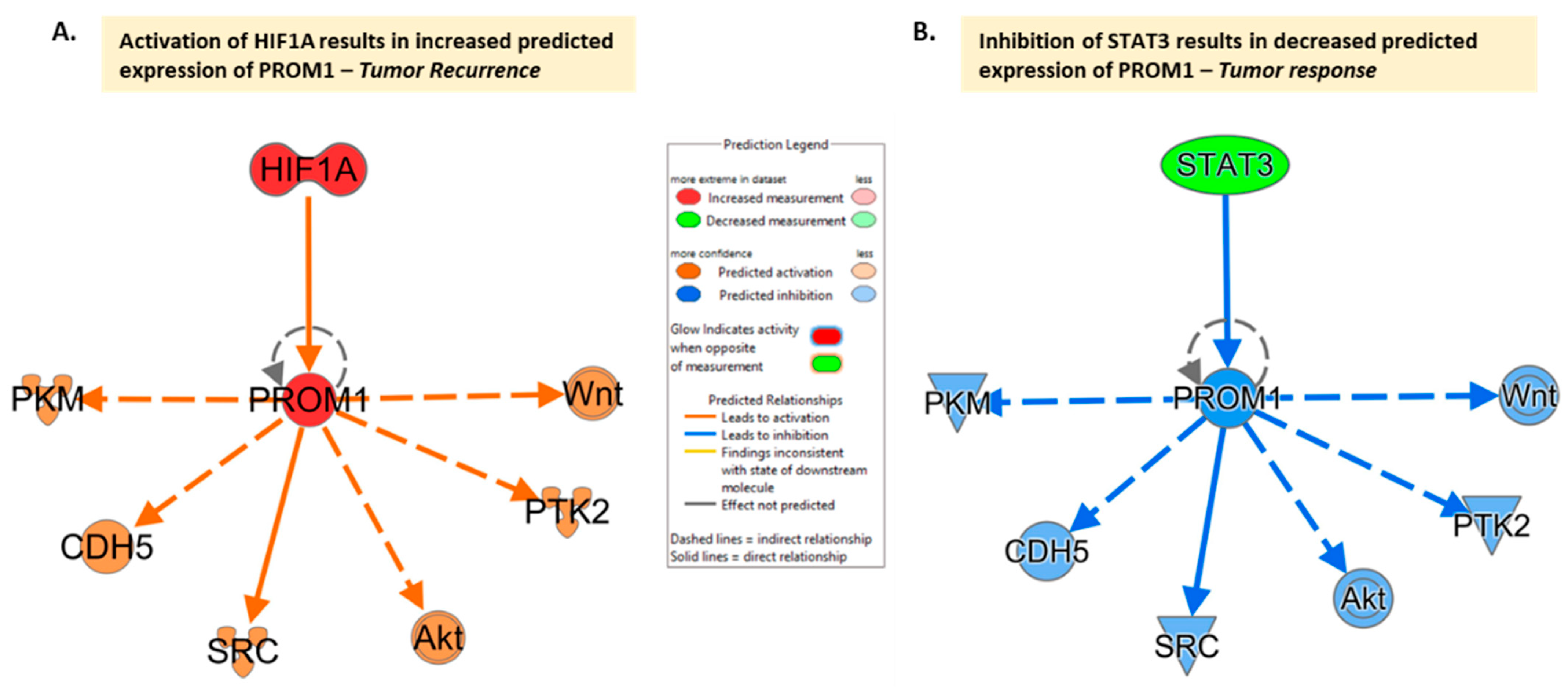
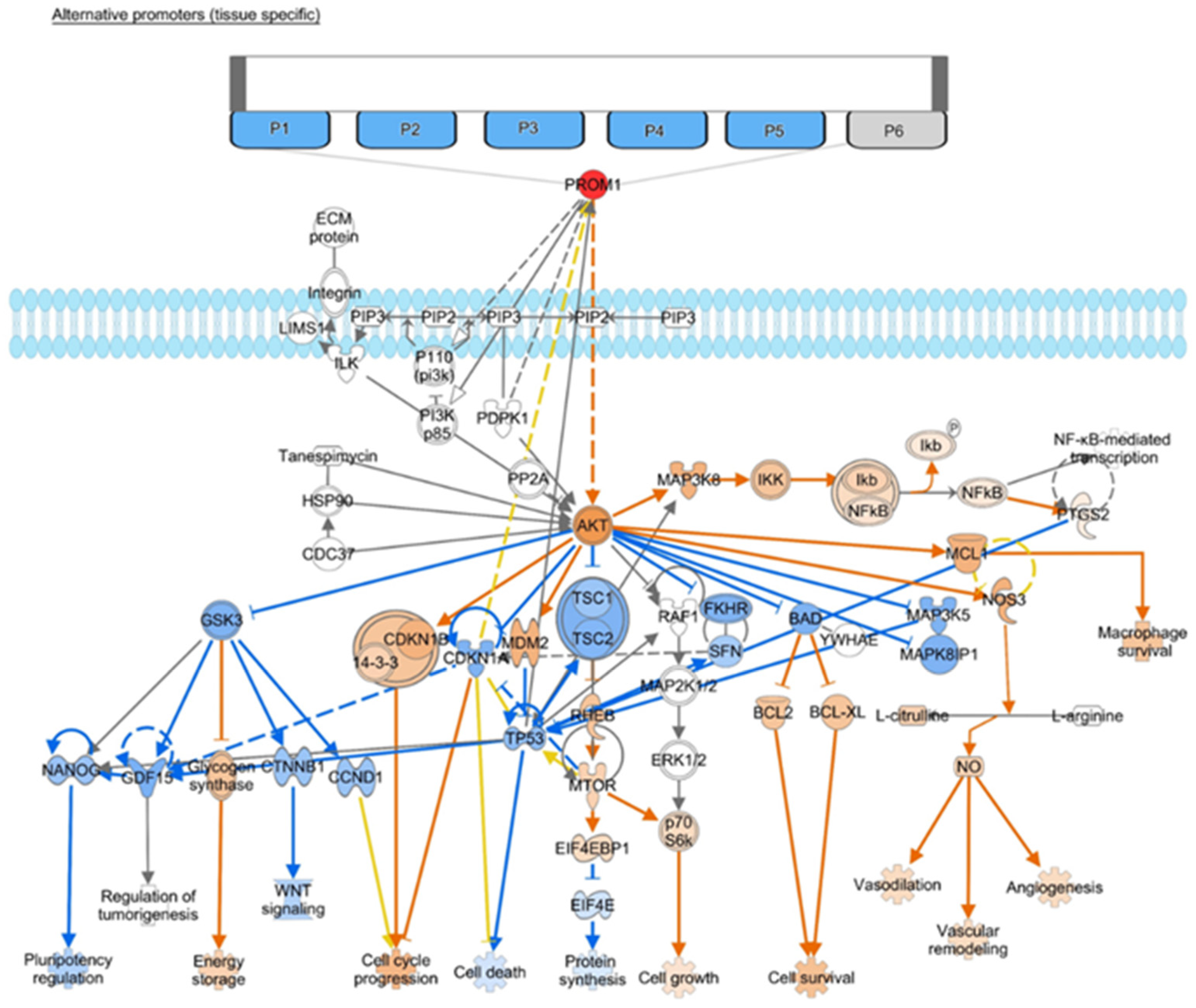
6. CD133-Relevant Linkages
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATRA | All trans retinoic acid |
| CNS | Central Nervous System |
| CDKs | Cyclin-dependent kinases |
| DNMT1 | DNA methyltransferase I |
| FRAT1 | Frequently rearranged in advanced T cell lymphomas-1 |
| GBM | Glioblastoma Multiforme |
| IPA | Ingenuity Pathway Analysis |
| OS | Overall Survival |
| PFS | Progression-free survival |
| PRPS1 | Phosphoribosylpyrophosphate synthetase 1 |
| TMZ | Temozolomide |
Appendix A
References
- Nie, S.; Gurrea, M.; Zhu, J.; Thakolwiboon, S.; Heth, J.A.; Muraszko, K.M.; Fan, X.; Lubman, D.M. Tenascin-C: A novel candidate marker for cancer stem cells in glioblastoma identified by tissue microarrays. J. Proteome Res. 2015, 14, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Chen, G.; Li, Y.; Xie, X.; Zhou, Y.; Du, Z. Aggressive invasion is observed in CD133(−)/A2B5(+) glioma-initiating cells. Oncol. Lett. 2015, 10, 3399–3406. [Google Scholar] [CrossRef]
- Mia-Jan, K.; Jung, S.Y.; Kim, I.Y.; Oh, S.S.; Choi, E.; Chang, S.J.; Kang, T.Y.; Cho, M.Y. CD133 expression is not an independent prognostic factor in stage II and III colorectal cancer but may predict the better outcome in patients with adjuvant therapy. BMC Cancer 2013, 13, 166. [Google Scholar] [CrossRef]
- Lee, G.; Auffinger, B.; Guo, D.; Hasan, T.; Deheeger, M.; Tobias, A.L.; Kim, J.Y.; Atashi, F.; Zhang, L.; Lesniak, M.S.; et al. Dedifferentiation of Glioma Cells to Glioma Stem-like Cells By Therapeutic Stress-induced HIF Signaling in the Recurrent GBM Model. Mol. Cancer Ther. 2016, 15, 3064–3076. [Google Scholar] [CrossRef]
- Salnikov, A.V.; Gladkich, J.; Moldenhauer, G.; Volm, M.; Mattern, J.; Herr, I. CD133 is indicative for a resistance phenotype but does not represent a prognostic marker for survival of non-small cell lung cancer patients. Int. J. Cancer 2010, 126, 950–958. [Google Scholar] [CrossRef]
- Yu, J.W.; Zhang, P.; Wu, J.G.; Wu, S.H.; Li, X.Q.; Wang, S.T.; Lu, R.Q.; Ni, X.C.; Jiang, B.J. Expressions and clinical significances of CD133 protein and CD133 mRNA in primary lesion of gastric adenocacinoma. J. Exp. Clin. Cancer Res. 2010, 29, 141. [Google Scholar] [CrossRef]
