Therapeutic Strategies and Oncological Outcome of Peritoneal Metastases from Lung Cancer: A Systematic Review and Pooled Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction and Synthesis
2.4. Outcome Measures
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
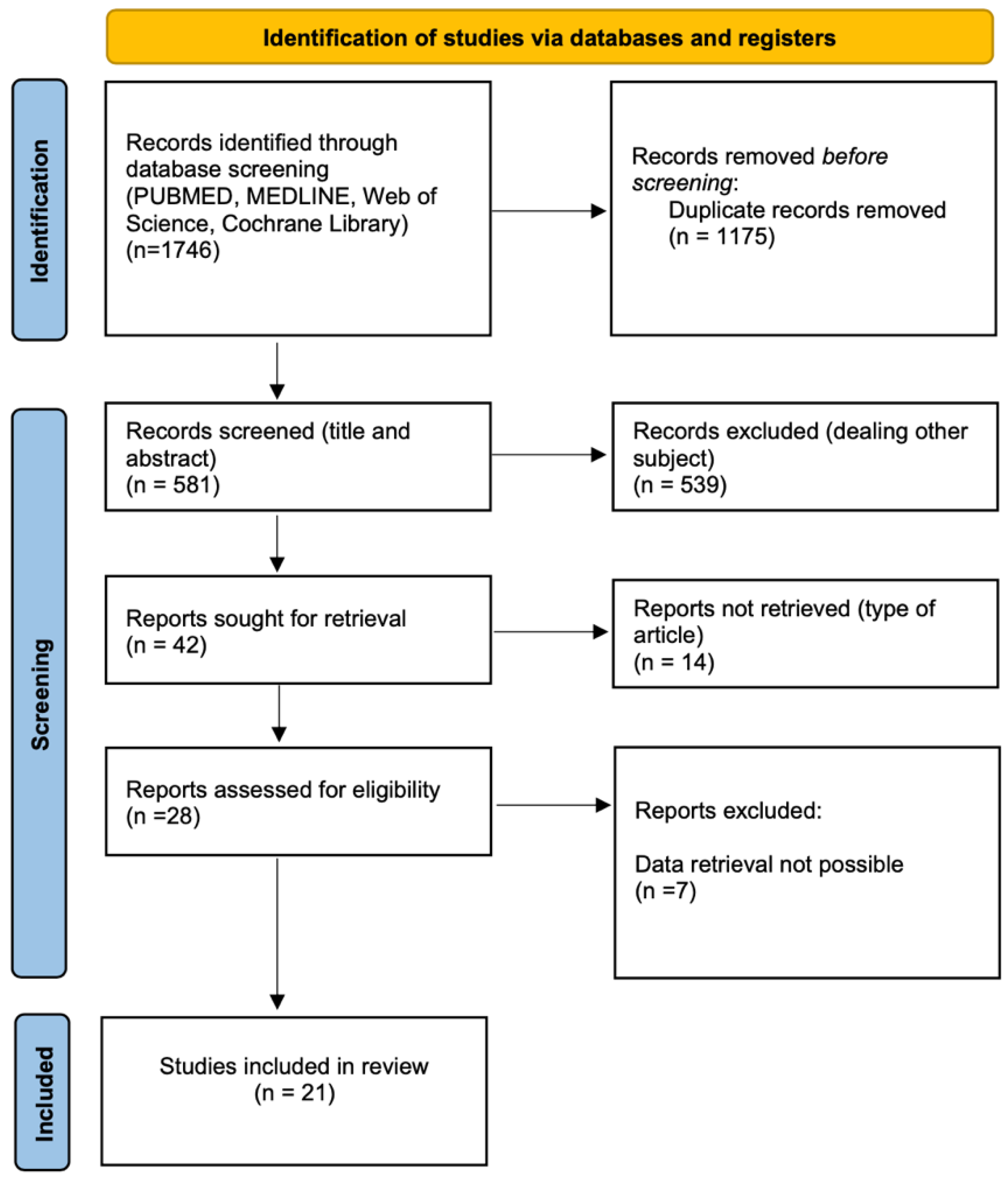
3.1. Systematic Search
3.2. Study Characteristics and Quality Assessment
3.3. Pooled Analysis
3.3.1. Baseline Characteristics
3.3.2. PCLC Management and Outcomes
3.4. Synchronous and Metachronous PCLC Characteristics Subanalysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Cancer Society. Cancer Facts & Figures 2023; American Cancer Society: Atlanta, GA, USA, 2023. [Google Scholar]
- Niu, F.-Y.; Zhou, Q.; Yang, J.-J.; Zhong, W.-Z.; Chen, Z.-H.; Deng, W.; He, Y.-Y.; Chen, H.-J.; Zeng, Z.; Ke, E.-E.; et al. Distribution and prognosis of uncommon metastases from non-small cell lung cancer. BMC Cancer 2016, 16, 149. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, A.; Cardi, M.; Biacchi, D.; Sibio, S.; Accarpio, F.; Ciardi, A.; Cornali, T.; Framarino, M.; Sammartino, P. Depth of colorectal-wall invasion and lymph-node involvement as major outcome factors influencing surgical strategy in patients with advanced and recurrent ovarian cancer with diffuse peritoneal metastases. World J. Surg. Oncol. 2013, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Kitai, T. The role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the treatment of peritoneal carcinomatosis: A systematic review including evidence from Japan. Surg. Today 2020, 51, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- REACCT Collaborative. Characteristics of Early-Onset vs. Late-Onset Colorectal Cancer: A Review. JAMA Surg. 2021, 156, 865–874. [Google Scholar] [CrossRef]
- Jiang, A.; Wang, J.; Liu, N.; Zheng, X.; Li, Y.; Ma, Y.; Zheng, H.; Chen, X.; Fan, C.; Zhang, R.; et al. Integration of Single-Cell RNA Sequencing and Bulk RNA Sequencing Data to Establish and Validate a Prognostic Model for Patients With Lung Adenocarcinoma. Front Genet. 2022, 13, 833797. [Google Scholar] [CrossRef]
- Brinkhof, S.; Groen, H.J.; Siesling, S.S.; Ijzerman, M.J. Resource utilization in lung cancer diagnostic procedures: Current use and budget consequences. PLoS ONE 2017, 12, e0189251. [Google Scholar] [CrossRef]
- Jiang, A.; Chen, X.; Zheng, H.; Liu, N.; Ding, Q.; Li, Y.; Fan, C.; Fu, X.; Liang, X.; Tian, T.; et al. Lipid metabolism-related gene prognostic index (LMRGPI) reveals distinct prognosis and treatment patterns for patients with early-stage pulmonary adenocarcinoma. Int. J. Med. Sci. 2022, 19, 711–728. [Google Scholar] [CrossRef]
- Jiang, A.; Liu, N.; Bai, S.; Wang, J.; Gao, H.; Zheng, X.; Fu, X.; Ren, M.; Zhang, X.; Tian, T.; et al. Identification and validation of an autophagy-related long non-coding RNA signature as a prognostic biomarker for patients with lung adenocarcinoma. J. Thorac. Dis. 2021, 13, 720–734. [Google Scholar] [CrossRef]
