Poor Muscle Status, Dietary Protein Intake, Exercise Levels, Quality of Life and Physical Function in Women with Metastatic Breast Cancer at Chemotherapy Commencement and during Follow-Up
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Participants
2.3. Data Collection
2.4. Nutritional Status
2.5. Body Composition
2.6. Physical Function Tests
2.7. Definition of Sarcopenia and Myosteatosis
2.8. Quality of Life
2.9. Statistics
2.10. Ethics Statement
3. Results
3.1. Nutritional status
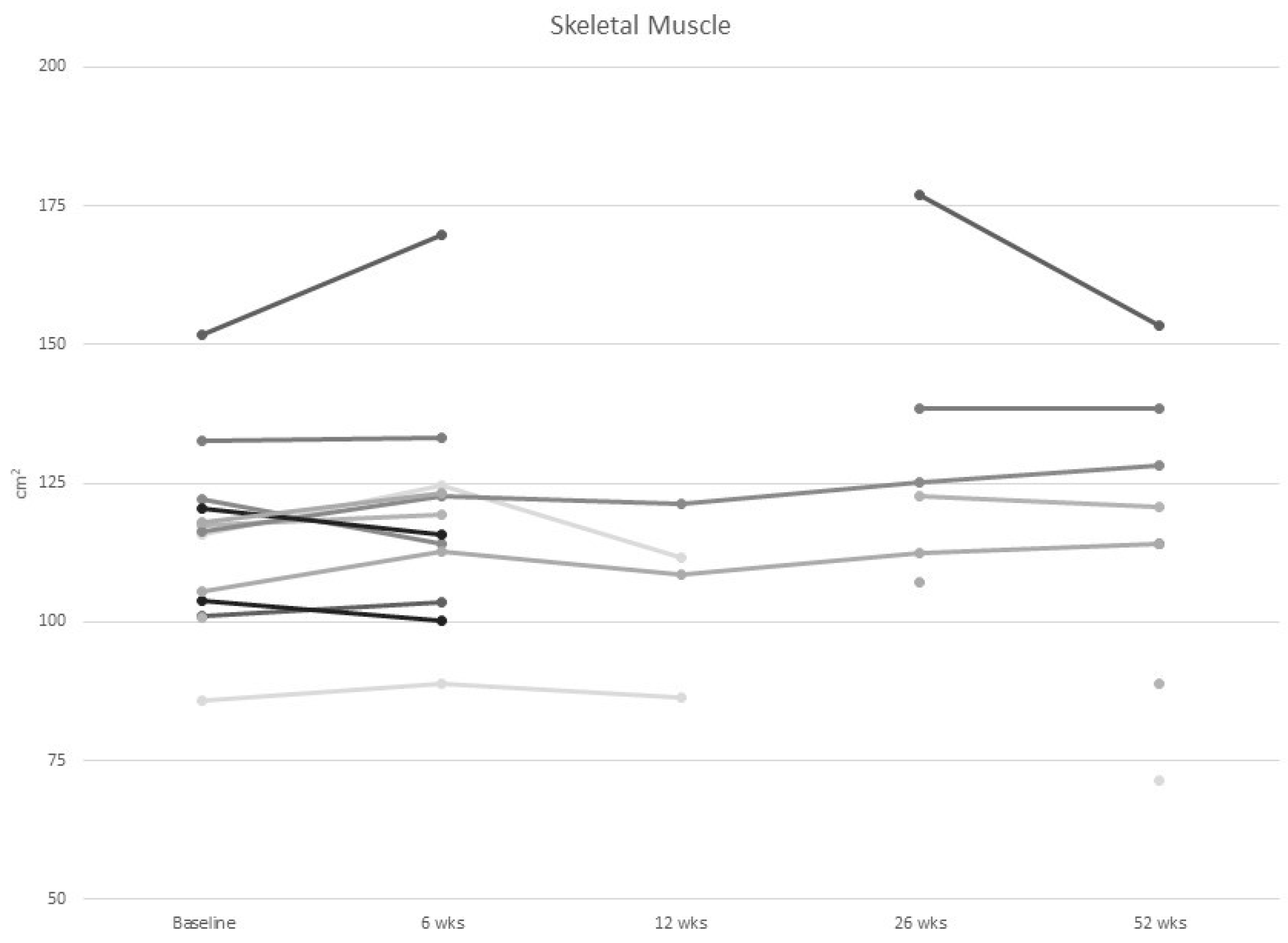
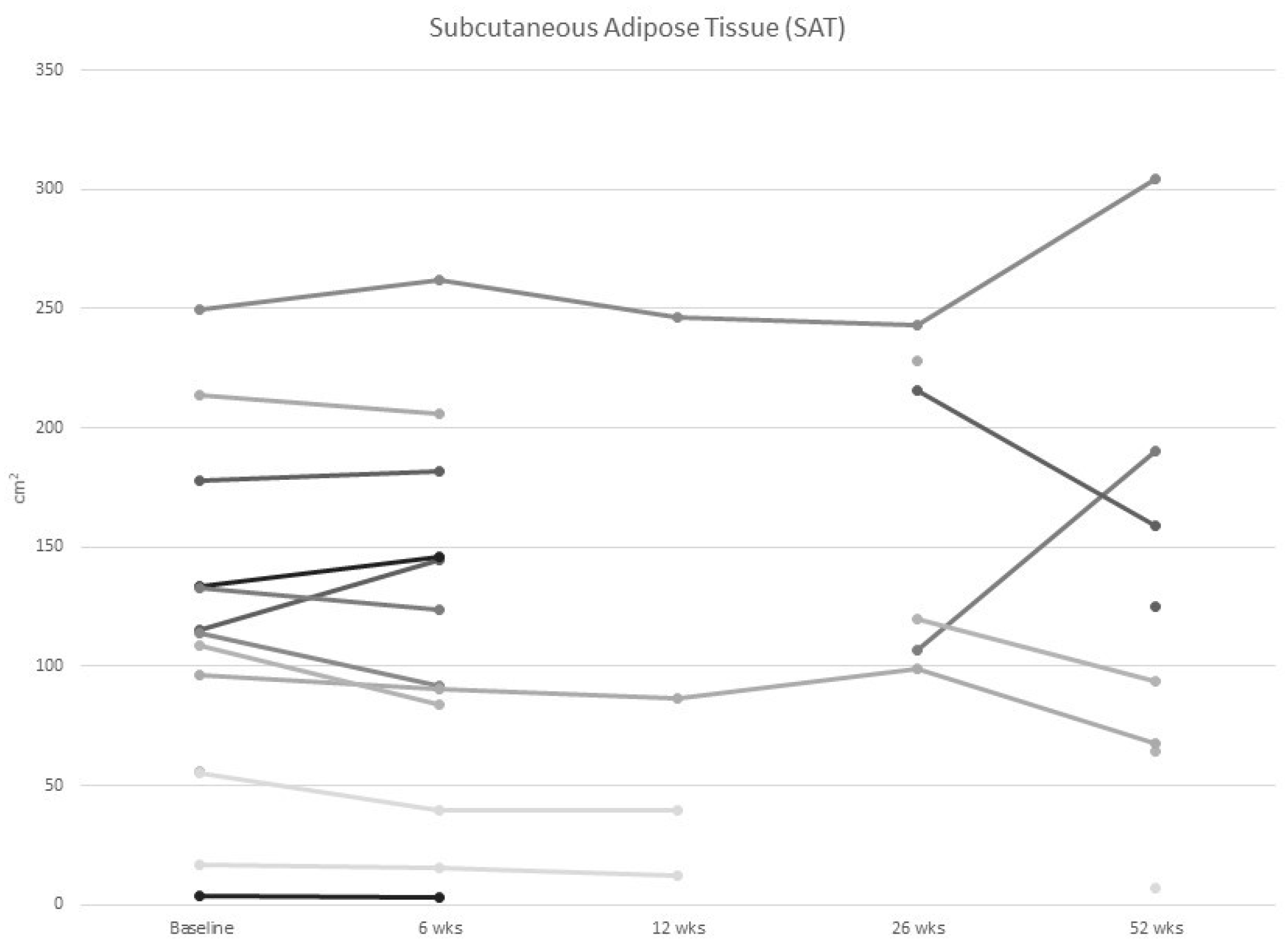
3.2. Body Composition
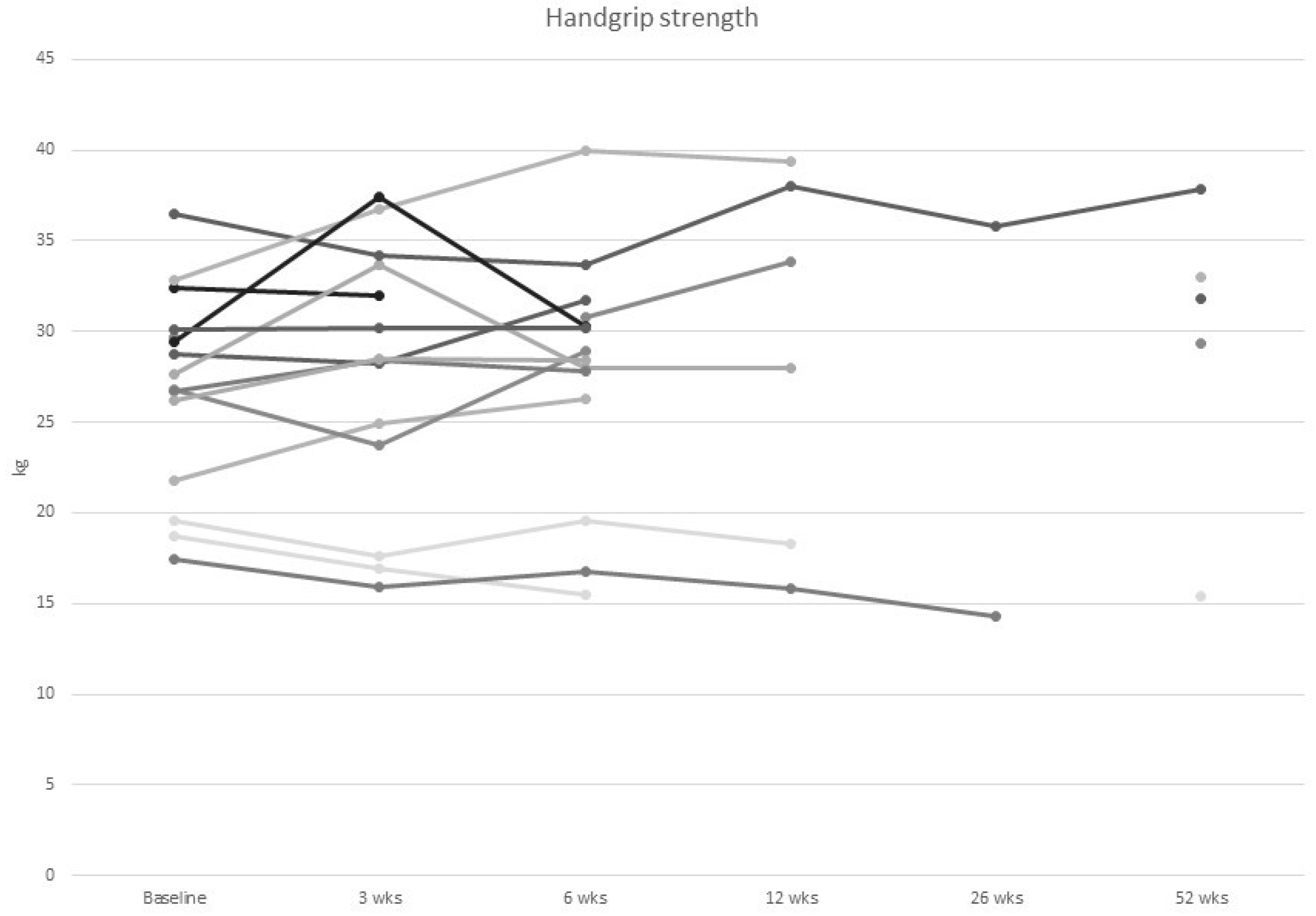
3.3. Physical Function Tests
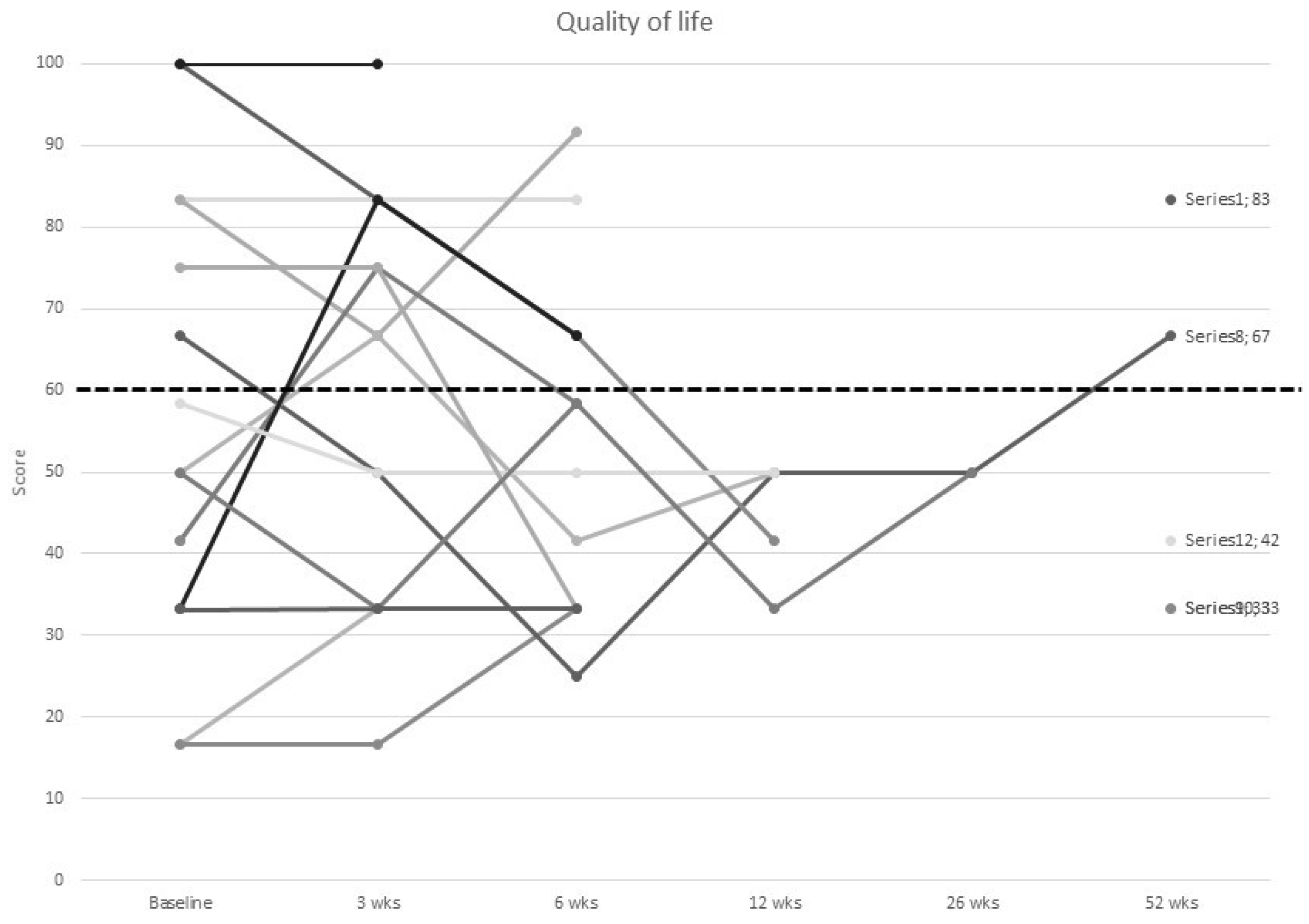
3.4. Quality of Life
3.5. Prognostic Impact of Malnutrition Risk at Baseline
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trestini, I.; Carbognin, L.; Monteverdi, S.; Zanelli, S.; De Toma, A.; Bonaiuto, C.; Nortilli, R.; Fiorio, E.; Pilotto, S.; Di Maio, M.; et al. Clinical Implication of Changes in Body Composition and Weight in Patients with Early-Stage and Metastatic Breast Cancer. Crit. Rev. Oncol. Hematol. 2018, 129, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Metastatic Breast Cancer. Available online: https://www.breastcancer.org/symptoms/types/recur_metast (accessed on 30 March 2020).
