Why We Should Look at Dinner Plates: Diet Changes in Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Malnutrition Is Common amongst Cancer Patients
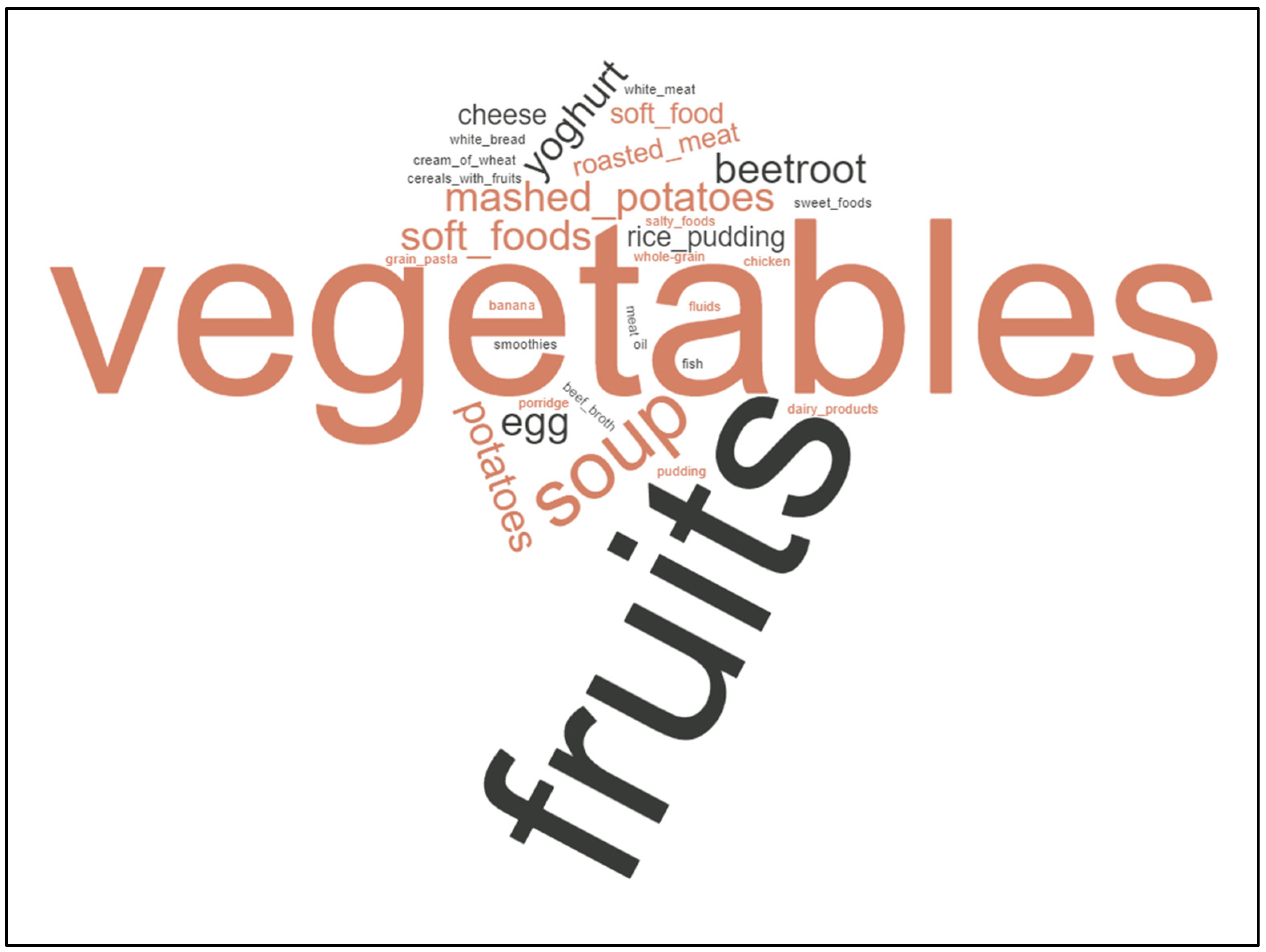
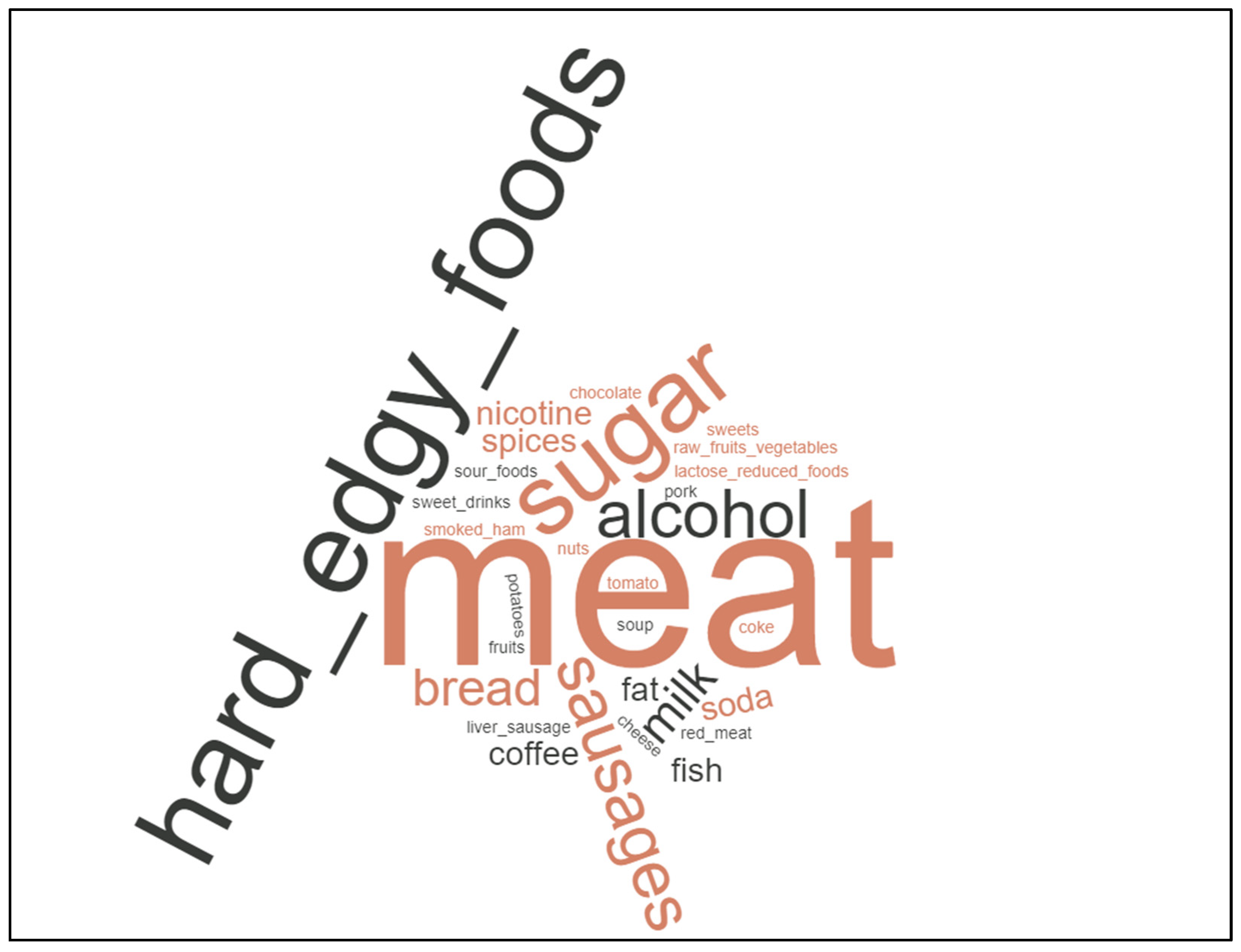
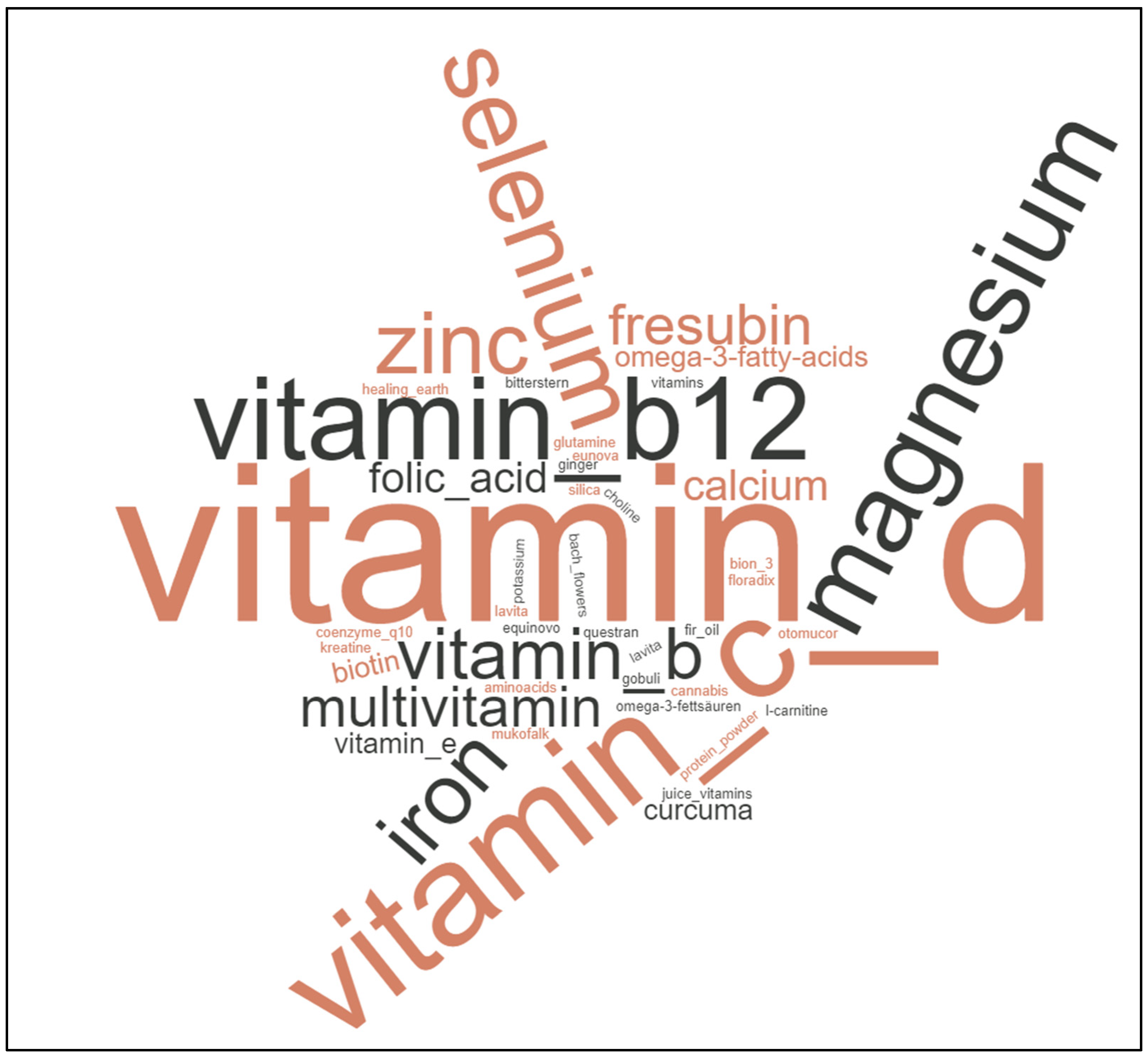
3.2. Diet Changes Are Common amongst Cancer Patients, Not Specific Cancer Diets
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| NRS2002 (Nach Nach Kondrup J et al., Clinical Nutrition 2003; 22: 415–421 [12] ) | |||||||
| Vorscreening | |||||||
| Ist der Body Mass Index < 20,5 kg/m2? | |||||||
| Hat der Patient die letzten 3 Monate an Gewicht verloren | x | ||||||
