Current Perspectives in Liver Transplantation for Perihilar Cholangiocarcinoma
Abstract
1. Introduction
2. Staging System
3. Results of Surgical Resection in pCCA
4. Adjuvant Therapy
5. Liver Transplantation in pCCA
6. Discussion and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef] [PubMed]
- Blechacz, B.; Komuta, M.; Roskams, T.; Gores, G.J. Clinical diagnosis and staging of cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Gores, G.J. Cholangiocarcinoma: Current concepts and insights. Hepatology 2003, 37, 961–969. [Google Scholar] [CrossRef]
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019, 39, 19–31. [Google Scholar] [CrossRef]
- DeOliveira, M.L.; Cunningham, S.C.; Cameron, J.L.; Kamangar, F.; Winter, J.M.; Lillemoe, K.D.; Choti, M.A.; Yeo, C.J.; Schulick, R.D. Cholangiocarcinoma: Thirty-one-year experience with 564 patients at a single institution. Ann. Surg. 2007, 245, 755–762. [Google Scholar] [CrossRef]
- Anderson, C.D.; Pinson, C.W.; Berlin, J.; Chari, R.S. Diagnosis and Treatment of Cholangiocarcinoma. Oncologist 2004, 9, 43–57. [Google Scholar] [CrossRef]
- Chapman, R.; Fevery, J.; Kalloo, A.; Nagorney, D.M.; Boberg, K.M.; Shneider, B.; Gores, G.J. American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010, 51, 660–678. [Google Scholar] [CrossRef] [PubMed]
- Tirotta, F.; Giovinazzo, F.; Hodson, J.; Yates, L.; Tan, D.; Salsano, M.; Baker, D.; Sundareyan, R.; Marudanayagam, R.; Dasari, B. Risk factors to differentiate between benign proximal biliary strictures and perihilar cholangiocarcinoma. HPB 2020, 22, 1753–1758. [Google Scholar] [CrossRef]
- Rizvi, S.; Gores, G.J. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 2013, 145, 1215–1229. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, M.H.; tong, T.Y.; Hwang, C.Y.; Kim, J.S.; Yun, S.C.; Lee, S.S.; Seo, D.W.; Lee, S.K. Clinical role of 18F-FDG PET-CT in suspected and potentially operable cholangiocarcinoma: A prospective study compared with conventional imaging. Am. J. Gastroenterol. 2008, 103, 1145–1151. [Google Scholar] [CrossRef]