- Demark-Wahnefried, W.; Kenyon, A.J.; Eberle, P.; Skye, A.; Kraus, W.E. Preventing Sarcopenic Obesity among Breast Cancer Patients Who Receive Adjuvant Chemotherapy: Results of a Feasibility Study. Clin. Exerc. Physiol. 2002, 4, 44–49. [Google Scholar] [PubMed]
- Sommer, I.; Griebler, U.; Mahlknecht, P.; Thaler, K.; Bouskill, K.; Gartlehner, G.; Mendis, S. Socioeconomic Inequalities in Non-Communicable Diseases and Their Risk Factors: An Overview of Systematic Reviews. BMC Public Health 2015, 15, 914. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Hu, W.; Xu, Y.; Wang, D.; Wang, Y.; Lv, W.; Xiong, M.; Yi, Y.; Wang, H.; Zhang, Q.; et al. Current Landscape: The Mechanism and Therapeutic Impact of Obesity for Breast Cancer. Front. Oncol. 2021, 11, 704893. [Google Scholar] [CrossRef]
- Alarfi, H.; Salamoon, M.; Kadri, M.; Alammar, M.; Haykal, M.A.; Alseoudi, A.; Youssef, L.A. The Impact of Baseline Body Mass Index on Clinical Outcomes in Metastatic Breast Cancer: A Prospective Study. BMC Res. Notes 2017, 10, 550. [Google Scholar] [CrossRef]
- Saleh, K.; Carton, M.; Dieras, V.; Heudel, P.-E.; Brain, E.; D’Hondt, V.; Mailliez, A.; Patsouris, A.; Mouret-Reynier, M.-A.; Goncalves, A.; et al. Impact of Body Mass Index on Overall Survival in Patients with Metastatic Breast Cancer. Breast 2021, 55, 16–24. [Google Scholar] [CrossRef]
- Prado, C.M.M.; Baracos, V.E.; McCargar, L.J.; Reiman, T.; Mourtzakis, M.; Tonkin, K.; Mackey, J.R.; Koski, S.; Pituskin, E.; Sawyer, M.B. Sarcopenia as a Determinant of Chemotherapy Toxicity and Time to Tumor Progression in Metastatic Breast Cancer Patients Receiving Capecitabine Treatment. Clin. Cancer Res. 2009, 15, 2920–2926. [Google Scholar] [CrossRef]
- Kutynec, C.L.; McCargar, L.; Barr, S.I.; Hislop, T.G. Energy Balance in Women with Breast Cancer during Adjuvant Treatment. J. Am. Diet. Assoc. 1999, 99, 1222–1227. [Google Scholar] [CrossRef]
- Pamoukdjian, F.; Bouillet, T.; Lévy, V.; Soussan, M.; Zelek, L.; Paillaud, E. Prevalence and Predictive Value of Pre-Therapeutic Sarcopenia in Cancer Patients: A Systematic Review. Clin. Nutr. 2018, 37, 1101–1113. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Carneiro, I.P.; Mazurak, V.C.; Prado, C.M. Clinical Implications of Sarcopenic Obesity in Cancer. Curr. Oncol. Rep. 2016, 18, 62. [Google Scholar] [CrossRef] [PubMed]
- Shachar, S.S.; Deal, A.M.; Weinberg, M.; Nyrop, K.A.; Williams, G.R.; Nishijima, T.F.; Benbow, J.M.; Muss, H.B. Skeletal Muscle Measures as Predictors of Toxicity, Hospitalization, and Survival in Patients with Metastatic Breast Cancer Receiving Taxane-Based Chemotherapy. Clin. Cancer Res. 2017, 23, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Caan, B.J.; Cespedes Feliciano, E.M.; Prado, C.M.; Alexeeff, S.; Kroenke, C.H.; Bradshaw, P.; Quesenberry, C.P.; Weltzien, E.K.; Castillo, A.L.; Olobatuyi, T.A.; et al. Association of Muscle and Adiposity Measured by Computed Tomography With Survival in Patients With Nonmetastatic Breast Cancer. JAMA Oncol. 2018, 4, 798–804. [Google Scholar] [CrossRef]
- Rier, H.N.; Jager, A.; Sleijfer, S.; van Rosmalen, J.; Kock, M.C.J.M.; Levin, M.-D. Changes in Body Composition and Muscle Attenuation during Taxane-Based Chemotherapy in Patients with Metastatic Breast Cancer. Breast Cancer Res. Treat. 2018, 168, 95–105. [Google Scholar] [CrossRef]
- Prado, C.M.; Purcell, S.A.; Laviano, A. Nutrition Interventions to Treat Low Muscle Mass in Cancer. J. Cachexia. Sarcopenia Muscle 2020, 11, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN Guidelines on Nutrition in Cancer Patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Foucaut, A.-M.; Morelle, M.; Kempf-Lépine, A.-S.; Baudinet, C.; Meyrand, R.; Guillemaut, S.; Metzger, S.; Bourne-Branchu, V.; Grinand, E.; Chabaud, S.; et al. Feasibility of an Exercise and Nutritional Intervention for Weight Management during Adjuvant Treatment for Localized Breast Cancer: The PASAPAS Randomized Controlled Trial. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2019, 27, 3449–3461. [Google Scholar] [CrossRef]