- Amelio, I.; Bertolo, R.; Bove, P.; Candi, E.; Chiocchi, M.; Cipriani, C.; Di Daniele, N.; Ganini, C.; Juhl, H.; Mauriello, A.; et al. Cancer predictive studies. Biol. Direct 2020, 15, 18. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinicalepidemiology/oxford.asp (accessed on 20 December 2022).
- Satoh, H.; Ishikawa, H.; Yamashita, Y.T.; Kurishima, K.; Ohtsuka, M.; Sekizawa, K. Peritoneal carcinomatosis in lung cancer patients. Oncol. Rep. 2001, 8, 1305–1307. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Iizasa, T.; Ishikawa, A.; Yoshino, M.; Shingyouji, M.; Kimura, M.; Hirata, T.; Odaka, A.; Matsubayasi, K. Eradication of intractable malignant ascites by abdominocentesis, reinfusion of concentrated ascites, and adoptive immunotherapy with dendritic cells and activated killer cells in a patient with recurrent lung cancer: A case report. J. Med. Case Rep. 2008, 2, 372. [Google Scholar] [CrossRef] [PubMed]
- SSu, H.-T.; Tsai, C.-M.; Perng, R.-P. Peritoneal carcinomatosis in lung cancer. Respirology 2008, 13, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Tanriverdi, O.; Barutca, S.; Meydan, N. Relapse with isolated peritoneal metastasis in lung adenocarcinoma: Case report and review of the literature. Contemp. Oncol. 2012, 16, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Sereno, M.; Rodríguez-Esteban, I.; Gómez-Raposo, C.; Merino, M.; López-Gómez, M.; Zambrana, F.; Casado, E. Lung cancer and peritoneal carcinomatosis. Oncol. Lett. 2013, 6, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Bazine, A.; Fetohi, M.; Khmamouch, M.R.; Namad, T.; Ichou, M.; Errihani, H. An Unusual Case of Isolated Peritoneal Metastases from Lung Adenocarcinoma. Case Rep. Oncol. 2014, 7, 600–604. [Google Scholar] [CrossRef]
- Li, B.; Lu, J.C.; He, D.; Wang, J.; Zhou, H.; Shen, L.; Zhang, C.; Duan, C. Rapid onset lung squamous cell carcinoma with prominent peritoneal carcinomatosis and an eosinophilic leukemoid reaction, with coexistence of the BRAF V600E and oncogenic KRAS G12A mutations: A case report. Oncol Lett. 2014, 8, 589–593. [Google Scholar] [CrossRef]
- Patil, T.; Aisner, D.L.; Noonan, S.A.; Bunn, P.A.; Purcell, W.T.; Carr, L.L.; Camidge, D.R.; Doebele, R.C. Malignant pleural disease is highly associated with subsequent peritoneal metastasis in patients with stage IV non-small cell lung cancer independent of oncogene status. Lung Cancer 2016, 96, 27–32. [Google Scholar] [CrossRef]
- Kobayashi, H.; Wakuda, K.; Takahashi, T. Effectiveness of afatinib in lung cancer with paralytic ileus due to peritoneal carcinomatosis. Respirol. Case Rep. 2016, 4, e00197. [Google Scholar] [CrossRef]
- Hanane, K.; Salma, B.; Khadija, B.; Ibrahim, E.; Saber, B.; Hind, M. Peritoneal carcinomatosis, an unusual and only site of metastasis from lung adenocarcinoma. Pan Afr. Med. J. 2016, 23, 60. [Google Scholar] [CrossRef]
