Ampullary Cancer: Histological Subtypes, Markers, and Clinical Behaviour—State of the Art and Perspectives
Abstract
1. Introduction
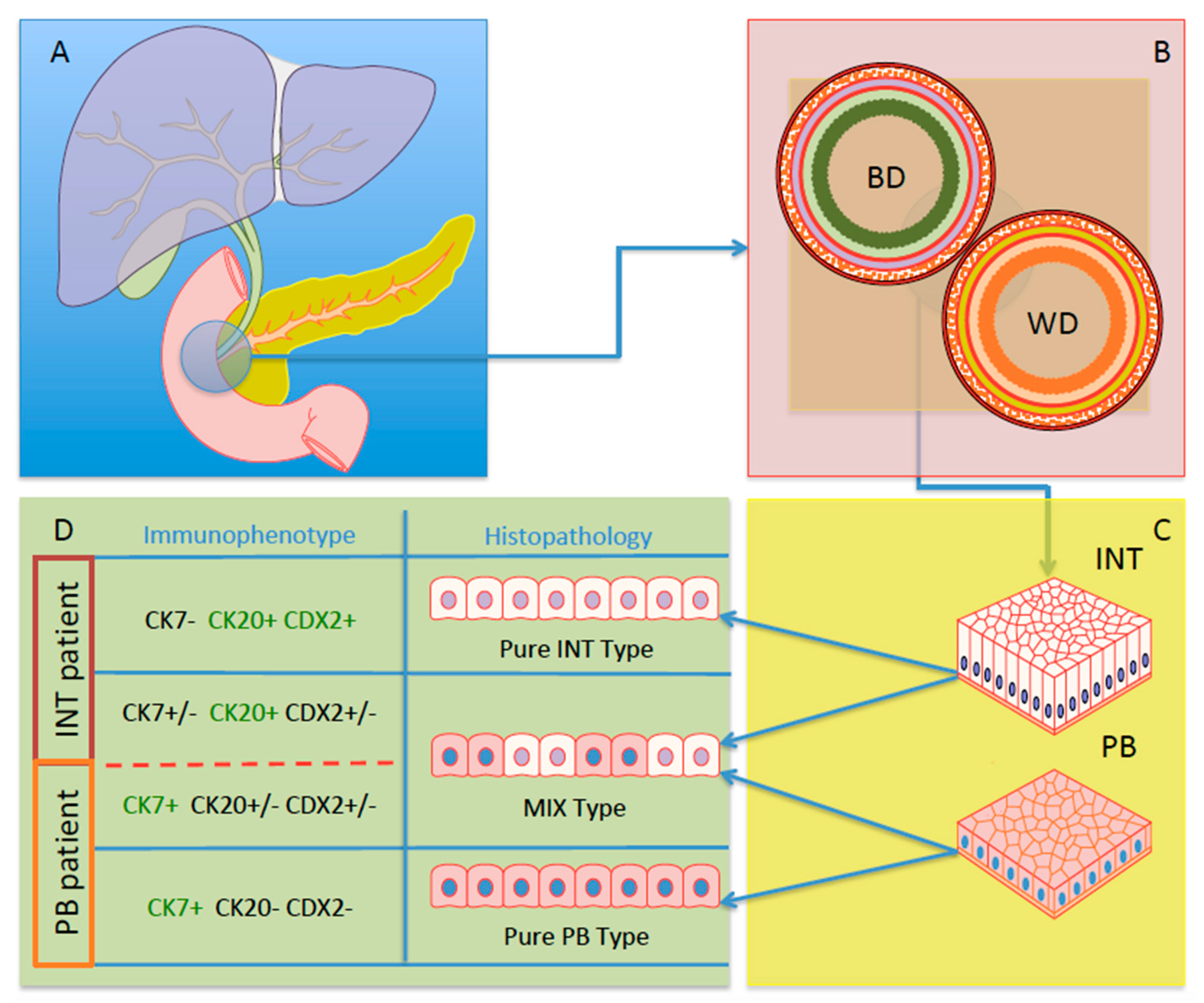
2. Definition of Different Subtypes of AAC
3. Prognosis of AACs
4. The Role of Adjuvant Therapy for Ampullary Cancer
5. The Role of Histological Biomarkers in AACs
6. IHC and Its Diagnostic Role in AACs
7. IHC and Its Prognostic Role in AACs
8. IHC and Its Predictive Role in AACs
9. Conclusions
10. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Kim, R.D.; Kundhal, P.S.; McGilvray, I.D.; Cattral, M.S.; Taylor, B.; Langer, B.; Grant, D.R.; Zogopoulos, G.; Shah, S.A.; Greig, P.D.; et al. Predictors of Failure after Pancreaticoduodenectomy for Ampullary Carcinoma. J. Am. Coll. Surg. 2006, 202, 112–119. [Google Scholar] [CrossRef]
- Henson, D.E.; Schwartz, A.M.; Nsouli, H.; Albores-Saavedra, J. Carcinomas of the Pancreas, Gallbladder, Extrahepatic Bile Ducts, and Ampulla of Vater Share a Field for Carcinogenesis: A Population-Based Study. Arch. Pathol. Lab. Med. 2009, 133, 67–71. [Google Scholar] [CrossRef]
- Albores-Saavedra, J.; Henson, D.E.; Sobin, L.H. The WHO Histological Classification of Tumors of the Gallbladder and Extrahepatic Bile Ducts. A Commentary on the Second Edition. Cancer 1992, 70, 410–414. [Google Scholar] [CrossRef]
- Kimura, W.; Futakawa, N.; Yamagata, S.; Wada, Y.; Kuroda, A.; Muto, T.; Esaki, Y. Different Clinicopathologic Findings in Two Histologic Types of Carcinoma of Papilla of Vater. Jpn. J. Cancer Res. 1994, 85, 161–166. [Google Scholar] [CrossRef]
- Outerbridge, G.W. Carcinoma of the papilla of vater. Ann. Surg. 1913, 57, 402–426. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.K.; Jamieson, N.B.; Johns, A.L.; Scarlett, C.J.; Pajic, M.; Chou, A.; Pinese, M.; Humphris, J.L.; Jones, M.D.; Toon, C.; et al. Histomolecular Phenotypes and Outcome in Adenocarcinoma of the Ampulla of Vater. J. Clin. Oncol. 2013, 31, 1348–1356. [Google Scholar] [CrossRef]
- Ang, D.C.; Shia, J.; Tang, L.H.; Katabi, N.; Klimstra, D.S. The Utility of Immunohistochemistry in Subtyping Adenocarcinoma of the Ampulla of Vater. Am. J. Surg. Pathol. 2014, 38, 1371–1379. [Google Scholar] [CrossRef]
- Westgaard, A.; Pomianowska, E.; Clausen, O.P.F.; Gladhaug, I.P. Intestinal-Type and Pancreatobiliary-Type Adenocarcinomas: How Does Ampullary Carcinoma Differ from Other Periampullary Malignancies? Ann. Surg. Oncol. 2013, 20, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.T.; Grenert, J.P.; Rubenstein, L.; Stewart, L.; Way, L.W. Tumors of the Ampulla of Vater: Histopathologic Classification and Predictors of Survival. J. Am. Coll. Surg. 2008, 207, 210–218. [Google Scholar] [CrossRef]
- Schirmacher, P.; Büchler, M.W. Ampullary Adenocarcinoma – Differentiation Matters. BMC Cancer 2008, 8, 251. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours of the Digestive System; Bosman, F.T.; World Health Organization, International Agency for Research on Cancer (Eds.) World Health Organization Classification of Tumours, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2010; ISBN 978-92-832-2432-7. [Google Scholar]
