Pathology of Cholangiocarcinomas
Abstract
:1. Introduction
2. Epidemiology and Risk Factors of Cholangiocarcinoma
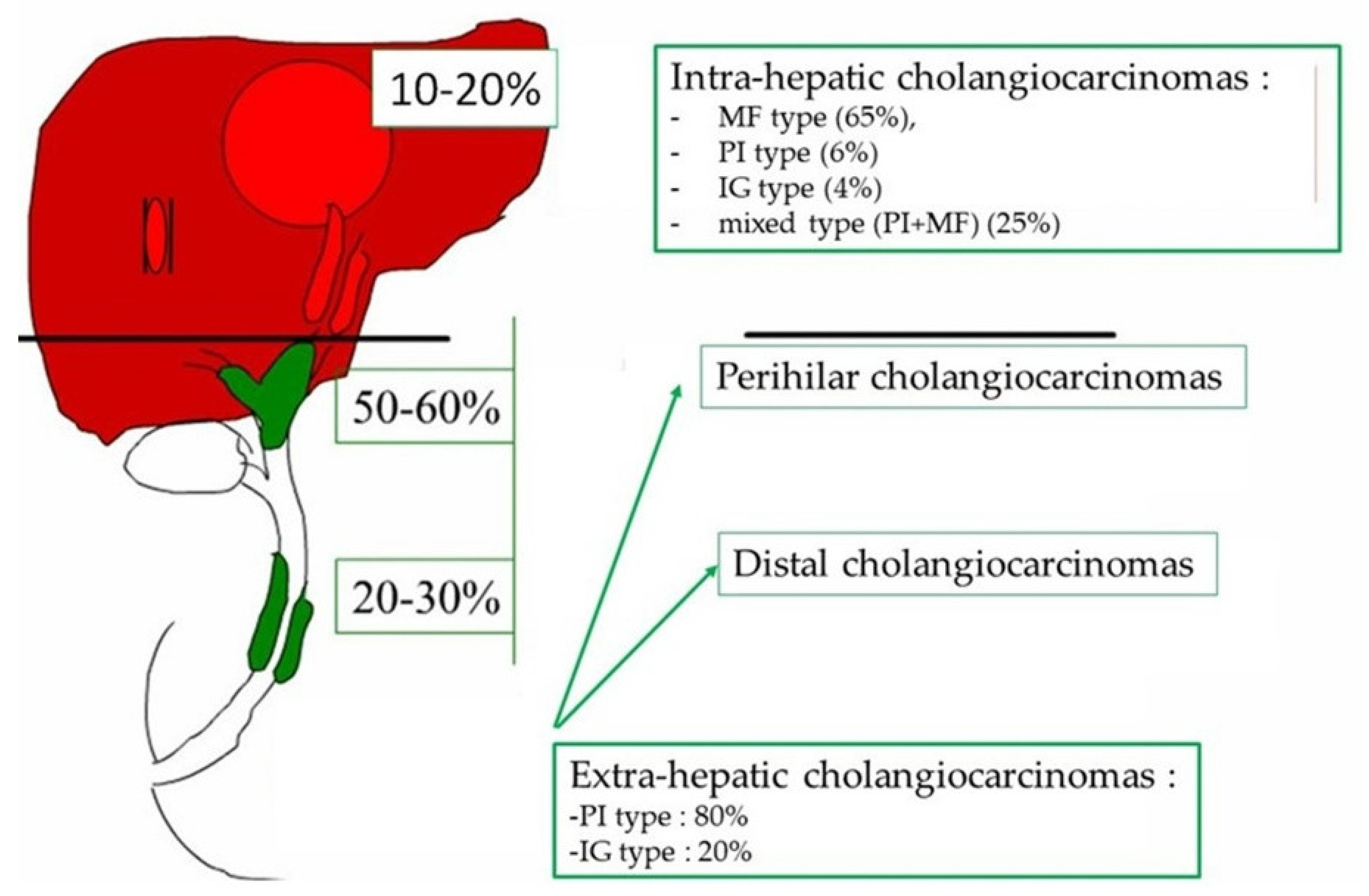
3. Macroscopic Aspects of Cholangiocarcinoma
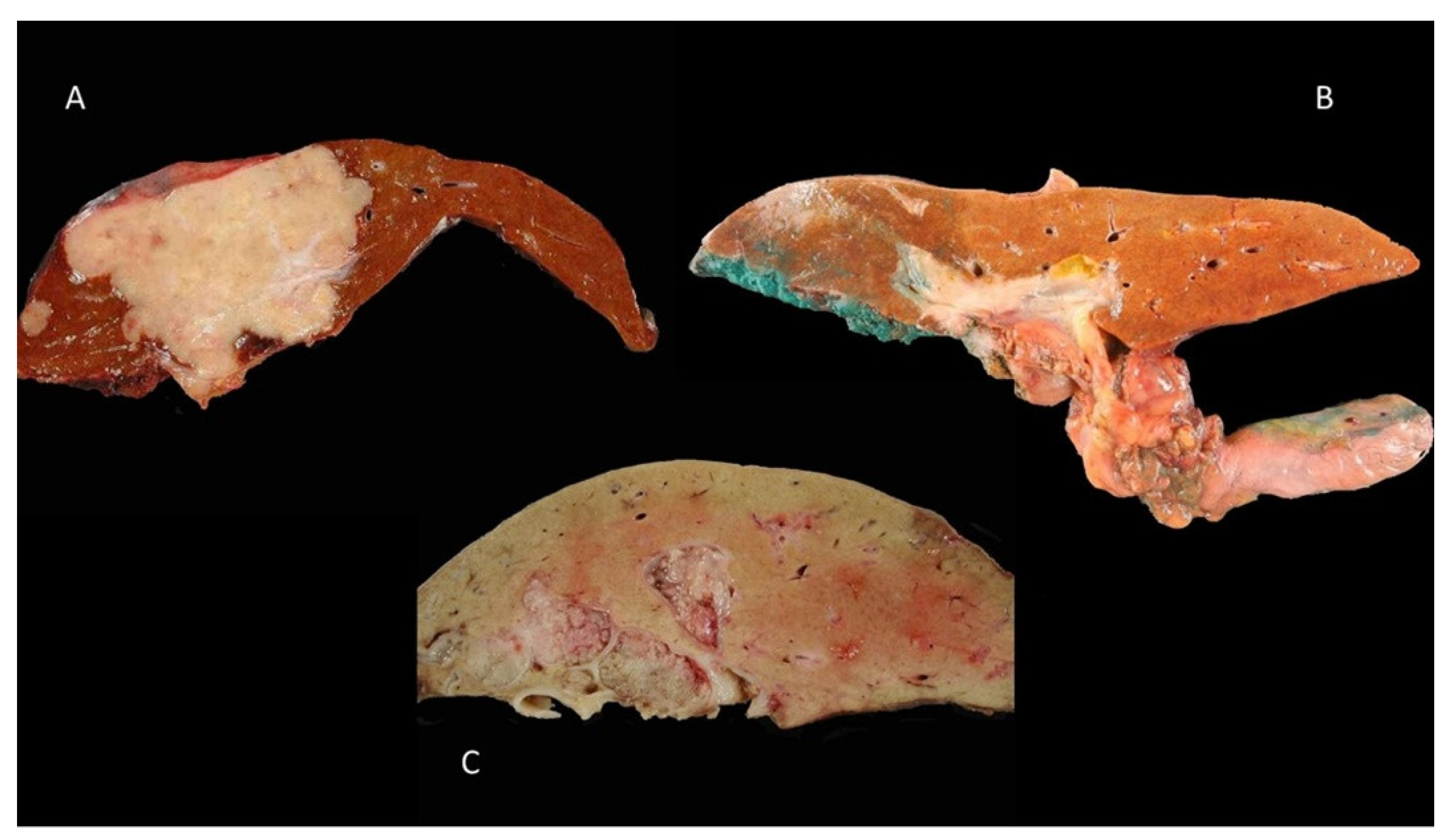
3.1. Mass-Forming Type
3.2. Periductal-Infiltrating Type
3.3. Intraductal-Growth Type
4. Microscopic Aspects of Cholangiocarcinomas
4.1. Intra-Hepatic Cholangiocarcinoma
4.2. Perihilar and Distal Cholangiocarcinomas
4.3. Targeted Therapy
5. Immunophenotype of Cholangiocarcinomas
6. Mode of Tumor Extension and Histoprognostic Factors of Cholangiocarcinomas
6.1. Intra-Hepatic Cholangiocarcinoma
6.2. Extra-Hepatic Cholangiocarcinoma
7. Role of Biopsy in the Diagnosis of Cholangiocarcinoma
7.1. Intra-Hepatic Cholangiocarcinoma
7.2. Extra-Hepatic Cholangiocarcinoma
8. Preneoplastic Lesions
9. Limitations and Future Direction
Funding
Acknowledgments
Conflicts of Interest
References
- Rodrigues, P.M.; Olaizola, P.; Paiva, N.A.; Olaizola, I.; Agirre-Lizaso, A.; Landa, A.; Bujanda, L.; Perugorria, M.J.; Banales, J.M. Pathogenesis of Cholangiocarcinoma. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 433–463. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blechacz, B. Cholangiocarcinoma: Current Knowledge and New Developments. Gut Liver 2017, 11, 13–26. [Google Scholar] [CrossRef]
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019, 39 (Suppl. 1), 19–31. [Google Scholar] [CrossRef] [Green Version]
- Casadio, M.; Biancaniello, F.; Overi, D.; Venere, R.; Carpino, G.; Gaudio, E.; Alvaro, D.; Cardinale, V. Molecular Landscape and Therapeutic Strategies in Cholangiocarcinoma: An Integrated Translational Approach towards Precision Medicine. Int. J. Mol. Sci. 2021, 22, 5613. [Google Scholar] [CrossRef]
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Khan, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.T.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Primers 2021, 7, 65. [Google Scholar] [CrossRef]
- Liver Cancer Study Group of Japan. The General Rules for the Clinical and Pathology Study of Primary Liver Cancer, 4th ed.; Kanehara: Tokyo, Japan, 2000.