- Yang, P.; Li, W.-L.; Zhou, J.-X.; Yang, Y.-B.; Jin, X.-X. Peritoneum as the sole distant metastatic site of lung adenosquamous cell carcinoma: A case report. J. Med. Case Rep. 2017, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Kamaleshwaran, K.K.; Joseph, J.; Kalarikal, R.K.; Shinto, A. Image findings of rare case of peritoneal carcinomatosis from non small cell lung cancer and response to erlotinib in F-18 FDG positron emission tomography/computed tomography. Indian J. Nucl. Med. 2017, 32, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, M.; Solon, J.; Chang, K.; Deady, S.; Moran, B.; Cahill, R.; Shields, C.; Mulsow, J. Peritoneal metastases from extra-abdominal cancer–A population-based study. Eur. J. Surg. Oncol. 2018, 44, 1811–1817. [Google Scholar] [CrossRef]
- Hsu, J.-F.; Lee, Y.-L.; Chang, H.-L.; Wei, P.-J.; Shen, Y.-T.; Lin, C.-M.; Li, C.-Y.; Chong, I.-W.; Yang, C.-J. Clinical efficacy of concurrent bevacizumab for malignant ascites in nonsquamous cell carcinoma of the lung. Asia-Pac. J. Clin. Oncol. 2019, 15, e126–e131. [Google Scholar] [CrossRef] [PubMed]
- Sibio, S.; Sica, G.S.; Di Carlo, S.; Cardi, M.; Di Giorgio, A.; Sollazzo, B.M.; Sammartino, P. Surgical treatment of intraperitoneal metastases from lung cancer: Two case reports and a review of the literature. J. Med. Case Rep. 2019, 13, 262. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Hanaoka, J.; Hayashi, H.; Fukuda, Y.; Iihara, H.; Suzuki, A.; Sugiyama, T. Clinical efficacy of osimertinib for a patient with ileus due to peritoneal carcinomatosis. Clin. Case Rep. 2019, 8, 347–350. [Google Scholar] [CrossRef]
- Abbate, M.I.; Cortinovis, D.L.; Tiseo, M.; Vavalà, T.; Cerea, G.; Toschi, L.; Canova, S.; Colonese, F.; Bidoli, P. Peritoneal carcinomatosis in non-small-cell lung cancer: Retrospective multicentric analysis and literature review. Future Oncol. 2019, 15, 989–994. [Google Scholar] [CrossRef]
- Kazakova, V.; Velasco, S.V.A.; Perepletchikov, A.; Lathan, C.S. ROS1-rearranged lung adenocarcinoma with peritoneal carcinomatosis on initial presentation. BMJ Case Rep. 2020, 13, e233864. [Google Scholar] [CrossRef]
- Lurvink, R.J.; Rijken, A.; Bakkers, C.; Aarts, M.J.; Kunst, P.W.A.; van de Borne, B.E.; van Erning, F.N.; de Hingh, I.H.J.T. Synchronous peritoneal metastases from lung cancer: Incidence, associated factors, treatment and survival: A Dutch population-based study. Clin. Exp. Metastasis 2021, 38, 295–303. [Google Scholar] [CrossRef]
- Tani, T.; Nakachi, I.; Ikemura, S.; Ohgino, K.; Kuroda, A.; Terai, H.; Masuzawa, K.; Shinozaki, T.; Ishioka, K.; Funatsu, Y.; et al. Clinical Characteristics and Therapeutic Outcomes of Metastatic Peritoneal Carcinomatosis in Non-Small-Cell Lung Cancer. Cancer Manag. Res. 2021, 13, 7497–7503. [Google Scholar] [CrossRef]
- Yagami, Y.; Nakahara, Y.; Manabe, H.; Yamamoto, H.; Otani, S.; Sato, T.; Igawa, S.; Kubota, M.; Sasaki, J.; Naoki, K. Promising Response to Dabrafenib Plus Trametinib in a Patient with Peritoneal Carcinomatosis from Non Small Lung Cancer Harboring BRAF V600E Mutation. OncoTargets Ther. 2022, 15, 1369–1374. [Google Scholar] [CrossRef]