- Adsay, V.; Ohike, N.; Tajiri, T.; Kim, G.E.; Krasinskas, A.; Balci, S.; Bagci, P.; Basturk, O.; Bandyopadhyay, S.; Jang, K.-T.; et al. Ampullary Region Carcinomas: Definition and Site Specific Classification With Delineation of Four Clinicopathologically and Prognostically Distinct Subsets in an Analysis of 249 Cases. Am. J. Surg. Pathol. 2012, 36, 1592–1608. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.-P.; Zhou, H. Pathogenesis of Carcinoma of the Papilla of Vater. J. Hepatobiliary. Pancreat. Surg. 2004, 11, 301–309. [Google Scholar] [CrossRef]
- Takashima, M.; Ueki, T.; Nagai, E.; Yao, T.; Yamaguchi, K.; Tanaka, M.; Tsuneyoshi, M. Carcinoma of the Ampulla of Vater Associated With or Without Adenoma: A Clinicopathologic Analysis of 198 Cases With Reference to P53 and Ki-67 Immunohistochemical Expressions. Mod. Pathol. 2000, 13, 1300–1307. [Google Scholar] [CrossRef]
- Vogelstein, B.; Kinzler, K.W. The Multistep Nature of Cancer. Trends Genet. 1993, 9, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Fearon, E.R.; Vogelstein, B. A Genetic Model for Colorectal Tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Reid, M.D.; Balci, S.; Ohike, N.; Xue, Y.; Kim, G.E.; Tajiri, T.; Memis, B.; Coban, I.; Dolgun, A.; Krasinskas, A.M.; et al. Ampullary Carcinoma Is Often of Mixed or Hybrid Histologic Type: An Analysis of Reproducibility and Clinical Relevance of Classification as Pancreatobiliary versus Intestinal in 232 Cases. Mod. Pathol. 2016, 29, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Basturk, O.; Hong, S.-M.; Wood, L.D.; Adsay, N.V.; Albores-Saavedra, J.; Biankin, A.V.; Brosens, L.A.A.; Fukushima, N.; Goggins, M.; Hruban, R.H.; et al. A Revised Classification System and Recommendations From the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am. J. Surg. Pathol. 2015, 39, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Albores-Saavedra, J.; Schwartz, A.M.; Batich, K.; Henson, D.E. Cancers of the Ampulla of Vater: Demographics, Morphology, and Survival Based on 5,625 Cases from the SEER Program: Cancer of the Ampulla of Vater. J. Surg. Oncol. 2009, 100, 598–605. [Google Scholar] [CrossRef]
- Xue, Y.; Reid, M.D. Approaches to Biopsy and Resection Specimens from the Ampulla. Surg. Pathol. Clin. 2020, 13, 453–467. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The WHO Classification of Tumours Editorial Board The 2019 WHO Classification of Tumours of the Digestive System. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Beghelli, S.; Orlandini, S.; Moore, P.S.; Talamini, G.; Capelli, P.; Zamboni, G.; Falconi, M.; Scarpa, A. Ampulla of Vater Cancers: T-Stage and Histological Subtype but Not Dpc4 Expression Predict Prognosis. Virchows Arch. 2002, 441, 19–24. [Google Scholar] [CrossRef]