- Missol-Kolka, E.; Karbanova, J.; Janich, P.; Haase, M.; Fargeas, C.A.; Huttner, W.B.; Corbeil, D. Prominin-1 (CD133) is not restricted to stem cells located in the basal compartment of murine and human prostate. Prostate 2011, 71, 254–267. [Google Scholar] [CrossRef]
- Wu, B.; Sun, C.; Feng, F.; Ge, M.; Xia, L. Do relevant markers of cancer stem cells CD133 and Nestin indicate a poor prognosis in glioma patients? A systematic review and meta-analysis. J. Exp. Clin. Cancer Res. 2015, 34, 44. [Google Scholar] [CrossRef]
- Han, M.; Guo, L.; Zhang, Y.; Huang, B.; Chen, A.; Chen, W.; Liu, X.; Sun, S.; Wang, K.; Liu, A.; et al. Clinicopathological and Prognostic Significance of CD133 in Glioma Patients: A Meta-Analysis. Mol. Neurobiol. 2016, 53, 720–727. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, H.; Lv, S.; Yang, H. High CD133 Expression Is Associated with Worse Prognosis in Patients with Glioblastoma. Mol. Neurobiol. 2016, 53, 2354–2360. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; McCrudden, C.M.; Yuen, H.F.; Xi, X.; Lyu, P.; Chan, K.W.; Zhang, S.D.; Kwok, H.F. CD133 in brain tumor: The prognostic factor. Oncotarget 2017, 8, 11144–11159. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Cortez, E.G.; Vargas Felix, G.; Rangel López, E.; Sotelo, J.; Martínez-Canseco, C.; Pérez-de la Cruz, V.; Pineda, B. Production and Evaluation of an Avian IgY Immunotoxin against CD133+ for Treatment of Carcinogenic Stem Cells in Malignant Glioma: IgY Immunotoxin for the Treatment of Glioblastoma. J. Oncol. 2019, 2019, 2563092. [Google Scholar] [CrossRef] [PubMed]
- Abdoli Shadbad, M.; Hosseinkhani, N.; Asadzadeh, Z.; Brunetti, O.; Silvestris, N.; Baradaran, B. The Prognostic Value of CD133 in Predicting the Relapse and Recurrence Pattern of High-Grade Gliomas on MRI: A Meta-Analysis. Front. Oncol. 2021, 11, 722833. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.V.; Filiz, G.; Daniel, P.M.; Hollande, F.; Dworkin, S.; Amiridis, S.; Kountouri, N.; Ng, W.; Morokoff, A.P.; Mantamadiotis, T. Expression of CD133 and CD44 in glioblastoma stem cells correlates with cell proliferation, phenotype stability and intra-tumor heterogeneity. PLoS ONE 2017, 12, e0172791. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, J.K.; Jeon, H.Y.; Ham, S.W.; Kim, H. CD133 Regulates IL-1β Signaling and Neutrophil Recruitment in Glioblastoma. Mol. Cells 2017, 40, 515–522. [Google Scholar] [CrossRef]