- Iaculli, E.; Agostini, M.; Biancone, L.; Fiorani, C.; Di Vizia, A.; Montagnese, F.; Sibio, S.; Manzelli, A.; Tesauro, M.; Rufini, A.; et al. C-reactive protein levels in the perioperative period as a predictive marker of endoscopic recurrence after ileo-colonic resection for Crohn’s disease. Cell Death Discov. 2016, 2, 16032. [Google Scholar] [CrossRef] [PubMed]
- Sibio, S.; Sammartino, P.; Accarpio, F.; Biacchi, D.; Cornali, T.; Cardi, M.; Iafrate, F.; Di Giorgio, A. Metastasis of Pleural Mesothelioma Presenting as Bleeding Colonic Polyp. Ann. Thorac. Surg. 2011, 92, 1898–1901. [Google Scholar] [CrossRef] [PubMed]
- Aarnink, A.; Fumet, J.D.; Favier, L.; Truntzer, C.; Ghiringhelli, F. Role of pleural and peritoneal metastasis in immune checkpoint inhibitors efficacy patients with non-small cell lung cancer: Real-world data from a large cohort in France. J. Cancer Res. Clin. Oncol. 2020, 146, 2699–2707. [Google Scholar] [CrossRef]
- Onali, S.; Calabrese, E.; Petruzziello, C.; Lolli, E.; Ascolani, P.; Ruffa, A.; Sica, G.; Rossi, A.; Chiaramonte, C.; Pallone, F.; et al. Post-operative recurrence of Crohn’s disease: A prospective study at 5 years. Dig Liver Dis. 2016, 48, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Di Carlo, S.; Cavallaro, G.; La Rovere, F.; Usai, V.; Siragusa, L.; Izzo, P.; Izzo, L.; Fassari, A.; Izzo, S.; Franceschilli, M.; et al. Synchronous liver and peritoneal metastases from colorectal cancer: Is cytoreductive surgery and hyperthermic intraperitoneal chemotherapy combined with liver resection a feasible option? Front. Surg. 2022, 9, 1006591. [Google Scholar] [CrossRef]
- Sibio, S.; Fiorani, C.; Stolfi, C.; Divizia, A.; Pezzuto, R.; Montagnese, F.; Bagaglini, G.; Sammartino, P.; Sica, G.S. Detection methods and clinical significance of free peritoneal tumor cells found during colorectal cancer surgery. World J. Gastrointest. Surg. 2015, 7, 178–184. [Google Scholar] [CrossRef]
- Di Giorgio, A.; Sammartino, P.; Cardini, C.L.; Al Mansour, M.; Accarpio, F.; Sibio, S.; Di Seri, M. Lung cancer and skeletal muscle metastases. Ann. Thorac. Surg. 2004, 78, 709–711. [Google Scholar] [CrossRef]
- COVIDSurg Collaborative; GlobalSurg Collaborative. SARS-CoV-2 infection and venous thromboembolism after surgery: An international prospective cohort study. Anaesthesia 2022, 77, 28–39. [Google Scholar]
| Authors | Year | Country/Region | Journal | Study Design | N° Patients | NOS Score |
|---|---|---|---|---|---|---|
| Satoh et al. [13] | 2001 | Japan | Oncology Reports | Retrospective | 12 | 7 |
| Kimura et al. [14] | 2008 | Japan | Journal of Medical Case Reports | Case Report | 1 | - |
| Su et al. [15] | 2008 | Taiwan | Respirology | Retrospective | 30 | 7 |
| Tanriverdi et al. [16] | 2012 | Turkey | Wspolczesna Onkol | Case Report | 1 | - |
| Sereno et al. [17] | 2013 | Spain | Oncology letters | Case Series | 4 | - |
| Bazine et al. [18] | 2014 | Morocco | Case Reports in Oncology | Case Report | 1 | - |
| Li et al. [19] | 2014 | China | Oncology letters | Case Report | 1 | - |
| Patil et al. [20] | 2016 | Colorado | Lung Cancer | Retrospective | 33 | 7 |
| Kobayashi et al. [21] | 2016 | Japan | Respirology Case Reports | Case Report | 1 | - |