- Ruemmele, P.; Dietmaier, W.; Terracciano, L.; Tornillo, L.; Bataille, F.; Kaiser, A.; Wuensch, P.-H.; Heinmoeller, E.; Homayounfar, K.; Luettges, J.; et al. Histopathologic Features and Microsatellite Instability of Cancers of the Papilla of Vater and Their Precursor Lesions. Am. J. Surg. Pathol. 2009, 33, 691–704. [Google Scholar] [CrossRef]
- Kim, W.S.; Choi, D.W.; Choi, S.H.; Heo, J.S.; You, D.D.; Lee, H.G. Clinical Significance of Pathologic Subtype in Curatively Resected Ampulla of Vater Cancer: Ampulla of Vater Cancer. J. Surg. Oncol. 2012, 105, 266–272. [Google Scholar] [CrossRef]
- Robert, P.-E.; Leux, C.; Ouaissi, M.; Miguet, M.; Paye, F.; Merdrignac, A.; Delpero, J.R.; Schwarz, L.; Carrere, N.; Muscari, F.; et al. Predictors of Long-Term Survival Following Resection for Ampullary Carcinoma: A Large Retrospective French Multicentric Study. Pancreas 2014, 43, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Schueneman, A.; Goggins, M.; Ensor, J.; Saka, B.; Neishaboori, N.; Lee, S.; Maitra, A.; Varadhachary, G.; Rezaee, N.; Wolfgang, C.; et al. Validation of Histomolecular Classification Utilizing Histological Subtype, MUC1, and CDX2 for Prognostication of Resected Ampullary Adenocarcinoma. Br. J. Cancer 2015, 113, 64–68. [Google Scholar] [CrossRef]
- Zimmermann, C.; Wolk, S.; Aust, D.E.; Meier, F.; Saeger, H.-D.; Ehehalt, F.; Weitz, J.; Welsch, T.; Distler, M. The Pathohistological Subtype Strongly Predicts Survival in Patients with Ampullary Carcinoma. Sci. Rep. 2019, 9, 12676. [Google Scholar] [CrossRef] [PubMed]
- Sessa, F.; Furlan, D.; Zampatti, C.; Carnevali, I.; Franzi, F.; Capella, C. Prognostic Factors for Ampullary Adenocarcinomas: Tumor Stage, Tumor Histology, Tumor Location, Immunohistochemistry and Microsatellite Instability. Virchows Arch. 2007, 451, 649–657. [Google Scholar] [CrossRef]
- De Paiva Haddad, L.B.; Patzina, R.A.; Penteado, S.; Montagnini, A.L.; Da Cunha, J.E.M.; Machado, M.C.C.; Jukemura, J. Lymph Node Involvement and Not the Histophatologic Subtype Is Correlated with Outcome After Resection of Adenocarcinoma of the Ampulla of Vater. J. Gastrointest. Surg. 2010, 14, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Bowitz Lothe, I.M.; Kleive, D.; Pomianowska, E.; Cvancarova, M.; Kure, E.; Dueland, S.; Gladhaug, I.P.; Labori, K.J. Clinical Relevance of Pancreatobiliary and Intestinal Subtypes of Ampullary and Duodenal Adenocarcinoma: Pattern of Recurrence, Chemotherapy, and Survival after Pancreatoduodenectomy. Pancreatology 2019, 19, 316–324. [Google Scholar] [CrossRef]