- Tang, F.; Wang, J.; Tang, Z.; Kang, M.; Deng, Q.; Yu, J. Quality of Life and Its Association with Physical Activity among Different Types of Cancer Survivors. PLoS ONE 2016, 11, e0164971. [Google Scholar] [CrossRef]
- Desbiens, C.; Filion, M.; Brien, M.-C.; Hogue, J.-C.; Laflamme, C.; Lemieux, J. Impact of Physical Activity in Group versus Individual Physical Activity on Fatigue in Patients with Breast Cancer: A Pilot Study. Breast 2017, 35, 8–13. [Google Scholar] [CrossRef]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and Physical Activity Guidelines for Cancer Survivors. CA Cancer J. Clin. 2012, 62, 243–274. [Google Scholar] [CrossRef]
- Furmaniak, A.C.; Menig, M.; Markes, M.H. Exercise for Women Receiving Adjuvant Therapy for Breast Cancer. Cochrane Database Syst. Rev. 2016, 9, CD005001. [Google Scholar] [CrossRef] [PubMed]
- Sheean, P.; Kabir, C.; Rao, R.; Hoskins, K.; Stolley, M. Exploring Diet, Physical Activity, and Quality of Life in Females with Metastatic Breast Cancer: A Pilot Study to Support Future Intervention. J. Acad. Nutr. Diet. 2015, 115, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Visovsky, C. Muscle Strength, Body Composition, and Physical Activity in Women Receiving Chemotherapy for Breast Cancer. Integr. Cancer Ther. 2006, 5, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Delrieu, L.; Pialoux, V.; Pérol, O.; Morelle, M.; Martin, A.; Friedenreich, C.; Febvey-Combes, O.; Pérol, D.; Belladame, E.; Clémençon, M.; et al. Feasibility and Health Benefits of an Individualized Physical Activity Intervention in Women With Metastatic Breast Cancer: Intervention Study. JMIR mHealth uHealth 2020, 8, e12306. [Google Scholar] [CrossRef] [PubMed]
- Hébuterne, X.; Lemarié, E.; Michallet, M.; de Montreuil, C.B.; Schneider, S.M.; Goldwasser, F. Prevalence of Malnutrition and Current Use of Nutrition Support in Patients with Cancer. JPEN. J. Parenter. Enter. Nutr. 2014, 38, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Arends, J. The Causes and Consequences of Cancer-Associated Malnutrition. Eur. J. Oncol. Nurs. 2005, 9 (Suppl. S2), S51–S63. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M. Cancer-Associated Malnutrition. Eur. J. Oncol. Nurs. 2005, 9 (Suppl. S2), S39–S50. [Google Scholar] [CrossRef]
- Harvie, M.N.; Howell, A.; Thatcher, N.; Baildam, A.; Campbell, I. Energy Balance in Patients with Advanced NSCLC, Metastatic Melanoma and Metastatic Breast Cancer Receiving Chemotherapy--a Longitudinal Study. Br. J. Cancer 2005, 92, 673–680. [Google Scholar] [CrossRef][Green Version]
- Rier, H.N.; Jager, A.; Sleijfer, S.; van Rosmalen, J.; Kock, M.C.J.M.; Levin, M.-D. Low Muscle Attenuation Is a Prognostic Factor for Survival in Metastatic Breast Cancer Patients Treated with First Line Palliative Chemotherapy. Breast 2017, 31, 9–15. [Google Scholar] [CrossRef]
- Solomayer, E.-F.; Braun, E.-M.; Zimmermann, J.S.M.; Radosa, J.C.; Stroeder, J.; Endrikat, J.; Gerlinger, C. Muscle Mass Loss in Patients with Metastatic Breast Cancer. Arch. Gynecol. Obstet. 2019, 300, 201–206. [Google Scholar] [CrossRef]
- Aleixo, G.F.P.; Shachar, S.S.; Deal, A.M.; Nyrop, K.A.; Muss, H.B.; Chen, Y.T.; Yu, H.; Williams, G.R. The Association of Body Composition Parameters and Adverse Events in Women Receiving Chemotherapy for Early Breast Cancer. Breast Cancer Res. Treat. 2020, 182, 631–642. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Isenring, E.; Cross, G.; Daniels, L.; Kellett, E.; Koczwara, B. Validity of the Malnutrition Screening Tool as an Effective Predictor of Nutritional Risk in Oncology Outpatients Receiving Chemotherapy. Support. Care Cancer 2006, 14, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Jager-Wittenaar, H.; Ottery, F.D. Assessing Nutritional Status in Cancer: Role of the Patient-Generated Subjective Global Assessment. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 322–329. [Google Scholar] [CrossRef]
- NEMO Estimating Energy, Protein & Fluid Requirements for Adult Clinical Conditions. Available online: https://www.health.qld.gov.au/__data/assets/pdf_file/0022/144175/est-req.pdf (accessed on 5 December 2022).