| Hanane et al. [22] | 2016 | Morocco | PanAfrican Medical Journal | Case Report | 1 | - |
| Yang et al. [23] | 2017 | China | Journal of Medical Case Report | Case Report | 1 | - |
| Kamaleshwaran et al. [24] | 2017 | India | Indian Journal of Nuclear Medicine | Case Report | 1 | - |
| Flanagan et al. [25] | 2018 | Ireland | European Journal of surgical oncology | Retrospective | 139 | 8 |
| Jui-Feng Hsu et al. [26] | 2018 | Taiwan | Asia-Pacific Journal of Clinical Oncology | Case Series | 3 | - |
| Sibio et al. [27] | 2019 | Italy | Journal of Medical Case Reports | Case Series | 2 | - |
| Kawaguchi et al. [28] | 2019 | Japan | Clinical Case Reports | Case Report | 1 | - |
| Abbate et al. [29] | 2019 | Italy | Future Oncology | Retrospective | 60 | 7 |
| Kazakova et al. [30] | 2020 | USA | Unusual presentation of more common disease/injury | Case Report | 1 | - |
| Lurvink et al. [31] | 2021 | Netherlands | Clinical & Experimental Metastasis | Retrospective | 2533 | 8 |
| Tani et al. [32] | 2021 | Japan | Cancer Management and Research | Retrospective | 46 | 7 |
| Yagami et al. [33] | 2022 | Japan | Oncotargets and therapy | Case report | 1 | - |
| Authors | Number of Patients | Male (n %) | Age Median (Range) (Years) | Smoker (n %) | Incidence of PC (n %) | Time from Diagnosis to PC Median (Range) (Months) | Ascites (n%) | Stage at Diagnosis (n %) | Other M (n %) | Peritoneal Single M Site (n, %) | Histological Type (n %) | Mutations (n, %) | Clinical Presentation (n %) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Satoh et al. [13] | 12 | 6 50% | 54 (34–74) | na | 12/1041 1.2% | 9 (0–36) | na | na | Pleura 9 75% Lung 6 50% Liver 4 33.3% Bone 5 41.7% Brain 3 25% Distant node 3 25% | na | Adenocarcinoma 7 58.3% SCLC 1 8.3% SCC 2 16.7% NSCLC 2 16.7% | na | Synchronous 1 8.3% Metachronous 11 91.7% |
| Kimura et al. [14] | 1 | 0 0% | 52 | na | na | 25 | 1 100% | IV | Lung 1 100% Pleura 1 100% | na | Adenocarcinoma 1 100% | na | Metachronous 1 100% |
| Su et al. [15] | 30 | 20 66.7% | 59 (29–83) | na | na | 8.5 (0–38) | 30 100% | IIIb 6 20% IV 24 80% | Lung 9 30% Liver 11 36.7% Bone 13 43.3% Brain 5 16.7% Pleura 24 80% Adrenal 3 10% Soft tissue 1 3.3% Eye 1 3.3% Pancreas 2 6.7% Pericardial effusion 3 10% Spleen 1 3.3% | 0% | Adenocarcinoma 25 83.4% SCLC 3 10% SCC 1 3.3% Mixed small cell/squamous cell carcinoma 1 3.3% | na | na |
| Tanriverdi et al. [16] | 1 | 1 100% | 59 | 1 100% | na | 3 | 1 100% | IIIa 1 100% | Pericardium 1 100% | 0% | Adenocarcinoma 1 100% | na | Metachronous 1 100% |
| Sereno et al. [17] | 4 | 3 75 % | 64 (52–67) Mean 61.5 | 2 100% | na | 3 (0–12) | 1 25% | IVb 1 25% (1 pt) | Lung 1 25% Liver 1 25% Pleura 3 75% Adrenal gland 1 25% | 0% | Adenocarcinoma 4 100% | EGFR 2 50% | Metachronous 3 75% Synchronous 1 25% |
| Bazine et al. [18] | 1 | 0 0% | 55 | 0 0% | na | 0 | na | na | 0 0% | 1 100% | Adenocarcinoma 1 100% | None 1 100% | Synchronous 1 100% |
| Li et al. [19] | 1 | 1 100% | 63 | 1 100% | na | 0 | 1 100% | na | 0 0% | 1 100% | SCC 1 100% | BRAF 1 100% kRAS 1 100% | Synchronous 1 100% |