- Moekotte, A.L.; Lof, S.; Van Roessel, S.; Fontana, M.; Dreyer, S.; Shablak, A.; Casciani, F.; Mavroeidis, V.K.; Robinson, S.; Khalil, K.; et al. Histopathologic Predictors of Survival and Recurrence in Resected Ampullary Adenocarcinoma: International Multicenter Cohort Study. Ann. Surg. 2020, 272, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Moekotte, A.L.; Van Roessel, S.; Malleo, G.; Rajak, R.; Ecker, B.L.; Fontana, M.; Han, H.-S.; Rabie, M.; Roberts, K.J.; Khalil, K.; et al. Development and External Validation of a Prediction Model for Survival in Patients with Resected Ampullary Adenocarcinoma. Eur. J. Surg. Oncol. 2020, 46, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, D.; Wu, L.; Si, X. The Histopathologic Type Predicts Survival of Patients with Ampullary Carcinoma after Resection: A Meta-Analysis. Pancreatology 2017, 17, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Moore, M.J.; Cox, T.F.; Valle, J.W.; Palmer, D.H.; McDonald, A.C.; Carter, R.; Tebbutt, N.C.; Dervenis, C.; Smith, D.; et al. Effect of Adjuvant Chemotherapy With Fluorouracil Plus Folinic Acid or Gemcitabine vs Observation on Survival in Patients With Resected Periampullary Adenocarcinoma: The ESPAC-3 Periampullary Cancer Randomized Trial. JAMA 2012, 308, 147. [Google Scholar] [CrossRef] [PubMed]
- Narang, A.K.; Miller, R.C.; Hsu, C.C.; Bhatia, S.; Pawlik, T.M.; Laheru, D.; Hruban, R.H.; Zhou, J.; Winter, J.M.; Haddock, M.G.; et al. Evaluation of Adjuvant Chemoradiation Therapy for Ampullary Adenocarcinoma: The Johns Hopkins Hospital - Mayo Clinic Collaborative Study. Radiat. Oncol. 2011, 6, 126. [Google Scholar] [CrossRef]
- Nassour, I.; Hynan, L.S.; Christie, A.; Minter, R.M.; Yopp, A.C.; Choti, M.A.; Mansour, J.C.; Porembka, M.R.; Wang, S.C. Association of Adjuvant Therapy with Improved Survival in Ampullary Cancer: A National Cohort Study. J. Gastrointest. Surg. 2018, 22, 695–702. [Google Scholar] [CrossRef]
- Ha, H.R.; Oh, D.-Y.; Kim, T.-Y.; Lee, K.; Kim, K.; Lee, K.-H.; Han, S.-W.; Chie, E.K.; Jang, J.-Y.; Im, S.-A.; et al. Survival Outcomes According to Adjuvant Treatment and Prognostic Factors Including Host Immune Markers in Patients with Curatively Resected Ampulla of Vater Cancer. PLOS ONE 2016, 11, e0151406. [Google Scholar] [CrossRef]
- Jin, Z.; Hartgers, M.L.; Sanhueza, C.T.; Shubert, C.R.; Alberts, S.R.; Truty, M.J.; Muppa, P.; Nagorney, D.M.; Smyrk, T.C.; Hassan, M.; et al. Prognostic Factors and Benefits of Adjuvant Therapy after Pancreatoduodenectomy for Ampullary Adenocarcinoma: Mayo Clinic Experience. Eur. J. Surg. Oncol. 2018, 44, 677–683. [Google Scholar] [CrossRef]
- Showalter, T.N.; Zhan, T.; Anne, P.R.; Chervoneva, I.; Mitchell, E.P.; Yeo, C.J.; Rosato, E.L.; Kennedy, E.P.; Berger, A.C. The Influence of Prognostic Factors and Adjuvant Chemoradiation on Survival After Pancreaticoduodenectomy for Ampullary Carcinoma. J. Gastrointest. Surg. 2011, 15, 1411–1416. [Google Scholar] [CrossRef]
- Chavez, M.T.; Sharpe, J.P.; O’Brien, T.; Patton, K.T.; Portnoy, D.C.; VanderWalde, N.A.; Deneve, J.L.; Shibata, D.; Behrman, S.W.; Dickson, P.V. Management and Outcomes Following Pancreaticoduodenectomy for Ampullary Adenocarcinoma. Am. J. Surg. 2017, 214, 856–861. [Google Scholar] [CrossRef]