- Guedj, N.; Blaise, L.; Cauchy, F.; Albuquerque, M.; Soubrane, O.; Paradis, V. Prognostic value of desmoplastic stroma in intrahepatic cholangiocarcinoma. Mod. Pathol. 2021, 34, 408–416. [Google Scholar] [CrossRef]
- Vijgen, S.; Terris, B.; Rubbia-Brandt, L. Pathology of intrahepatic cholangiocarcinoma. Hepatobiliary Surg. Nutr. 2017, 6, 22–34. [Google Scholar] [CrossRef]
- Fabris, L.; Perugorria, M.J.; Mertens, J.; Björkström, N.K.; Cramer, T.; Lleo, A.; Solinas, A.; Sänger, H.; Lukacs-Kornek, V.; Moncsek, A.; et al. The tumour microenvironment and immune milieu of cholangiocarcinoma. Liver Int. 2019, 39 (Suppl. 1), 63–78. [Google Scholar] [CrossRef] [Green Version]
- Mar, W.A.; Chan, H.K.; Trivedi, S.B.; Berggruen, S.M. Imaging of Intrahepatic Cholangiocarcinoma. Semin. Ultrasound. CT MRI 2021, 42, 366–380. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Sato, Y.; Harada, K.; Sasaki, M.; Xu, J.; Ikeda, H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J. Hepatol. 2010, 2, 419–427. [Google Scholar] [CrossRef]
- Chung, T.; Park, Y.N. Up-to-Date Pathologic Classification and Molecular Characteristics of Intrahepatic Cholangiocarcinoma. Front. Med. 2022, 9, 857140. [Google Scholar] [CrossRef]
- Shiota, K.; Taguchi, J.; Nakashima, O.; Nakashima, M.; Kojiro, M. Clinicopathologic study on cholangiolocellular carcinoma. Oncol. Rep. 2001, 8, 263–268. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, Y.; Wang, S.-M.; Hu, A.-Y.; Pan, Y.-C.; Zhang, S.-H. Histopathological evidence of intrahepatic cholangiocarcinoma occurring in ductal plate malformation: A clinicopathologic study of 5 cases. Ann. Diagn. Pathol. 2021, 55, 151828. [Google Scholar] [CrossRef]
- Anatomical, Histomorphological and Molecular Classification of Cholangiocarcinoma—PubMed. Available online: https://pubmed-ncbi-nlm-nih-gov.proxy.insermbiblio.inist.fr/30882996/ (accessed on 20 October 2022).