- Vogel, A.; Bridgewater, J.; Edeline, J.; Kelley, R.; Klümpen, H.; Malka, D.; Primrose, J.; Rimassa, L.; Stenzinger, A.; Valle, J.; et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Gaspersz, M.P.; Buettner, S.; van Vugt, J.L.A.; de Jonge, J.; Polak, W.G.; Doukas, M.; Ijzermans, J.N.M.; Koerkamp, B.G.; Willemssen, F.E.J.A. Evaluation of the New American Joint Committee on Cancer Staging Manual 8th Edition for Perihilar Cholangiocarcinoma. J. Gastrointest Surg 2019, 24, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Rocha, F.G.; Ito, K.; D’Angelica, M.I.; Allen, P.J.; Fong, Y.; DeMatteo, R.P.; Gonen, M.; Endo, I.; Jarnagin, W.R. The Blumgart Preoperative Staging System for Hilar Cholangiocarcinoma: Analysis of Resectability and Outcomes in 380 Patients. J. Am. Coll. Surg. 2012, 215, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Launois, B.; Reding, R.; Lebeau, G.; Buard, J.L. Surgery for hilar cholangiocarcinoma: French experience in a collective survey of 552 extrahepatic bile duct cancers. J. Hepato-Biliary-Pancreat. Surg. 2000, 7, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Olthof, P.B.; Coelen, R.J.; Wiggers, J.K.; Koerkamp, B.G.; Malago, M.; Hernandez-Alejandro, R.; Topp, S.A.; Vivarelli, M.; Aldrighetti, L.A.; Campos, R.R. High mortality after ALPPS for perihilar cholangiocarcinoma: Case-control analysis including the first series from the international ALPPS registry. HPB 2017, 19, 381–387. [Google Scholar] [CrossRef]
- Jarnagin, W.R.; Fong, Y.; DeMatteo, R.P.; Gonen, M.; Burke, E.C.; Bodniewicz, B.J.; Youssef, B.M.; Klimstra, D.; Blumgart, L.H. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann. Surg. 2001, 234, 507–519. [Google Scholar] [CrossRef]
- Burke, E.C.; Jarnagin, W.R.; Hochwald, S.N.; Pisters, P.W.T.; Fong, Y.; Blumgart, L.H. Hilar cholangiocarcinoma: Patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann. Surg. 1998, 228, 385–394. [Google Scholar] [CrossRef]
- van Keulen, A.-M.; Olthof, P.B.; Cescon, M.; Guglielmi, A.; Jarnagin, W.R.; Nadalin, S.; Pratschke, J.; Ratti, F.; Troisi, R.I.; Koerkamp, B.G. Actual 10-Year Survival after Resection of Perihilar Cholangiocarcinoma: What Factors Preclude a Chance for Cure? Cancers 2021, 13, 6260. [Google Scholar] [CrossRef]
- Cillo, U.; Fondevila, C.; Donadon, M.; Gringeri, E.; Mocchegiani, F.; Schlitt, H.J.; Ijzermans, J.N.M.; Vivarelli, M.; Zieniewicz, K.; Olde Damink, S.W.M. Surgery for cholangiocarcinoma. Liver Int. 2019, 39, 143–155. [Google Scholar] [CrossRef]