- Palta, M.; Patel, P.; Broadwater, G.; Willett, C.; Pepek, J.; Tyler, D.; Zafar, S.Y.; Uronis, H.; Hurwitz, H.; White, R.; et al. Carcinoma of the Ampulla of Vater: Patterns of Failure Following Resection and Benefit of Chemoradiotherapy. Ann. Surg. Oncol. 2012, 19, 1535–1540. [Google Scholar] [CrossRef]
- Kamarajah, S.K. Adjuvant Radiotherapy Following Pancreaticoduodenectomy for Ampullary Adenocarcinoma Improves Survival in Node-Positive Patients: A Propensity Score Analysis. Clin. Transl. Oncol. 2018, 20, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Stiles, Z.E.; Behrman, S.W.; Deneve, J.L.; Glazer, E.S.; Dong, L.; Wan, J.Y.; Martin, M.G.; Dickson, P.V. Ampullary Adenocarcinoma: Defining Predictors of Survival and the Impact of Adjuvant Therapy Following Surgical Resection for Stage I Disease. J. Surg. Oncol. 2018, 117, 1500–1508. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, B.H.; Kim, K.; Chie, E.K.; Ha, S.W. Survival Benefit of Adjuvant Chemoradiotherapy in Patients With Ampulla of Vater Cancer: A Systematic Review and Meta-Analysis. Ann. Surg. 2015, 262, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Bonet, M.; Rodrigo, A.; Vázquez, S.; Carrizo, V.; Vilardell, F.; Mira, M. Adjuvant Therapy for True Ampullary Cancer: A Systematic Review. Clin. Transl. Oncol. 2020, 22, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Bolm, L.; Ohrner, K.; Nappo, G.; Rückert, F.; Zimmermann, C.; Rau, B.M.; Petrova, E.; Honselmann, K.C.; Lapshyn, H.; Bausch, D.; et al. Adjuvant Therapy Is Associated with Improved Overall Survival in Patients with Pancreatobiliary or Mixed Subtype Ampullary Cancer after Pancreatoduodenectomy - A Multicenter Cohort Study. Pancreatology 2020, 20, 433–441. [Google Scholar] [CrossRef]
- Moekotte, A.L.; Malleo, G.; Van Roessel, S.; Bonds, M.; Halimi, A.; Zarantonello, L.; Napoli, N.; Dreyer, S.B.; Wellner, U.F.; Bolm, L.; et al. Gemcitabine-Based Adjuvant Chemotherapy in Subtypes of Ampullary Adenocarcinoma: International Propensity Score-Matched Cohort Study. Br. J. Surg. 2020, 107, 1171–1182. [Google Scholar] [CrossRef]
- Schiergens, T.S.; Reu, S.; Neumann, J.; Renz, B.W.; Niess, H.; Boeck, S.; Heinemann, V.; Bruns, C.J.; Jauch, K.-W.; Kleespies, A. Histomorphologic and Molecular Phenotypes Predict Gemcitabine Response and Overall Survival in Adenocarcinoma of the Ampulla of Vater. Surgery 2015, 158, 151–161. [Google Scholar] [CrossRef]
- Palmeri, M.; Funel, N.; Franco, G.D.; Furbetta, N.; Gianardi, D.; Guadagni, S.; Bianchini, M.; Pollina, L.E.; Ricci, C.; Chiaro, M.D.; et al. Tissue Microarray-Chip Featuring Computerized Immunophenotypical Characterization More Accurately Subtypes Ampullary Adenocarcinoma than Routine Histology. World J. Gastroenterol. 2020, 26, 6822–6836. [Google Scholar] [CrossRef]
- Luchini, C.; Scarpa, A. Microsatellite Instability in Pancreatic and Ampullary Carcinomas: Histology, Molecular Pathology, and Clinical Implications. Hum. Pathol. 2023, 132, 176–182. [Google Scholar] [CrossRef]