| Patil et al. [20] | 33 | 12 36% | 58 (51–91) | 13 39% | 33/410 8% | 16.5 (0.6–108) | na | na | Lung 5 15% Liver 3 9% Bone 14 42% Brain 10 30% Pleura 26 79% Adrenal 4 12% Soft tissue 4 12% | na | NSCLC 33 100% | EGFR 17 51% kRAS 5 15% MET 1 3% ALK 5 15% None 5 15% | Metachronous 33 100% |
| Kobayashi et al. [21] | 1 | 0 0% | 61 | 0 0% | na | na | 1 100% | IV 100% | Lung 1 100% Pleura 1 100% | 0 0% | Adenocarcinoma 1 100% | EGFR 1 100% | Metachronous 1 100% |
| Hanane et al. [22] | 1 | 1 100% | 56 | 1 100% | na | 14 | 1 100% | IIIa 1 100% | 0 0% | 1 100% | Adenocarcinoma 1 100% | None 1 0% | Metachronous 1 100% |
| Yang et al. [23] | 1 | 1 100% | 82 | 1 100% | na | 1.7 | 1 100% | IIIa 1 100% | 0 0% | 1 100% | SCC 1 100% | kRAS 1 100% | Metachronous 1 100% |
| Kamaleshwaran et al. [24] | 1 | 1 100% | 45 | na | na | 0 | na | IV 1 100% | 0 0% | 1 100% | NSCLC 1 100% | EGFR 1 100% | Synchronous 1 100% |
| Flanagan et al. [25] | 139 | 80 57% | na | na | 139/41,789 0.3% | 8.5 (1–9) | na | IV 139 100% | Liver 37 26.6% Bone 10 7.2% Brain 9 6.5% Distant node 8 5.8% Adrenal 18 12.9% | 34 24.4% | Adenocarcinoma 51 37% SCLC 27 19% SCC 21 15% NSCLC 12 9% Unspecified 28 20% | na | Synchronous 99 71 % Metachronous 40 29 % |
| Jui-Feng Hsu et al. [26] | 3 | 2 67% | 66 (53–67) Mean 62 | 1 33.3% | 3/265 1.1% | 21 (0–28) Mean 16.3 | 3 100% | IV 3 100% | Liver 1 33.3% Pericardium 1 33.3% | 2 66.7% | Adenocarcinoma 3 100% | EGFR 3 100% | Synchronous 1 33.3% Metachronous 2 66.7% |
| Sibio et al. [27] | 2 | 2 100% | 52 (44–59) | 1 50% | na | 42 (36–48) | 1 50% | IIb 2 100% | Brain 1 50% Colon 1 50% Small bowel 1 50% Spleen 1 50% | 0 0% | Adenocarcinoma 2 100% | na | Metachronous 2 100% |
| Kawaguchi et al. [28] | 1 | 1 100% | 42 | 1 100% | na | 21 | 1 100% | IV 1 100% | Lung 1 100% Brain 1 100% | 0 0% | Adenocarcinoma 1 100% | EGFR 1 100% | Metachronous 1 100% |
| Abbate et al. [29] | 60 | na | 60 (25–75) | 43 72% | na | na | na | na | na | na | Adenocarcinoma 48 80% SCC 1 2% Unspecified 11 18% | EGFR 7/23 30% ALK 3/17 18% MET 2/4 50% ROS 1/3 33% 3 | Synchronous 20 33.3% Metachronous 40 66.7% |
| Kazakova et al. [30] | 1 | 1 100% | 56 | 0 0% | na | 0 | 1 100% | IV 1 100% | 0 0% | 1 100% | Adenocarcinoma 1 100% | ROS1 1 100% | Synchronous 1 100% |
| Lurvink et al. [31] | 2533 | 1483 58.5% | Mean 67 ± 10 | na | 2533/129,651 2% | 0 | na | IV 2533 100% | na | 326 12.9% | Adenocarcinoma 1122 44.3% SCLC 500 19.7% SCC 258 10.2% NSCLC 653 25.8% | na | Synchronous 2533 100% |
| Tani et al. [32] | 46 | 33 71.7% | 66 (59–71) | 36 78% | na | na | 15 32.6% | na | Brain 5 10% Pleural 17 37% | na | Adenocarcinoma 40 87% NSCLC 4 8.6% SCC 1 2.2% Pleomorphic carcinoma 1 2.2% | EGFR 14 30.4% ALK 1 2.2% None 31 67.4% | Synchronous 12 26.1% Metachronous 34 73.9% |
| Yagami et al. [33] | 1 | 1 100% | 67 | 0 0% | na | 33 | 1 100% | I 100% | Pleural 1 100% | 0 0% | Adenocarcinoma | BRAF 100% | Metachronous 1 100% |