- Musah-Eroje, A.; Watson, S. Adaptive Changes of Glioblastoma Cells Following Exposure to Hypoxic (1% Oxygen) Tumour Microenvironment. Int. J. Mol. Sci. 2019, 20, 2091. [Google Scholar] [CrossRef]
- Miao, W.; Liu, X.; Wang, H.; Fan, Y.; Lian, S.; Yang, X.; Wang, X.; Guo, G.; Li, Q.; Wang, S. p53 upregulated modulator of apoptosis sensitizes drug-resistant U251 glioblastoma stem cells to temozolomide through enhanced apoptosis. Mol. Med. Rep. 2015, 11, 4165–4173. [Google Scholar] [CrossRef]
- Perazzoli, G.; Prados, J.; Ortiz, R.; Caba, O.; Cabeza, L.; Berdasco, M.; Gonzalez, B.; Melguizo, C. Temozolomide Resistance in Glioblastoma Cell Lines: Implication of MGMT, MMR, P-Glycoprotein and CD133 Expression. PLoS ONE 2015, 10, e0140131. [Google Scholar] [CrossRef]
- Song, W.S.; Yang, Y.P.; Huang, C.S.; Lu, K.H.; Liu, W.H.; Wu, W.W.; Lee, Y.Y.; Lo, W.L.; Lee, S.D.; Chen, Y.W.; et al. Sox2, a stemness gene, regulates tumor-initiating and drug-resistant properties in CD133-positive glioblastoma stem cells. J. Chin. Med. Assoc. 2016, 79, 538–545. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, M.; Cheng, K.; Li, P.; Han, S.; Li, R.; Su, M.; Zeng, W.; Liu, J.; Guo, J.; et al. Resistance of glioma cells to nutrient-deprived microenvironment can be enhanced by CD133-mediated autophagy. Oncotarget 2016, 7, 76238–76249. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M.; Bandopadhyay, G.; Coyle, B.; Grabowska, A. A HIF-independent, CD133-mediated mechanism of cisplatin resistance in glioblastoma cells. Cell. Oncol. 2018, 41, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Juric, V.; Düssmann, H.; Lamfers, M.L.M.; Prehn, J.H.M.; Rehm, M.; Murphy, B.M. Transcriptional CDK Inhibitors CYC065 and THZ1 Induce Apoptosis in Glioma Stem Cells Derived from Recurrent GBM. Cells 2021, 10, 1182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.P.; Chen, Q.X. Effects of HMGB1 on proliferation and apoptosis of human brain glioma CD133 cells. Bratisl. Lek. Listy 2015, 116, 480–485. [Google Scholar] [CrossRef]