- Koerkamp, B.G.; Wiggers, J.K.; Allen, P.J.; Besselink, M.G.; Blumgart, L.H.; Busch, O.R.; Coelen, R.J.; D’Angelica, M.I.; DeMatteo, R.P.; Gouma, D.J. Recurrence Rate and Pattern of Perihilar Cholangiocarcinoma after Curative Intent Resection. J. Am. Coll. Surg. 2015, 221, 1041–1049. [Google Scholar] [CrossRef]
- Matsuyama, R.; Mori, R.; Ota, Y.; Homma, Y.; Yabusita, Y.; Hiratani, S.; Murakami, T.; Sawada, Y.; Miyake, K.; Shimizu, Y.; et al. Impact of Gemcitabine Plus S1 Neoadjuvant Chemotherapy on Borderline Resectable Perihilar Cholangiocarcinoma. Ann. Surg. Oncol. 2022, 29, 2393–2405. [Google Scholar] [CrossRef] [PubMed]
- Halder, R.; Amaraneni, A.; Shroff, R.T. Cholangiocarcinoma: A review of the literature and future directions in therapy. HepatoBiliary Surg. Nutr. 2022, 11, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Nagino, M.; Ebata, T.; Yokoyama, Y.; Igami, T.; Sugawara, G.; Takahashi, Y.; Nimura, Y. Evolution of Surgical Treatment for Perihilar Cholangiocarcinoma: A Single-Center 34-Year Review of 574 Consecutive Resections. Ann. Surg. 2013, 258, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Shimada, K.; Sakamoto, Y.; Yamamoto, J.; Yamasaki, S.; Kosuge, T. One Hundred Two Consecutive Hepatobiliary Resections for Perihilar Cholangiocarcinoma With Zero Mortality. Ann. Surg. 2006, 244, 240–247. [Google Scholar] [CrossRef]
- Unno, M.; Katayose, Y.; Rikiyama, T.; Yoshida, H.; Yamamoto, K.; Morikawa, T.; Hayashi, H.; Motoi, F.; Egawa, S. Major hepatectomy for perihilar cholangiocarcinoma. J. Hepato-Biliary-Pancreat. Sci. 2009, 17, 463–469. [Google Scholar] [CrossRef]
- De Bellis, M.; Mastrosimini, M.G.; Conci, S.; Pecori, S.; Campagnaro, T.; Castelli, C.; Capelli, P.; Scarpa, A.; Guglielmi, A.; Ruzzenente, A. The Prognostic Role of True Radical Resection in Perihilar Cholangiocarcinoma after Improved Evaluation of Radial Margin Status. Cancers 2022, 14, 6126. [Google Scholar] [CrossRef]
- Yohanathan, L.; Yamashita, T.; Croome, K.; Cleary, S.; Grotz, T.; Kendrick, M.; Truty, M.; Smoot, R.; Nagorney, D. Is mandatory routine caudate lobe resection indicated in hilar cholangiocarcinoma? HPB 2020, 22, S23–S24. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Sahara, K.; Wu, L.; Moris, D.; Bagante, F.; Guglielmi, A.; Aldrighetti, L.; Weiss, M.; Bauer, T.W.; Alexandrescu, S. Very Early Recurrence After Liver Resection for Intrahepatic Cholangiocarcinoma: Considering Alternative Treatment Approaches. JAMA Surg. 2020, 155, 823–831. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Beal, E.W.; Chakedis, J.; Chen, Q.; Lv, Y.; Ethun, C.G.; Salem, A.; Weber, S.M.; Tran, T.; Poultsides, G.; et al. Defining Early Recurrence of Hilar Cholangiocarcinoma After Curative-intent Surgery: A Multi-institutional Study from the US Extrahepatic Biliary Malignancy Consortium. World J. Surg. 2018, 42, 2919–2929. [Google Scholar] [CrossRef]
- Komaya, K.; Ebata, T.; Shirai, K.; Ohira, S.; Morofuji, N.; Akutagawa, A.; Yamaguchi, R.; Nagino, M.; Aoba, T.; Kaneoka, Y. Recurrence after resection with curative intent for distal cholangiocarcinoma. Br. J. Surg. 2017, 104, 426–433. [Google Scholar] [CrossRef]
- Benson, A.B.; D’Angelica, M.I.; Abbott, D.E.; Anaya, D.A.; Anders, R.; Are, C.; Bachini, M.; Borad, M.; Brown, D.; Burgoyne, A.; et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2021, 19, 541–565. [Google Scholar] [CrossRef] [PubMed]
- Luvira, V.; Satitkarnmanee, E.; Pugkhem, A.; Kietpeerakool, C.; Lumbiganon, P.; Pattanittum, P. Postoperative adjuvant chemotherapy for resectable cholangiocarcinoma. Cochrane Database Syst. Rev. 2021, 2021, CD012814. [Google Scholar] [CrossRef]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Edeline, J.; Benabdelghani, M.; Bertaut, A.; Watelet, J.; Hammel, P.; Joly, J.-P.; Boudjema, K.; Fartoux, L.; Bouhier-Leporrier, K.; Jouve, J.-L. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomised Phase III Study. J. Clin. Oncol. 2019, 37, 658–667. [Google Scholar] [CrossRef]
- Munir, M.M.; Ruff, S.M.; Endo, Y.; Lima, H.A.; Alaimo, L.; Moazzam, Z.; Shaikh, C.; Pawlik, T.M. Does Adjuvant Therapy Benefit Low-Risk Resectable Cholangiocarcinoma? An Evaluation of the NCCN Guidelines. J. Gastrointest. Surg. 2022. [Google Scholar] [CrossRef]
- Li, J.; Rocha, F.G.; Mayo, S.C. Past, Present, and Future Management of Localized Biliary Tract Malignancies. Surg. Oncol. Clin. N. Am. 2023, 32, 83–99. [Google Scholar] [CrossRef]
- Vugts, J.J.A.; Gaspersz, M.P.; Roos, E.; Franken, L.C.; Olthof, P.B.; Coelen, R.J.S.; van Vugt, J.L.A.; Labeur, T.A.; Brouwer, L.; Besselink, M.G.H. Eligibility for Liver Transplantation in Patients with Perihilar Cholangiocarcinoma. Ann. Surg. Oncol. 2020, 28, 1483–1492. [Google Scholar] [CrossRef]
- Meyer, C.G.; Penn, I.; James, L. Liver transplantation for cholangiocarcinoma: Results in 207 patients. Transplantation 2000, 69, 1633–1637. [Google Scholar] [CrossRef]