| Total | 2873 | 1649/2873 57.4% | Median range 52–66 (range 25–91) | 101/157 64.3% | 2720/173,476 1.5% | Median range 0–42 m (range 0–108) | 59/94 63% | II 2/2873 0.1% III 9/2873 0.3% IV 2706/2720 99.6% | Pleural 81/280 28.9% Liver 57/280 20.4% Bone 42/280 15% Brain 34/280 12.1% Adrenal 26/280 9.3% Lung 24/280 8.6% Distant node 11/280 3.9% Pericardium 5/280 1.9% Soft tissue 5/280 1.9% Pancreas 2/280 0.7% Spleen 2/280 0.7% Small bowel 1/280 0.4% Colon 1/280 0.4% Eye 1/280 0.4% | 368/2721 13.5% | Adenocarcinoma 1310/2873 45.6% NSCLC 705/2873 24.5% SCLC 531/2873 18.5% SCC 286/2873 10% Pleomorphic carcinoma 1/2873 0.03% Mixed small cell/squamous cell carcinoma 1/2873 0.03% Unspecified 39/2873 13.6 | EGFR 46/117 39.3% ALK 9/111 8.1% kRAS 7/95 7.5% MET 3/98 3.1% ROS 2/97 2.1% BRAF 2/95 2.1% None 38/95 40% | Synchronous 2671/2843 94% Metachronous 172/2843 6% |
| Authors | Number of Patients | Chemotherapy (n %) | Type of Chemotherapy (n %) | Surgical Intervention (n %) | OS from Lung Cancer Diagnosis Median (Range) (Months) | OS PC Median/Mean (Range) (Months) | Death (n %) |
|---|---|---|---|---|---|---|---|
| Satoh et al. [13] | 12 | 1 8.3% | Platin-based agents 1 8.3% | 0 0% | na | 2 (1–9) | 12 100% |
| Kimura et al. [14] | 1 | 1 100% | Dendritic cell immunotherapy 1 100% | 0 0% | 35 | 10 | 0% |
| Su et al. [15] | 30 | 9/25 36% | na | 0 0% | 9 (0.2–42.7) (25 pt) | 0.5 (0–11.3) (25 pt) | 25/26 96.1% |
| Tanriverdi et al. [16] | 1 | 1 100% | Docetaxel 1 100% | 0 0% | na | 2 | 1 100% |
| Sereno et al. [17] | 4 | 4 100% | Docetaxel 3 75% Pemetrexed 3 75% Carboplatin 3 75% Cisplatin 1 25% Paclitaxel 2 50% Bevacizumab 2 50% Erlotinib 2 50% | 0 0% | na | na | 1 25% |
| Bazine et al. [18] | 1 | 1 100% | Pemetrexed 1 100% Carboplatin 1 100% Paclitaxel 1 100% Bevacizumab 1 100% | 0 0% | 10 | 10 | 0 0% |
| Li et al. [19] | 1 | 0 0% | na | 0 0% | 0.2 | 0.2 | 1 100% |
| Patil et al. [20] | 33 | na | na | 0 0% | 20.5 (1–88) | 2 (0–78) | 33 100% |
| Kobayashi et al. [21] | 1 | 1 100% | Afatinib 1 100% | 0 0% | na | 12 | 0 0% |
| Hanane et al. [22] | 1 | 1 100% | Gemcitabine 1 100% Cisplatin 1 100% Bevacizumab 1 100% | 0 0% | na | 6 | 1 100% |
| Yang et al. [23] | 1 | 1 100% | Cisplatin 1 100% Recombinant human endostatin 1 100% | 0 0% | 2.1 | 0.4 | 1 100% |
| Kamaleshwaran et al. [24] | 1 | 1 100% | Erlotinib 1 100% | 0 0% | na | na | na |
| Flanagan et al. [25] | 139 | 50 35% | na | 11 7% | 10 | 1.3 (0–16.2) | 139 100% |
| Jui-Feng Hsu et al. [26] | 3 | 3 100% | Gemcitabine 1 33.3% Bevacizumab 3 100% Erlotinib 1 33.3% Afatinib 1 33.3% | 0 0% | 65.6 | 41.3 | 1 33.3% |
| Sibio et al. [27] | 2 | 2 100% | Cisplatin 1 50% Gemcitabile 1 50% | 2 100% | 74.5 (65–84) | 32.5 (29–36) | 1 50% |
| Kawaguchi et al. [28] | 1 | 1 100% | Osimertinib 1 100% | 0 0% | 25 | 4 | 1 100% |
| Abbate et al. [29] | 60 | 58 96.7% | na | 0 0% | 17.5 | 3.5 | na |
| Kazakova et al. [30] | 1 | 1 100% | Crizotinib 1 100% | 0 0% | 6 | 6 | 0 0% |
| Lurvink et al. [31] | 2533 | 590 23.3% | na | 189 7% | na | 2.5 | na |
| Tani et al. [32] | 46 | 25 54.3% | Cytotoxic agents 13 28.3% EGFR/ALK-tyrosine kinase inhibitors 10 21.7% Immune-checkpoint inhibitors 2 4.3% Bevacizumab 3 6.5% | 0 0% | na | 5.2 (2.1–6.3) | na |