- Fernández Moro, C.; Fernandez-Woodbridge, A.; Alistair D’souza, M.; Zhang, Q.; Bozoky, B.; Kandaswamy, S.V.; Catalano, P.; Heuchel, R.; Shtembari, S.; Del Chiaro, M.; et al. Immunohistochemical Typing of Adenocarcinomas of the Pancreatobiliary System Improves Diagnosis and Prognostic Stratification. PLOS ONE 2016, 11, e0166067. [Google Scholar] [CrossRef]
- Washington, M.K.; Goldberg, R.M.; Chang, G.J.; Limburg, P.; Lam, A.K.; Salto-Tellez, M.; Arends, M.J.; Nagtegaal, I.D.; Klimstra, D.S.; Rugge, M.; et al. Diagnosis of Digestive System Tumours. Int. J. Cancer 2021, 148, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.; Yonezawa, S.; Tanaka, S.; Kim, Y.S.; Sato, E. Expression of Mucin Carbohydrates and Core Proteins in Carcinomas of the Ampulla of Vater: Their Relationship to Prognosis. Jpn. J. Cancer Res. 1996, 87, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Seno, H.; Oshima, M.; Taniguchi, M.-A.; Usami, K.; Ishikawa, T.-O.; Chiba, T.; Taketo, M. CDX2 Expression in the Stomach with Intestinal Metaplasia and Intestinal-Type Cancer: Prognostic Implications. Int. J. Oncol. 2002. [Google Scholar] [CrossRef]
- Hansel, D.E.; Maitra, A.; Lin, J.W.; Goggins, M.; Argani, P.; Yeo, C.J.; Piantadosi, S.; Leach, S.D.; Biankin, A.V. Expression of the Caudal-Type Homeodomain Transcription Factors CDX 1/2 and Outcome in Carcinomas of the Ampulla of Vater. J. Clin. Oncol. 2005, 23, 1811–1818. [Google Scholar] [CrossRef]
- Chu, P.G.; Schwarz, R.E.; Lau, S.K.; Yen, Y.; Weiss, L.M. Immunohistochemical Staining in the Diagnosis of Pancreatobiliary and Ampulla of Vater Adenocarcinoma: Application of CDX2, CK17, MUC1, and MUC2. Am. J. Surg. Pathol. 2005, 29, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.Y.; Kim, T.H.; Park, S.D.; Yun, K.J.; Choi, S.C.; Kim, H.C.; Nah, Y.H. Acral Metastasis in a Patient with Ampullary Carcinoma. Korean J. Intern. Med. 2007, 22, 55. [Google Scholar] [CrossRef]
- Lamarca, A.; Martinez-Marin, V.; Feliu, J. Cutaneous Relapse of an Ampullary Carcinoma: An Unusual Presentation. Case Rep. 2012, 2012, bcr0120125580. [Google Scholar] [CrossRef]
- Fernandez-Flores, A.; Cassarino, D.S. Cutaneous Metastasis of Adenocarcinoma of the Ampulla of Vater. Am. J. Dermatopathol. 2018, 40, 758–761. [Google Scholar] [CrossRef]
- Bansal, A.; Dalal, V.; Kaur, M.; Siraj, F. Periampullary Carcinoma: Unusual Sites of Metastasis. Ochsner J. 2017, 17, 426–429. [Google Scholar]
- Yun, S.P.; Seo, H.I. Prognostic Impact of Immunohistochemical Expression of CK7 and CK20 in Curatively Resected Ampulla of Vater Cancer. BMC Gastroenterol. 2015, 15, 165. [Google Scholar] [CrossRef]
- Cen, P.; Wray, C.J.; Zhang, S.; Thosani, N.C.; Dinh, B.C.; Gonzalez, A.; Mohlere, V.; Bynon, J.S. Durable Response for Ampullary and Duodenal Adenocarcinoma with a Nab-paclitaxel plus Gemcitabine ± Cisplatin Combination. Cancer Med. 2019, 8, 3464–3470. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; Varadhachary, G.R.; Kopetz, S.; Adinin, R.; Lin, E.; Morris, J.S.; Eng, C.; Abbruzzese, J.L.; Wolff, R.A. Phase II Study of Capecitabine and Oxaliplatin for Advanced Adenocarcinoma of the Small Bowel and Ampulla of Vater. J. Clin. Oncol. 2009, 27, 2598–2603. [Google Scholar] [CrossRef] [PubMed]