- Kubal, C.; Mihaylov, P.; Holden, J. Oncologic indications of liver transplantation and deceased donor liver allocation in the United States. Curr. Opin. Organ Transplant. 2021, 26, 168–175. [Google Scholar] [CrossRef]
- Heimbach, J.; Gores, G.; Haddock, M.; Alberts, S.; Nyberg, S.; Ishitani, M.; Rosen, C. Liver transplantation for unresectable perihilar cholangiocarcinoma. Semin. Liver Dis. 2004, 24, 201–207. [Google Scholar] [CrossRef]
- Sudan, D.; DeRoover, A.; Chinnakotla, S.; Fox, I.; Shaw, B.J.r.; MCCAhland, T.; Sorrell, M.; Tempero, M.; Langnas, A. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am. J. Transplant. 2002, 2, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Associazione Italiana di Oncologia Medica (AIOM). Linee Guida “Tumori delle Vie Biliari”. 2019. Available online: https://www.aiom.it/wp-content/uploads/2019/10/2019_LG_AIOM_Vie_biliari.pdf (accessed on 2 November 2022).
- Rosen, C.B.; Heimbach, J.K.; Gores, G.J. Liver transplantation for cholangiocarcinoma. Transpl. Int. 2010, 23, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Murad, S.D.; Kim, W.R.; Harnois, D.M.; Douglas, D.D.; Burton, J.; Kulik, L.M.; Botha, J.F.; Mezrich, J.D.; Chapman, W.C.; Schwartz, J.J.; et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012, 143, 88–98.e3. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Haddock, M.G.; Alberts, S.R.; Nyberg, S.L.; Ishitani, M.B.; Rosen, C.B.; Gores, G.J. Transplantation for hilar cholangiocarcinoma. Liver Transplant. 2004, 10, S65–S68. [Google Scholar] [CrossRef] [PubMed]
- Cambridge, W.A.; Fairfield, C.; Powell, J.J.; Harrison, E.M.; Søreide, K.; Wigmore, S.J.; Guest, R.V. Meta-analysis and Meta-regression of Survival After Liver Transplantation for Unresectable Perihilar Cholangiocarcinoma. Ann. Surg. 2021, 273, 240–250. [Google Scholar] [CrossRef]
- Loveday, B.P.T.; Knox, J.J.; Dawson, L.A.; Metser, U.; Brade, A.; Horgan, A.M.; Gallinger, S.; Greig, P.D.; Moulton, C.A. Neoadjuvant hyperfractionated chemoradiation and liver transplantation for unresectable perihilar cholangiocarcinoma in Canada. J. Surg. Oncol. 2018, 117, 213–219. [Google Scholar] [CrossRef]
- Welling, T.H.; Feng, M.; Wan, S.; Hwang, S.Y.; Volk, M.L.; Lawrence, T.S.; Zalupski, M.M.; Sonnenday, C.J. Neoadjuvant stereotactic body radiation therapy, capecitabine, and liver transplantation for unresectable hilar cholangiocarcinoma. Liver Transplant. 2014, 20, 81–88. [Google Scholar] [CrossRef]