| Yagami et al. [33] | 1 | 1 100% | Dabrafenib 1 100% Trametinib1 100% | 0% | 33 | 40 | 0 % |
| Total | 2873 | 753/2835 26.6% | Cytotoxic agents 25/45 55.5% EGFR/ALK-tyrosine kinase inhibitors 18/45 40% Bevacizumab 10/45 22.2% Platinum based agents 9/45 20% Immune-checkpoint inhibitors 2/45 4.4% Recombinant human endostatin 1/45 2.2% BRAF-tyrosine kinase inhibitors 1/45 2.2% MEK-tyrosine kinase inhibitors 1/45 2.2% Dendritic cell immunotherapy 1/45 2.2% | 202/2873 7% | Median range 9–20.5 (range 0.1–88) | Median range 0.5–5.2 (range 0–78) | 220/229 96.1% |
| Male (n %) | Age Mean (Years) | Smoker (n %) | Ascites (n%) | Stage at Diagnosis (n %) | Other M (n %) | Peritoneal Single M Site (n, %) | Histological Type (n %) | Mutations (n, %) | |
|---|---|---|---|---|---|---|---|---|---|
| Synchronous (n = 2671) | 1488/2538 59% | 67 | 1/5 20% | 3/4 75% | IV 2671/2671 100% | Pleura 1/6 17% | 5/6 83% | Adenocarcinoma 1126/2539 44% NSCLC 654/2539 26% SCLC 500/2539 20% SCC 259/2539 10% | None 2/6 33% EGFR 2/6 33% BRAF 1/6 17% kRAS 1/6 17% ROS1 1/6 17% |
| Metachronous (n = 172) | 22/47 47% | 58 | 21/46 46% | 12/14 86% | II 2 1.2% III 9 5.2% IV 161 93.6% | Pleura 31/47 66% Bone 14/47 30% Brain 12/47 26% Lung 9/47 19% Adrenal gland 5/47 11% Soft tissue 4/47 8% Pericardium 2/47 4% Bowel 2/47 4% | 3/46 7% | NSCLC 33/47 70% Adenocarcinoma 13/47 28% SCC 1/47 2% | None 6/43 14% EGFR 23/43 53% kRAS 6/43 14% ALK 5/43 12% BRAF 1/43 2% MET 1/43 2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siragusa, L.; Di Carlo, S.; Fassari, A.; Sensi, B.; Riccetti, C.; Izzo, L.; Cavallaro, G.; Fiori, E.; Sapienza, P.; Mallia, L.; et al. Therapeutic Strategies and Oncological Outcome of Peritoneal Metastases from Lung Cancer: A Systematic Review and Pooled Analysis. Curr. Oncol. 2023, 30, 2928-2941. https://doi.org/10.3390/curroncol30030224
Siragusa L, Di Carlo S, Fassari A, Sensi B, Riccetti C, Izzo L, Cavallaro G, Fiori E, Sapienza P, Mallia L, et al. Therapeutic Strategies and Oncological Outcome of Peritoneal Metastases from Lung Cancer: A Systematic Review and Pooled Analysis. Current Oncology. 2023; 30(3):2928-2941. https://doi.org/10.3390/curroncol30030224
Chicago/Turabian StyleSiragusa, Leandro, Sara Di Carlo, Alessia Fassari, Bruno Sensi, Camilla Riccetti, Luciano Izzo, Giuseppe Cavallaro, Enrico Fiori, Paolo Sapienza, Letizia Mallia, and et al. 2023. "Therapeutic Strategies and Oncological Outcome of Peritoneal Metastases from Lung Cancer: A Systematic Review and Pooled Analysis" Current Oncology 30, no. 3: 2928-2941. https://doi.org/10.3390/curroncol30030224
APA StyleSiragusa, L., Di Carlo, S., Fassari, A., Sensi, B., Riccetti, C., Izzo, L., Cavallaro, G., Fiori, E., Sapienza, P., Mallia, L., Pernazza, G., & Sibio, S. (2023). Therapeutic Strategies and Oncological Outcome of Peritoneal Metastases from Lung Cancer: A Systematic Review and Pooled Analysis. Current Oncology, 30(3), 2928-2941. https://doi.org/10.3390/curroncol30030224