- Chung, H.; Cobzas, D.; Lieffers, J.; Birdsell, L.; Baracos, V. Automated Segmentation of Muscle and Adipose Tissue on CT Images for Human Body Composition Analysis. In Medical Imaging 2009: Visualization, Image-Guided Procedures, and Modeling; SPIE: Bellingham, WA, USA, 2009; Volume 7261. [Google Scholar]
- Mourtzakis, M.; Prado, C.M.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A Practical and Precise Approach to Quantification of Body Composition in Cancer Patients Using Computed Tomography Images Acquired during Routine Care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetic: Champaign, IL, USA, 1998; ISBN 0-88011-623-4. [Google Scholar]
- Jones, L.W.; Hornsby, W.E.; Goetzinger, A.; Forbes, L.M.; Sherrard, E.L.; Quist, M.; Lane, A.T.; West, M.; Eves, N.D.; Gradison, M.; et al. Prognostic Significance of Functional Capacity and Exercise Behavior in Patients with Metastatic Non-Small Cell Lung Cancer. Lung Cancer 2012, 76, 248–252. [Google Scholar] [CrossRef]
- Kasymjanova, G.; Correa, J.A.; Kreisman, H.; Dajczman, E.; Pepe, C.; Dobson, S.; Lajeunesse, L.; Sharma, R.; Small, D. Prognostic Value of the Six-Minute Walk in Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2009, 4, 602–607. [Google Scholar] [CrossRef]
- Amireault, S.; Godin, G. The Godin-Shephard Leisure-Time Physical Activity Questionnaire: Validity Evidence Supporting Its Use for Classifying Healthy Adults into Active and Insufficiently Active Categories. Percept. Mot. Skills 2015, 120, 604–622. [Google Scholar] [CrossRef]
- Amireault, S.; Godin, G.; Lacombe, J.; Sabiston, C.M. The Use of the Godin-Shephard Leisure-Time Physical Activity Questionnaire in Oncology Research: A Systematic Review. BMC Med. Res. Methodol. 2015, 15, 60. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer Cachexia in the Age of Obesity: Skeletal Muscle Depletion Is a Powerful Prognostic Factor, Independent of Body Mass Index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Deluche, E.; Leobon, S.; Desport, J.C.; Venat-Bouvet, L.; Usseglio, J.; Tubiana-Mathieu, N. Impact of Body Composition on Outcome in Patients with Early Breast Cancer. Support. Care Cancer 2018, 26, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.; Fayers, P.; Aaronson, N.; Bottomley, A.; de Graeff, A.; Grønvold, M.; Gundy, C.; Koller, M.; Petersen, M.; Sprangers, M. EORTC QLQ-C30 Reference Values, 2nd ed.; EORTC Quality of Life Group: Brussels, Belgium, 2008. [Google Scholar]
- Sheean, P.; Gomez-Perez, S.; Joyce, C.; Vasilopoulos, V.; Bartolotta, M.B.; Robinson, P.; Lo, S.; Lomasney, L. Body Composition, Serum Biomarkers of Inflammation and Quality of Life in Clinically Stable Women with Estrogen Receptor Positive Metastatic Breast Cancer. Nutr. Cancer 2019, 71, 981–991. [Google Scholar] [CrossRef] [PubMed]
- de Kruif, A.J.; Westerman, M.J.; Winkels, R.M.; Koster, M.S.; van der Staaij, I.M.; van den Berg, M.M.G.A.; de Vries, J.H.M.; de Boer, M.R.; Kampman, E.; Visser, M. Exploring Changes in Dietary Intake, Physical Activity and Body Weight during Chemotherapy in Women with Breast Cancer: A Mixed-Methods Study. J. Hum. Nutr. Diet. 2021, 34, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and Clinical Implications of Sarcopenic Obesity in Patients with Solid Tumours of the Respiratory and Gastrointestinal Tracts: A Population-Based Study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.M.; Power, D.G.; Daly, L.; Cushen, S.J.; Ní Bhuachalla, Ē.; Prado, C.M. Cancer-Associated Malnutrition, Cachexia and Sarcopenia: The Skeleton in the Hospital Closet 40 Years Later. Proc. Nutr. Soc. 2016, 75, 199–211. [Google Scholar] [CrossRef]