- Sun, B.; Wan, Z.; Shen, J.; Ni, L.; Chen, J.; Cui, M.; Ni, H.; Shi, W.; Shi, J. DNA hypomethylation of CD133 promoter is associated with recurrent glioma. Oncol. Rep. 2016, 36, 1062–1068. [Google Scholar] [CrossRef]
- Chang, C.H.; Liu, W.T.; Hung, H.C.; Gean, C.Y.; Tsai, H.M.; Su, C.L.; Gean, P.W. Synergistic inhibition of tumor growth by combination treatment with drugs against different subpopulations of glioblastoma cells. BMC Cancer 2017, 17, 905. [Google Scholar] [CrossRef]
- Polat, B.; Wohlleben, G.; Kosmala, R.; Lisowski, D.; Mantel, F.; Lewitzki, V.; Lohr, M.; Blum, R.; Herud, P.; Flentje, M.; et al. Differences in stem cell marker and osteopontin expression in primary and recurrent glioblastoma. Cancer Cell Int. 2022, 22, 87. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, Q.; Huang, S.; Liu, Y.; Li, Y.; Xing, Y.; Shi, D.; Xu, W.; Liu, W.; Ji, Z.; et al. The Interaction between DNMT1 and High-Mannose CD133 Maintains the Slow-Cycling State and Tumorigenic Potential of Glioma Stem Cell. Adv. Sci. 2022, 9, e2202216. [Google Scholar] [CrossRef]
- Bien-Moller, S.; Balz, E.; Herzog, S.; Plantera, L.; Vogelgesang, S.; Weitmann, K.; Seifert, C.; Fink, M.A.; Marx, S.; Bialke, A.; et al. Association of Glioblastoma Multiforme Stem Cell Characteristics, Differentiation, and Microglia Marker Genes with Patient Survival. Stem Cells Int. 2018, 2018, 9628289. [Google Scholar] [CrossRef]
- Shevchenko, V.; Arnotskaya, N.; Korneyko, M.; Zaytsev, S.; Khotimchenko, Y.; Sharma, H.; Bryukhovetskiy, I. Proteins of the Wnt signaling pathway as targets for the regulation of CD133+ cancer stem cells in glioblastoma. Oncol. Rep. 2019, 41, 3080–3088. [Google Scholar] [CrossRef]
- Guo, G.; Liu, J.; Ren, Y.; Mao, X.; Hao, Y.; Zhong, C.; Chen, X.; Wang, X.; Wu, Y.; Lian, S.; et al. FRAT1 Enhances the Proliferation and Tumorigenesis of CD133(+)Nestin(+) Glioma Stem Cells In Vitro and In Vivo. J. Cancer 2020, 11, 2421–2430. [Google Scholar] [CrossRef]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Gorman, M.J.; McKenzie, L.D.; Chai, J.N.; Hubert, C.G.; Prager, B.C.; Fernandez, E.; Richner, J.M.; Zhang, R.; Shan, C.; et al. Correction: Zika virus has oncolytic activity against glioblastoma stem cells. J. Exp. Med. 2017, 214, 3145. [Google Scholar] [CrossRef]