- Gulhati, P.; Raghav, K.; Shroff, R.T.; Varadhachary, G.R.; Kopetz, S.; Javle, M.; Qiao, W.; Wang, H.; Morris, J.; Wolff, R.A.; et al. Bevacizumab Combined with Capecitabine and Oxaliplatin in Patients with Advanced Adenocarcinoma of the Small Bowel or Ampulla of Vater: A Single-center, Open-label, Phase 2 Study. Cancer 2017, 123, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
| Author | Ref. | N. of Patients | Histological Subtypes | Median OS | Median DFS |
|---|---|---|---|---|---|
| Beghelli et al. | [22] | 89 | Pb-AC: 56 (62.9%) In-AC: 28 (31.5%) | Pb-AC: 19 months In-AC: 70 months | - |
| Carter et al. | [9] | 118 | Pb-AC: 53 (44.9%) In-AC: 54 (45.8%) | Pb-AC: 22 months In-AC: 60 months | - |
| Ruemmele et al. | [23] | 118 | Pb-AC: 27 (22.9%) In-AC: 56 (47.5%) | Pb-AC: 41 months In-AC: 97 months | - |
| Kim et al. | [24] | 104 | Pb-AC: 62 (59.6%) In-AC: 42 (40.4%) | - | Pb-AC: 5y 47.8% In-AC: 5y 73.1% |
| Chang et al. | [6] | 208 | Pb-AC: 89 (42.8%) In-AC: 119 (57.2%) | Pb-AC: 22.0 months In-AC: 115.0 months | Pb-AC: 23.9 months In-AC: 69.9 months |
| Robert et al. | [25] | 319 | Pb-AC: 105 (32.9%) In-AC: 106 (32.2%) | - | Pb-AC: 25.3 months In-AC: 58.9 months |
| Schueneman et al. | [26] | 163 | Pb-AC: 75 (46.0%) In-AC: 50 (30.7%) | Pb-AC: 21.2 months In-AC: 106.4 months | - |
| Zimmermann et al. | [27] | 170 | Pb-AC: 69 (58%) In-AC: 41 (34.5%) | Pb-AC: 52.5 months In-AC: 115 months | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nappo, G.; Funel, N.; Laurenti, V.; Stenner, E.; Carrara, S.; Bozzarelli, S.; Spaggiari, P.; Zerbi, A. Ampullary Cancer: Histological Subtypes, Markers, and Clinical Behaviour—State of the Art and Perspectives. Curr. Oncol. 2023, 30, 6996-7006. https://doi.org/10.3390/curroncol30070507
Nappo G, Funel N, Laurenti V, Stenner E, Carrara S, Bozzarelli S, Spaggiari P, Zerbi A. Ampullary Cancer: Histological Subtypes, Markers, and Clinical Behaviour—State of the Art and Perspectives. Current Oncology. 2023; 30(7):6996-7006. https://doi.org/10.3390/curroncol30070507
Chicago/Turabian StyleNappo, Gennaro, Niccola Funel, Virginia Laurenti, Elisabetta Stenner, Silvia Carrara, Silvia Bozzarelli, Paola Spaggiari, and Alessandro Zerbi. 2023. "Ampullary Cancer: Histological Subtypes, Markers, and Clinical Behaviour—State of the Art and Perspectives" Current Oncology 30, no. 7: 6996-7006. https://doi.org/10.3390/curroncol30070507
APA StyleNappo, G., Funel, N., Laurenti, V., Stenner, E., Carrara, S., Bozzarelli, S., Spaggiari, P., & Zerbi, A. (2023). Ampullary Cancer: Histological Subtypes, Markers, and Clinical Behaviour—State of the Art and Perspectives. Current Oncology, 30(7), 6996-7006. https://doi.org/10.3390/curroncol30070507