- Duignan, S.; Maguire, D.; Ravichand, C.S.; Geoghegan, J.; Hoti, E.; Fennelly, D.; Armstrong, J.; Rock, K.; Mohan, H.; Traynor, O. Neoadjuvant chemoradiotherapy followed by liver transplantation for unresectable cholangiocarcinoma: A single-centre national experience. HPB 2014, 16, 91–98. [Google Scholar] [CrossRef]
- Marchan, E.M.; Landry, J.C. Neoadjuvant chemoradiation followed by orthotopic liver transplantation in cholangiocarcinomas: The emory experience. J. Gastrointest. Oncol. 2016, 7, 248–254. [Google Scholar] [CrossRef]
- Koedijk, M.S.; Heijmen, B.J.M.; Groot Koerkamp, B.; Eskens, F.A.; Sprengers, D.; Poley, J.W.; van Gent, D.; van der Laan, L.; Holt, B.; Willemssen, F.; et al. Protocol for the STRONG trial: Stereotactic body radiation therapy following chemotherapy for unresectable perihilar cholangiocarcinoma, a phase I feasibility study. BMJ Open 2018, 8, e020731. [Google Scholar] [CrossRef]
- Schüle, S.; Altendorf-Hofmann, A.; Uteß, F.; Rauchfuß, F.; Freesmeyer, M.; Knösel, T.; Dittmar, Y.; Settmacher, U. Liver transplantation for hilar cholangiocarcinoma—A single-centre experience. Langenbeck’s Arch. Surg. 2012, 398, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Giovinazzo, F.; Schlegel, A.; Dasary, B.; Chatzizacharias, N.; Roberts, K.; Marudanayagam, R.; Mirza, D.; Isaac, J.; Muiesan, P. Outcomes in Hilar Cholangiocarcinoma Management through a Clinical Pathway Implementation: A 30-year Single Centre Experience. HPB 2021, 23, S84. [Google Scholar] [CrossRef]
- Ethun, C.G.; Lopez-Aguiar, A.G.; Anderson, D.J.; Adams, A.B.; Fields, R.C.; Doyle, M.B.; Chapman, W.C.; Krasnick, B.A.; Weber, S.M.; Mezrich, J.D. Transplantation Versus Resection for Hilar Cholangiocarcinoma: An Argument for Shifting Treatment Paradigms for Resectable Disease. Ann. Surg. 2018, 267, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, N.; Usui, M.; Gyoten, K.; Hayasaki, A.; Fujii, T.; Iizawa, Y.; Kato, H.; Murata, Y.; Tanemura, A.; Kishiwada, M. Neoadjuvant chemotherapy followed by curative-intent surgery for perihilar cholangiocarcinoma based on its anatomical resectability classification and lymph node status. BMC Cancer 2020, 20, 405. [Google Scholar] [CrossRef] [PubMed]