- Grzmil, M.; Morin, P., Jr.; Lino, M.M.; Merlo, A.; Frank, S.; Wang, Y.; Moncayo, G.; Hemmings, B.A. MAP kinase-interacting kinase 1 regulates SMAD2-dependent TGF-beta signaling pathway in human glioblastoma. Cancer Res. 2011, 71, 2392–2402. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.S.; Grinshpun, B.; Feng, Y.; Ung, T.H.; Neira, J.A.; Samanamud, J.L.; Canoll, P.; Shen, Y.; Sims, P.A.; Bruce, J.N. Diversity and divergence of the glioma-infiltrating T-cell receptor repertoire. Proc. Natl. Acad. Sci. USA 2016, 113, E3529–E3537. [Google Scholar] [CrossRef]
- Ahmed, S.I.; Javed, G.; Laghari, A.A.; Bareeqa, S.B.; Farrukh, S.; Zahid, S.; Samar, S.S.; Aziz, K. CD133 Expression in Glioblastoma Multiforme: A Literature Review. Cureus 2018, 10, e3439. [Google Scholar] [CrossRef]
- Wu, X.; Wu, F.; Xu, D.; Zhang, T. Prognostic significance of stem cell marker CD133 determined by promoter methylation but not by immunohistochemical expression in malignant gliomas. J. Neuro-Oncol. 2016, 127, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, L.; Xiao, Z.; Wang, Y.; Liu, T.; Zhang, T.; Zhang, Y. Screening glioma stem cells in U251 cells based on the P1 promoter of the CD133 gene. Oncol. Lett. 2016, 12, 2457–2462. [Google Scholar] [CrossRef]
- Huang, W.; Zhong, Z.; Luo, C.; Xiao, Y.; Li, L.; Zhang, X.; Yang, L.; Xiao, K.; Ning, Y.; Chen, L.; et al. The miR-26a/AP-2α/Nanog signaling axis mediates stem cell self-renewal and temozolomide resistance in glioma. Theranostics 2019, 9, 5497–5516. [Google Scholar] [CrossRef]
- Li, C.; Yan, Z.; Cao, X.; Zhang, X.; Yang, L. Phosphoribosylpyrophosphate Synthetase 1 Knockdown Suppresses Tumor Formation of Glioma CD133+ Cells Through Upregulating Cell Apoptosis. J. Mol. Neurosci. 2016, 60, 145–156. [Google Scholar] [CrossRef]
- Li, N.; Guo, Q.; Zhang, Q.; Chen, B.J.; Li, X.A.; Zhou, Y. Comprehensive Analysis of Differentially Expressed Profiles of mRNA N6-Methyladenosine in Colorectal Cancer. Front. Cell Dev. Biol. 2021, 9, 760912. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, J.; Zhu, Y.; Li, Y.; Wang, Y.; Chen, H.; Wang, J.; Li, X.; Liu, Y.; Li, B.; et al. CD163, a novel therapeutic target, regulates the proliferation and stemness of glioma cells via casein kinase 2. Oncogene 2019, 38, 1183–1199. [Google Scholar] [CrossRef] [PubMed]