- Bering, T.; Maurício, S.F.; da Silva, J.B.; Correia, M.I.T.D. Nutritional and Metabolic Status of Breast Cancer Women. Nutr. Hosp. 2014, 31, 751–758. [Google Scholar] [CrossRef]
- Mansour, F.; Mekhancha, D.; Kadi, H.; Yagoubi-Benatallah, L.; Karoune, K.; Colette-Dahel-Mekhancha, C.; Nezzal, L. Malnutrition in Patients with Breast Cancer during Treatments (Algeria, 2016). Nutr. Clin. Métabol. 2018, 32, 129–137. [Google Scholar] [CrossRef]
- Oostra, D.L.; Burse, N.R.; Wolf, L.J.; Schleicher, E.; Mama, S.K.; Bluethmann, S.; Schmitz, K.; Winkels, R.M. Understanding Nutritional Problems of Metastatic Breast Cancer Patients: Opportunities for Supportive Care Through EHealth. Cancer Nurs. 2021, 44, 154–162. [Google Scholar] [CrossRef]
- McClelland, S.I.; Holland, K.J.; Griggs, J.J. Quality of Life and Metastatic Breast Cancer: The Role of Body Image, Disease Site, and Time since Diagnosis. Qual. Life Res. 2015, 24, 2939–2943. [Google Scholar] [CrossRef]
- Ruden, E.; Reardon, D.A.; Coan, A.D.; Herndon, J.E., 2nd; Hornsby, W.E.; West, M.; Fels, D.R.; Desjardins, A.; Vredenburgh, J.J.; Waner, E.; et al. Exercise Behavior, Functional Capacity, and Survival in Adults with Malignant Recurrent Glioma. J. Clin. Oncol. 2011, 29, 2918–2923. [Google Scholar] [CrossRef] [PubMed]
- Ammitzbøll, G.; Søgaard, K.; Karlsen, R.V.; Tjønneland, A.; Johansen, C.; Frederiksen, K.; Bidstrup, P. Physical Activity and Survival in Breast Cancer. Eur. J. Cancer 2016, 66, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Delrieu, L.; Jacquet, E.; Segura-Ferlay, C.; Blanc, E.; Febvey-Combes, O.; Friedenreich, C.; Romieu, G.; Jacot, W.; Rios, M.; Heudel, P.-E.; et al. Analysis of the StoRM Cohort Reveals Physical Activity to Be Associated with Survival in Metastatic Breast Cancer. Sci. Rep. 2020, 10, 10757. [Google Scholar] [CrossRef] [PubMed]
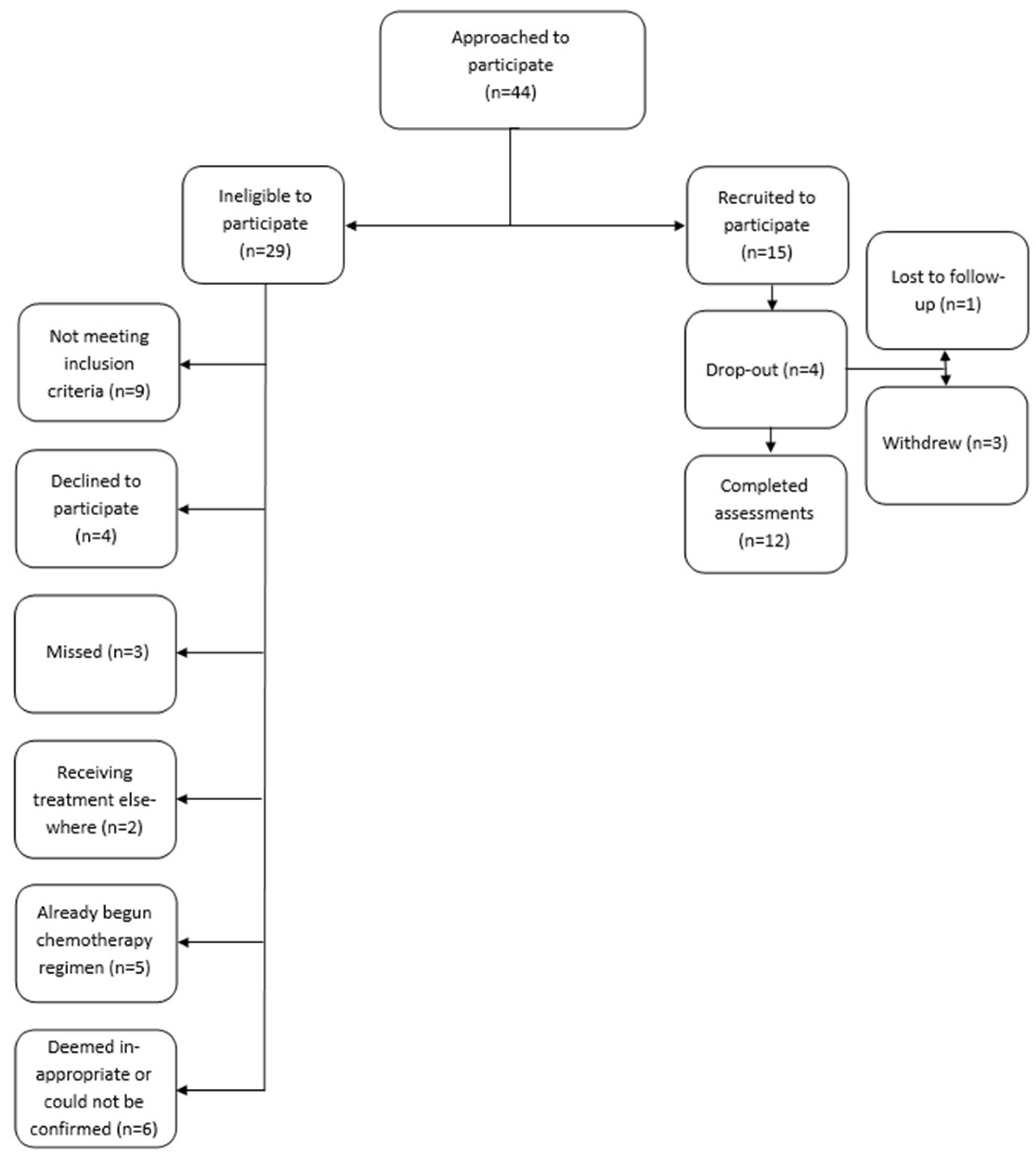
| Characteristic 1 | n = 15 |
|---|---|
| Age (y) | 53.73 (13.5) Range 33–76 |
| Height (m) | 1.62 (0.07) |
| Body Weight (kg) | 78.3 (18.2) |
| BMI (kg/m2) | 29.8 (7.4) |
| BMI categories, n (%) | |
| Underweight | 0 (0) |
| Normal weight | 5 (33.3) |
| Overweight | 3 (20.0) |
| Obese | 7 (46.7) |
| Race, n (%) | |
| Caucasian | 11 (73.3) |
| Asian | 2 (13.3) |
| Pacific Islander | 2 (13.3) |
| Treatment pathway, n (%) | |
| Oral | 1 (6.7) |
| Intravenous | 14 (93.3) |
| Chemotherapy agents | |
| Taxane | 4 |
| Anthracycline | 2 |
| Capecitabine | 2 |
| Trastuzumab | 3 |
| Carboplatin gemcitabine | 1 |
| Eribulin mesylate | 2 |
| Nutritional Supplements 2, n (%) | |
| Yes | 6 (40.0) |
| No | 9 (60.0) |
| Steroids, n (%) | |
| High dose | 0 (0.0) |
| Low dose | 2 (13.3) |
| Mean (SD) or n (%) | n = 15 |
|---|---|
| Dietary Intake | |
| Energy (% of requirements) mean (SD) | 57.9 (22.6) |
| Protein (% of requirements) mean (SD) | 58.2 (20.3) |
| Body Composition | |
| Bioimpedance Spectroscopy | |
| Fat free mass (kg) mean (SD) | 52.4 (8.4) |
| Fat free mass index (kg/m2) mean (SD) | 20.0 (3.6) |
| Fat mass (%) mean (SD) | 31.9 (7.2) |
| ICF (I) mean (SD) | 22.1 (3.8) |
| ECF (I) mean (SD) | 16.3 (2.5) |
| CT-scans | |
| Skeletal muscle (cm2) mean (SD) | 114.7 (16.4) |
| Sarcopenia n (%) | 3 (20.0) |
| IMAT (cm2) mean (SD) | 6.6 (4.0) |
| SAT (cm2) mean (SD) | 272.2 (108.5) |
| VAT (cm2) mean (SD) | 113.3 (71.8) |
| Radiodensity—skeletal muscle (HU) mean (SD) | 35.3 (7.0) |
| Muscle strength | |
| Handgrip strength (kg) | 27.0 (5.5) |
| Physical function | |
| 6 min walk test (m) | 480.2 (86.0) |
| 6 min walk test below cut off n (%) | 4 (26.7) |
| LAS median (range) | 6 (0–76) |