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Lendvai, G.; Szekerczés, T.; Illyés, I.; Dóra, R.; Kontsek, E.; Gógl, A.; Kiss, A.; Werling, K.; Kovalszky, I.; Schaff, Z.; et al. Cholangiocarcinoma: Classification, Histopathology and Molecular Carcinogenesis. Pathol. Oncol. Res. 2020, 26, 3–15. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- De Luca, A.; Esposito Abate, R.; Rachiglio, A.M.; Maiello, M.R.; Esposito, C.; Schettino, C.; Izzo, F.; Nasti, G.; Normanno, N. FGFR Fusions in Cancer: From Diagnostic Approaches to Therapeutic Intervention. Int. J. Mol. Sci. 2020, 21, 6856. [Google Scholar] [CrossRef]
- Guedj, N.; Bedossa, P.; Paradis, V. Pathology of cholangiocarcinoma. Ann. Pathol. 2010, 30, 455–463. [Google Scholar] [CrossRef]
- Sigel, C.S.; Drill, E.; Zhou, Y.; Basturk, O.; Askan, G.; Pak, L.M.; Vakiani, E.; Wang, T.; Boerner, T.; Do, R.K.G.; et al. Intrahepatic Cholangiocarcinomas Have Histologically and Immunophenotypically Distinct Small and Large Duct Patterns. Am. J. Surg. Pathol. 2018, 42, 1334–1345. [Google Scholar] [CrossRef]
- Hammill, C.W.; Wong, L.L. Intrahepatic cholangiocarcinoma: A malignancy of increasing importance. J. Am. Coll. Surg. 2008, 207, 594–603. [Google Scholar] [CrossRef]
- Silva, M.A.; Tekin, K.; Aytekin, F.; Bramhall, S.R.; Buckels, J.A.C.; Mirza, D.F. Surgery for hilar cholangiocarcinoma; a 10 year experience of a tertiary referral centre in the UK. Eur. J. Surg. Oncol. EJSO 2005, 31, 533–539. [Google Scholar] [CrossRef]
- Sakamoto, E.; Nimura, Y.; Hayakawa, N.; Kamiya, J.; Kondo, S.; Nagino, M.; Kanai, M.; Miyachi, M.; Uesaka, K. The pattern of infiltration at the proximal border of hilar bile duct carcinoma: A histologic analysis of 62 resected cases. Ann. Surg. 1998, 227, 405–411. [Google Scholar] [CrossRef]
- Takahashi, Y.; Dungubat, E.; Kusano, H.; Ganbat, D.; Tomita, Y.; Odgerel, S.; Fukusato, T. Application of Immunohistochemistry in the Pathological Diagnosis of Liver Tumors. Int. J. Mol. Sci. 2021, 22, 5780. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Lei, H.-J.; Chen, M.-H.; Ho, H.-L.; Chiu, L.-Y.; Li, C.-P.; Wang, Y.-C. C-Reactive Protein (CRP) is a Promising Diagnostic Immunohistochemical Marker for Intrahepatic Cholangiocarcinoma and is Associated With Better Prognosis. Am. J. Surg. Pathol. 2017, 41, 1630–1641. [Google Scholar] [CrossRef]
- Vasilieva, L.E.; Papadhimitriou, S.I.; Dourakis, S.P. Modern diagnostic approaches to cholangiocarcinoma. Hepatobiliary Pancreat. Dis. Int. 2012, 11, 349–359. [Google Scholar] [CrossRef]
- Zen, Y.; Fujii, T.; Itatsu, K.; Nakamura, K.; Minato, H.; Kasashima, S.; Kurumaya, H.; Katayanagi, K.; Kawashima, A.; Masuda, S.; et al. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology 2006, 44, 1333–1343. [Google Scholar] [CrossRef]





| Type | iCCA | eCCA | |
|---|---|---|---|
| Small Duct | Large Duct | d + p CCA | |
| Cells of origin | Interlobular bile duct | Peribiliary glands | Cholangiocytes Peribiliary glands |
| morphology | Small duct with cubic cells and little mucosecretion | Large duct or papillae lined with mucus secreting columnar cells | Same than large duct iCCA |
| phenotype | Cytoplasmic expression of CK7 and CK19 | ||
| Expression of CRP, N-cadherin and CD56 | Expression of S100P, MUC5AC and MUC6 | Same than large duct iCCA | |
| extension | High frequency of SN Low frequency of PNI Vascular invasion Metastatic lymph node | Low frequency of SN and high frequency of PNI Hepatic infiltration | Same than large duct iCCA |
| Molecular alterations | IDH1/2, BRCA1/2 and BAP 1 mutations, FGFR2 fusion | KRAS, TP53 mutations | Same than large duct iCCA with an higher frequency of KRAS mutations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guedj, N. Pathology of Cholangiocarcinomas. Curr. Oncol. 2023, 30, 370-380. https://doi.org/10.3390/curroncol30010030
Guedj N. Pathology of Cholangiocarcinomas. Current Oncology. 2023; 30(1):370-380. https://doi.org/10.3390/curroncol30010030
Chicago/Turabian StyleGuedj, Nathalie. 2023. "Pathology of Cholangiocarcinomas" Current Oncology 30, no. 1: 370-380. https://doi.org/10.3390/curroncol30010030
APA StyleGuedj, N. (2023). Pathology of Cholangiocarcinomas. Current Oncology, 30(1), 370-380. https://doi.org/10.3390/curroncol30010030