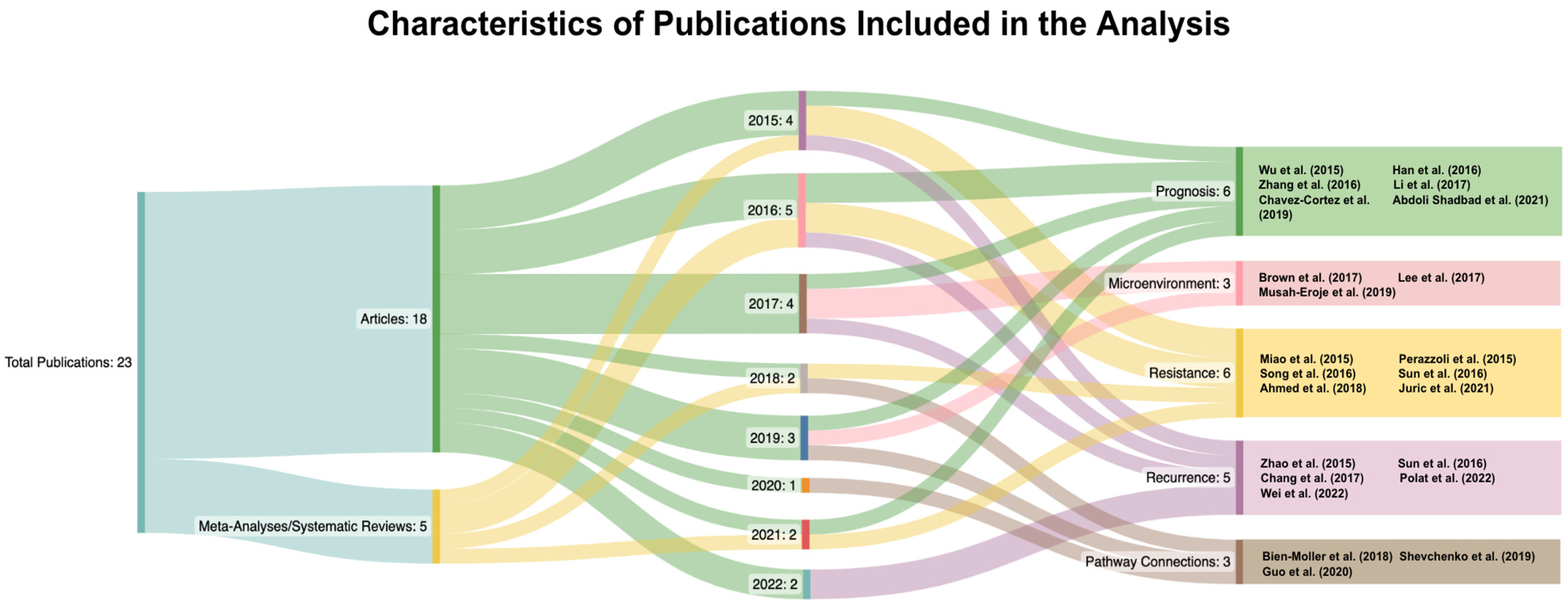

| Authors(s) | Category | Title | Overview of Topic |
|---|---|---|---|
| Wu et al., (2015) [9] | Related to Prognosis | Do relevant markers of cancer stem cells CD133 and Nestin indicate a poor prognosis in glioma patients? A systematic review and meta-analysis | Association between high CD133 expression and OS and PFS based on the grade of glioma |
| Han et al., (2016) [10] | Related to Prognosis | Clinicopathological and Prognostic Significance of CD133 in Glioma Patients: A Meta-Analysis | Association between high CD133 expression and OS and PFS |
| Zhang et al., (2016) [11] | Related to Prognosis | High CD133 Expression Is Associated with Worse Prognosis in Patients with Glioblastoma | Association of high CD133 expression to OS and PFS |
| Li et al., (2017) [12] | Related to Prognosis | CD133 in brain tumor: the prognostic factor | Connection to HOX gene stem cell factors (HOXA5, HOXA7, HOXA10, HOXC4, HOXC6) |
| Chavez- Cortez et al., (2019) [13] | Related to Prognosis | Production and Evaluation of an Avian IgY Immunotoxin against CD133+ for Treatment of Carcinogenic Stem Cells in Malignant Glioma: IgY Immunotoxin for the Treatment of Glioblastoma | Creation of CD133 immunotoxin from IgY |
| Abdoli Shadbad et al., (2021) [14] | Related to Prognosis | The Prognostic Value of CD133 in Predicting Relapse and Recurrence Pattern of High-Grade Gliomas on MRI: A Meta-Analysis | Association of high CD133 expression with PFS and recurrence |
| Brown et al., (2017) [15] | Related to Microenvironment | Expression of CD133 and CD144 in glioblastoma stem cells correlates with cell proliferation, phenotype stability and intra-tumor heterogeneity | Connection between CD44 and CD133 |
| Lee et al., (2017) [16] | Related to Microenvironment | CD133 Regulates IL-1Beta Signaling and Neutrophil Recruitment in Glioblastoma | Relationship between tumor microenvironment and IL-1β signaling pathway |