- Panayotova, G.; Lunsford, K.E.; Latt, N.L.; Paterno, F.; Guarrera, J.V.; Pyrsopoulos, N. Expanding indications for liver transplantation in the era of liver transplant oncology. World J. Gastrointest. Surg. 2021, 13, 392–405. [Google Scholar] [CrossRef]
- Bzeizi, K.I.; Abdullah, M.; Vidyasagar, K.; Alqahthani, S.A.; Broering, D. Hepatocellular Carcinoma Recurrence and Mortality Rate Post Liver Transplantation: Meta-Analysis and Systematic Review of Real-World Evidence. Cancers 2022, 14, 5114. [Google Scholar] [CrossRef]
- Lehrke, H.D.; Heimbach, J.K.; Wu, T.-T.; Jenkins, S.M.; Gores, G.J.; Rosen, C.B.; Mounajjed, T. Prognostic Significance of the Histologic Response of Perihilar Cholangiocarcinoma to Preoperative Neoadjuvant Chemoradiation in Liver Explants. Am. J. Surg. Pathol. 2016, 40, 510–518. [Google Scholar] [CrossRef]
- Baltatzis, M.; Jegatheeswaran, S.; Siriwardena, A.K. Neoadjuvant chemoradiotherapy before resection of perihilar cholangiocarcinoma: A systematic review. Hepatobiliary Pancreat. Dis. Int. 2020, 19, 103–108. [Google Scholar] [CrossRef]
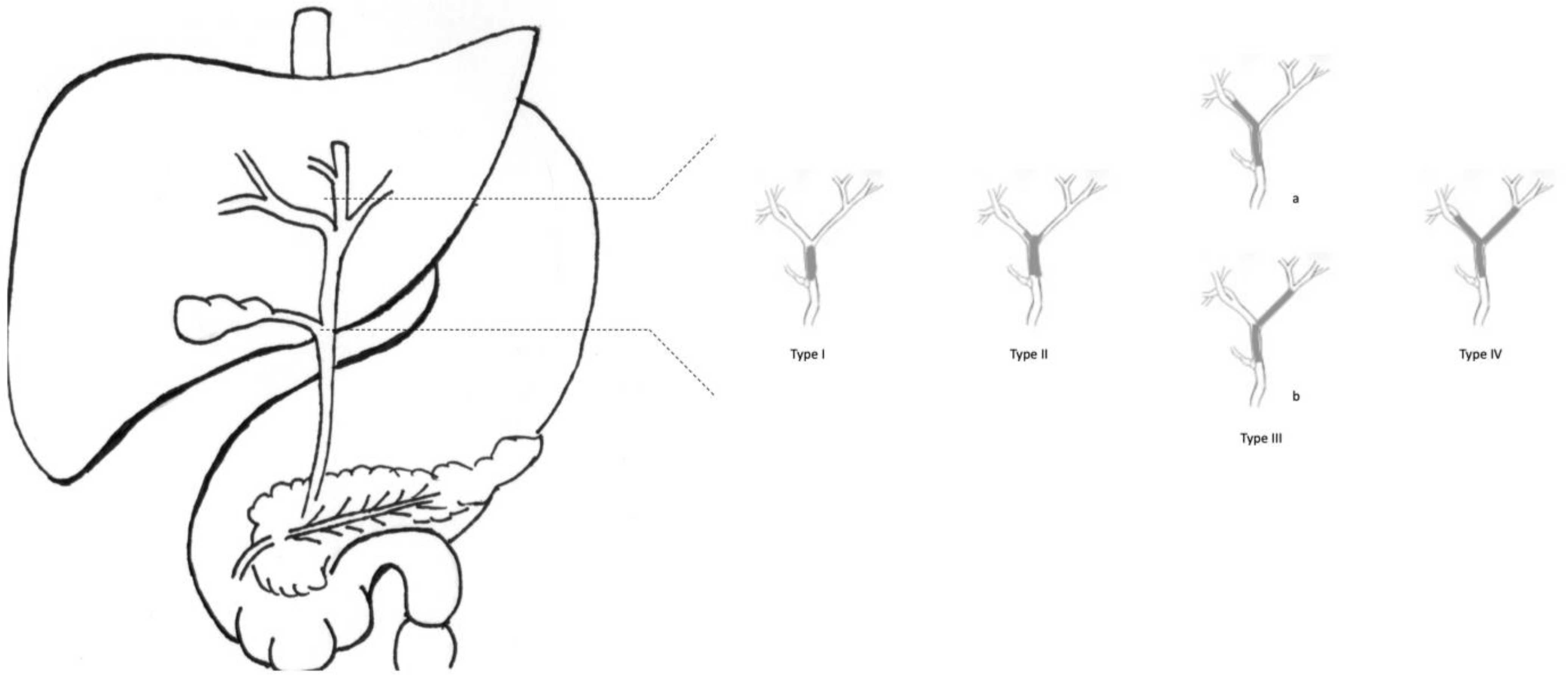
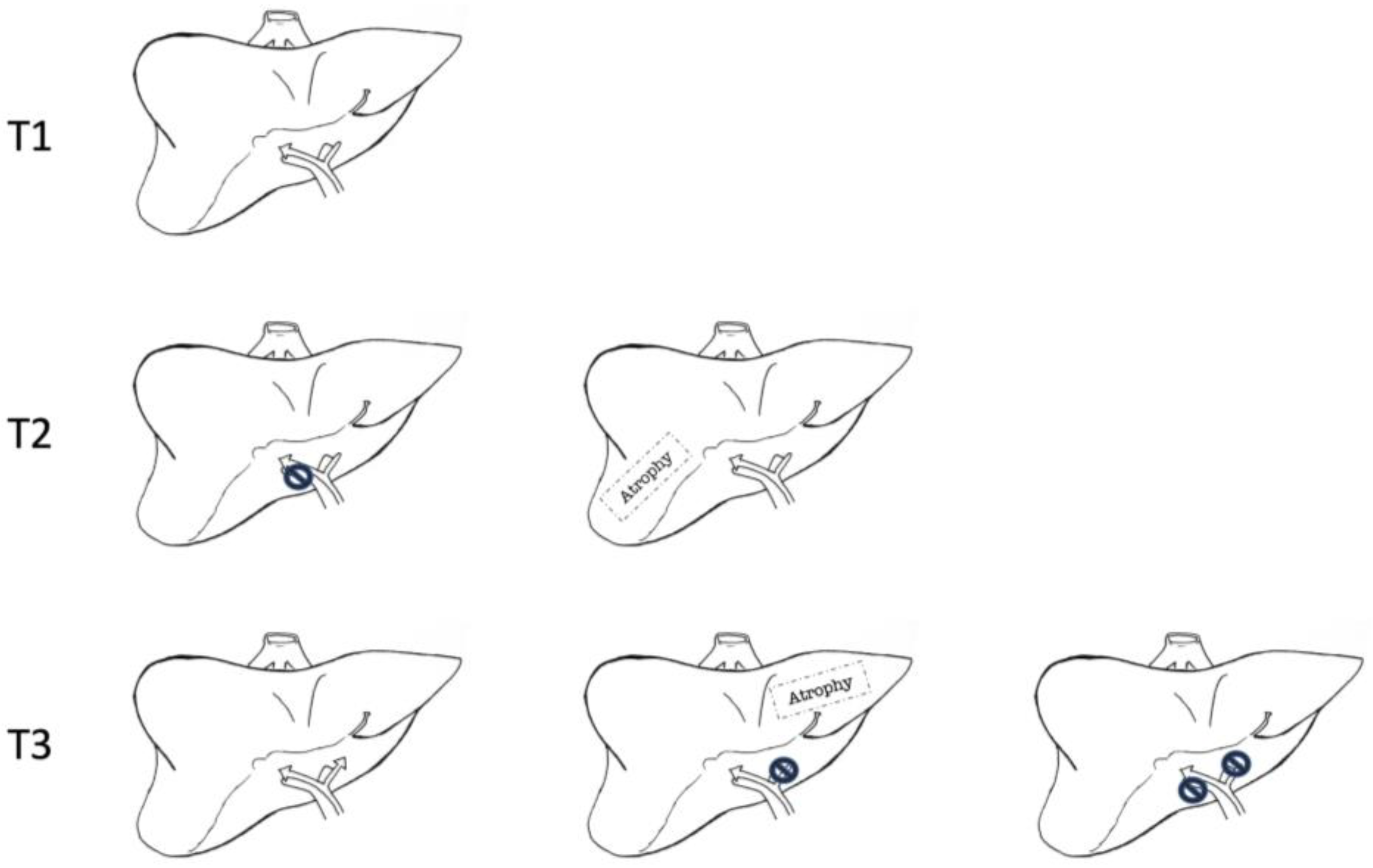



| Stage | T | N | M |
|---|---|---|---|
| 0 | is | 0 | 0 |
| I | 1 | 0 | 0 |
| II | 2a-b | 0 | 0 |
| IIIa | 3 | 0 | 0 |
| IIIb | 4 | 0 | 0 |
| IIIc | Any | 1 | 0 |
| IVa | Any | 2 | 0 |
| IVb | Any | Any | 1 |
| Neoadjuvant | LR (%) | LT n(%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Year | Number of Patients | Procedure | Protocol | Cholangitis n (%) | Drop-Out n (%) | Follow up Years, (Range) | Median Survival, Months (range) | OS n (%) | Disease-Free Survival n (%) | OS n (%) | Disease-Free Survival n (%) |
| Sudan et al. [41] | 2002 | 17 | LT | Nebraska | 5 (29) | 6 (35) | 7.5 (2.8–14.5) | 25 (4–174) | - | - | 5 (45) | 5 (45) |
| Heimbach et al. [45] | 2004 | 56 | LT | Mayo protocol | - | 14 (39) | 3.5 (0.5–10.9) | - | - | - | 11 (50) | 30 (90) |
| Sano et al. [24] | 2006 | 102 | LR | no | - | - | 2.8 (0.4–5.2) | 19 (4–62) | 62 (61) | 60 (50) | - | - |
| Michiaki et al. [25] | 2010 | 125 | LR | no | - | - | 1.5 (0.02–8.25) | 26.8 (nr) | 43 (35) | - | - | - |
| Murad et al. [44] | 2012 | 287 | LT | Mayo protocol | - | 71 (25) | 2.5 (0.1–17.8) | 14.4 (1–205.2) | - | - | 165 (57) | 244 (80) |
| Nagino et al. [23] | 2013 | 574 | LR | no | - | - | 5.8 (nr) | - | 83 (21.4) | - | - | - |