| Quality of life | |
| Global quality of life | 56.7 (27.3) |
| Physical function | 74.2 (19.7) |
| n (%) | Baseline n = 15 | 3 wks n = 14 | 6 wks n = 14 | 12 wks n = 5 | 26 wks n = 2 | 52 wks n = 5 |
|---|---|---|---|---|---|---|
| Nutritional status | ||||||
| Malnutrition screening (MST) | ||||||
| At risk (%) | 5 (33.3) | 2 (14.3) | 5 (35.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Malnutrition assessment (PG-SGA) | ||||||
| A—well nourished | 12 (80.0) | 12 (85.7) | 11 (78.6) | 5 (100.0) | n/a | 4 (80.0) |
| B—moderately malnourished | 3 (20.0) | 2 (14.3) | 3 (21.4) | 0 (0.0) | n/a | 1 (20.0) |
| C—severely malnourished | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | n/a | 0 (0.0) |
| Dietary intake | ||||||
| Met energy requirements | 2 (13.3) | 3 (21.4) | 2 (14.3) | 0 (0.0) | 0 (0.0) | 1 (20.0) |
| Met protein requirements | 2 (13.3) | 2 (14.3) | 3 (21.4) | 0 (0.0) | 0 (0.0) | 1 (20.0) |
| Body Composition | ||||||
| IMAT above cut-off (myosteatosis) | 11 (73.3) | n/a | 10 (71.4) | 4 (80.0) | 2 (100.0) | 5 (100.0) |
| Skeletal muscle radiodensity below cut-off (myosteatosis) | 7 (46.7%) | n/a | 7 (50.0) | 2 (40.0) | 2 (100.0) | 4 (50.0) |
| Sarcopenia (EWGSOP-criteria) | 1 (6.7) | 1 (7.1) | 2 (14.3) | 1 (20.0) | 1 (50.0) | 0 (0.0) |
| Sarcopenia (CT-scans) | 3 (20.0) | n/a | 2 (14.3) | 3 (60.0) | 0 (0.0) | 2 (40.0) |
| Physical function | ||||||
| Handgrip strength below cut-off | 0 (0.0) | 1 (7.1) | 1 (7.1) | 1 (20.0) | 1 (50.0) | 1 (20.0) |
| Walk test below cut-off | 4 (26.7) | 5 (35.7) | 4 (28.6) | 3 (60.0) | 1 (50.0) | 3 (60.0) |
| LAS below cut-off | 11 (73.3) | 10 (78.6) | 11 (78.6) | 3 (60.0) | 0 (0.0) | 3 (60.0) |
| Quality of life | ||||||
| Global QoL below cut-off | 9 (60.0) | 6 (42.9) | 9 (64.3) | 5 (100.0) | 2 (100.0) | 3 (60.0) |
| Physical function below cut-off | 10 (66.7) | 7 (50.0) | 8 (57.1) | 4 (80.0) | 1 (50.0) | 4 (80.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parkinson, J.; Bandera, A.; Crichton, M.; Shannon, C.; Woodward, N.; Hodgkinson, A.; Millar, L.; Teleni, L.; van der Meij, B.S. Poor Muscle Status, Dietary Protein Intake, Exercise Levels, Quality of Life and Physical Function in Women with Metastatic Breast Cancer at Chemotherapy Commencement and during Follow-Up. Curr. Oncol. 2023, 30, 688-703. https://doi.org/10.3390/curroncol30010054
Parkinson J, Bandera A, Crichton M, Shannon C, Woodward N, Hodgkinson A, Millar L, Teleni L, van der Meij BS. Poor Muscle Status, Dietary Protein Intake, Exercise Levels, Quality of Life and Physical Function in Women with Metastatic Breast Cancer at Chemotherapy Commencement and during Follow-Up. Current Oncology. 2023; 30(1):688-703. https://doi.org/10.3390/curroncol30010054
Chicago/Turabian StyleParkinson, Jessica, Amelia Bandera, Megan Crichton, Catherine Shannon, Natasha Woodward, Adam Hodgkinson, Luke Millar, Laisa Teleni, and Barbara S. van der Meij. 2023. "Poor Muscle Status, Dietary Protein Intake, Exercise Levels, Quality of Life and Physical Function in Women with Metastatic Breast Cancer at Chemotherapy Commencement and during Follow-Up" Current Oncology 30, no. 1: 688-703. https://doi.org/10.3390/curroncol30010054
APA StyleParkinson, J., Bandera, A., Crichton, M., Shannon, C., Woodward, N., Hodgkinson, A., Millar, L., Teleni, L., & van der Meij, B. S. (2023). Poor Muscle Status, Dietary Protein Intake, Exercise Levels, Quality of Life and Physical Function in Women with Metastatic Breast Cancer at Chemotherapy Commencement and during Follow-Up. Current Oncology, 30(1), 688-703. https://doi.org/10.3390/curroncol30010054