| Musah-Eroje et al., (2019) [17] | Related to Microenvironment | Adaptive Changes of Glioblastoma Cells Following Exposure to Hypoxic (1% Oxygen) Tumour Microenvironment | Connection between hypoxia and VEGF signaling |
| Miao et al., (2015) [18] | Related to Resistance | P53 upregulated modulator of apoptosis sensitizes drug-resistant U251 glioblastoma stem cells to temozolomide through enhanced apoptosis | Connection between PUMA, TMZ, and CD133 |
| Perazzoli et al., (2015) [19] | Related to Resistance | Temozolomide Resistance in Glioblastoma Cell Lines: Implication of MGMT, MMR, P-Glycoprotein, and CD133 Expression | The effect of CD133 on TMZ resistance |
| Song et al., (2016) [20] | Related to Resistance | Sox2, a stemness gene, regulates tumor-initiating and drug-resistant properties in CD133-positive glioblastoma stem cells | SOX2 connection to CD133(+) GBM cells |
| Sun et al., (2016) [21] | Related to Resistance | Resistance of glioma cells to nutrient-deprived microenvironment can be enhanced by CD133-mediated autophagy | CD133 and cell survival via autophagy |
| Ahmed et al., (2018) [22] | Related to Resistance | A HIF-independent, CD133-mediated mechanism of cisplatin resistance in glioblastoma cells | Hypoxia-induced CD133-mediated cisplatin resistance |
| Juric et al., (2021) [23] | Related to Resistance | Transcriptional CDK Inhibitors CYC065 and THZ1 Induce Apoptosis in Glioma Stem Cells Derived from Recurrent GBM | Cyclin-dependent kinases (CDKs) used to treat recurrent glioma |
| Zhao et al., (2015) [24] | Related to Recurrence | Effects of HMGB1 on proliferation and apoptosis of human brain glioma CD133 cells | Relationship between overexpression of HMGB1 gene and CD133 |
| Sun et al., (2016) [25] | Related to Recurrence | DNA hypomethylation of CD133 promoter is associated with recurrent glioma | Promoter hypomethylation and glioma recurrence |
| Chang et al., (2017) [26] | Related to Recurrence | Synergistic inhibition of tumor growth by combination treatment with drugs against different subpopulations of glioblastoma cells | Connection to STAT3 and cell viability based on CD133 expression |
| Polat et al., (2022) [27] | Related to Recurrence | Differences in stem cell marker and osteopontin expression in primary and recurrent glioblastoma | Comparison between CD133 expression in original and recurring tumors |