| Duignan et al. [49] | 2014 | 27 | LT | Mayo protocol | - | 7(26) | 3.1 (1.6–6.3) | - | - | - | 9 (45) | 9 (56) |
| Welling et al. [48] | 2014 | 17 | LT | Mayo protocol | 6 (50) | 10 (59) | 1.2 (nr) | - | - | - | * | * |
| Koerkamp et al. [32] | 2015 | 306 | LR | no | - | - | - | 40 (nr) | 145 (30) | 111 (31) | - | - |
| Marchan et al. [50] | 2016 | 10 | LT | Mayo protocol | - | 2 (20) | 2.5 (nr-3.1) | - | - | - | ° | - |
| Ethun et al. [54] | 2018 | 304 | LT/LR | Mayo protocol | - | 29 (41) | - | § | 35 (18) | - | 27 (65%) | - |
| Loveday et al. [47] | 2018 | 43 | LT | Toronto | 12 (67) | 11 (62) | 1.5 (0.5–1.8) | 17.7 (4.9–29.6) | - | - | & | & |
| Vugts et al. [37] | 2021 | 34 | LT | no | - | 32 (94) | - | - | 55 (36) | - | 1 (50) | 1 (50) |
| De Bellis et al. [26] | 2022 | 100 | LR | no | - | - | 3.4 | 40.5 | 36 (36) | 16 (16) | - | - |
| Matsuyama et al. [21] | 2022 | 60 | LR | Gemcitabine S1 | 27 (45) | 17 (28) | 2.5 (0.4–8.9) | 50.1 (nr) | 22 (36) | 33 (55) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovinazzo, F.; Pascale, M.M.; Cardella, F.; Picarelli, M.; Molica, S.; Zotta, F.; Martullo, A.; Clarke, G.; Frongillo, F.; Grieco, A.; et al. Current Perspectives in Liver Transplantation for Perihilar Cholangiocarcinoma. Curr. Oncol. 2023, 30, 2942-2953. https://doi.org/10.3390/curroncol30030225
Giovinazzo F, Pascale MM, Cardella F, Picarelli M, Molica S, Zotta F, Martullo A, Clarke G, Frongillo F, Grieco A, et al. Current Perspectives in Liver Transplantation for Perihilar Cholangiocarcinoma. Current Oncology. 2023; 30(3):2942-2953. https://doi.org/10.3390/curroncol30030225
Chicago/Turabian StyleGiovinazzo, Francesco, Marco Maria Pascale, Francesca Cardella, Matteo Picarelli, Serena Molica, Francesca Zotta, Annamaria Martullo, George Clarke, Francesco Frongillo, Antonio Grieco, and et al. 2023. "Current Perspectives in Liver Transplantation for Perihilar Cholangiocarcinoma" Current Oncology 30, no. 3: 2942-2953. https://doi.org/10.3390/curroncol30030225
APA StyleGiovinazzo, F., Pascale, M. M., Cardella, F., Picarelli, M., Molica, S., Zotta, F., Martullo, A., Clarke, G., Frongillo, F., Grieco, A., & Agnes, S. (2023). Current Perspectives in Liver Transplantation for Perihilar Cholangiocarcinoma. Current Oncology, 30(3), 2942-2953. https://doi.org/10.3390/curroncol30030225