| Wei et al., (2022) [28] | Related to Recurrence | The Interaction between DNMT1 and High-Mannose CD133 Maintains the Slow-Cycling State and Tumorigenic Potential of Glioma Stem Cell | Relation between CD133 and DNA methyltransferase 1 (DNMT1) and its impact on stem cells |
| Bien-Moller et al., (2018) [29] | Related to Pathways | Association of Glioblastoma Multiforme Stem Cell Characteristics, Differentiation, and Microglia Marker Genes with Patient Survival | Connection to CD44, CD95, CD133, ELF4, Nanog, Nestin, and Sparc and OS |
| Shevchenko et al., (2019) [30] | Related to Pathways | Proteins of the Wnt signaling pathway as targets for the regulation of CD133(+) cancer stem cells in glioblastoma | Differences identified in glycolysis/gluconeogenesis, focal adhesion, tight junction and Wnt signaling pathways |
| Guo et al., (2020) [31] | Related to Pathways | FRAT1 Enhances the Proliferation of Tumorigenesis of CD133(+) Nestin(+) Glioma Stem Cells In Vitro and In Vivo | Connection between FRAT1 and CD133 expression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joyce, T.; Jagasia, S.; Tasci, E.; Camphausen, K.; Krauze, A.V. An Overview of CD133 as a Functional Unit of Prognosis and Treatment Resistance in Glioblastoma. Curr. Oncol. 2023, 30, 8278-8293. https://doi.org/10.3390/curroncol30090601
Joyce T, Jagasia S, Tasci E, Camphausen K, Krauze AV. An Overview of CD133 as a Functional Unit of Prognosis and Treatment Resistance in Glioblastoma. Current Oncology. 2023; 30(9):8278-8293. https://doi.org/10.3390/curroncol30090601
Chicago/Turabian StyleJoyce, Thomas, Sarisha Jagasia, Erdal Tasci, Kevin Camphausen, and Andra Valentina Krauze. 2023. "An Overview of CD133 as a Functional Unit of Prognosis and Treatment Resistance in Glioblastoma" Current Oncology 30, no. 9: 8278-8293. https://doi.org/10.3390/curroncol30090601
APA StyleJoyce, T., Jagasia, S., Tasci, E., Camphausen, K., & Krauze, A. V. (2023). An Overview of CD133 as a Functional Unit of Prognosis and Treatment Resistance in Glioblastoma. Current Oncology, 30(9), 8278-8293. https://doi.org/10.3390/curroncol30090601