| War die Nahrungszufuhr in der letzten Woche vermindert? | |||||||
| Ist der Patient schwer erkrankt (z. B. Intensivtherapie)? | |||||||
| Screening | |||||||
| Störung des Ernährungszustandes | Punkte | + | Krankheitsschwere | Punkte | |||
| Keine | 0 | Keine | 0 | ||||
| Mild Gewichtsverlust > 5% in 3 Monaten oder Nahrungszufuhr < 50–75% des Bedarfs in der Vorwoche | 1 | Mild Schenkelhalsfraktur, chronische Erkrankung mit Komplikationen, Krebsleiden | 1 | ||||
| Mäßig Gewichtsverlust > 5% in 2 Monaten oder BMI 18,5–20,5 kg/m2 und reduzierter Allgemeinzustand oder Nahrungszufuhr 20–25% des Bedarfs in der Vorwoche | 2 | Mild Große Bauchchirurgie, Schlaganfall, Pneumonie, hämatologische Krebserkrankung | 2 | ||||
| Schwer Gewichtsverlust >5% in 1 Monat oder BMI < 18,5 kg/m2 und reduzierter Allgemeinzustand oder Nahrungszufuhr 0–25% des Bedarfs in der Vorwoche | 3 | Schwer Kopfverletzung, Knochenmark-transplantation, Intensivpflichtige Patienten | 3 | ||||
| +1 Punkt, wenn Alter 70 Jahre | |||||||
| 3 Punkte | Risiko für Malnutrition liegt vor, Erstellung eines Ernährungsplans | ||||||
| 3 Punkte | wöchentlich Screening wiederholen | ||||||
| Haben Sie Ihre Ernährungsgewohnheiten verändert, seitdem Sie von Ihrer Krebsdiagnose wissen? | |||||||
| Ja | Nein | ||||||
| Verzichten oder vermeiden Sie bestimme Nahrungsmittel? | JA | NEIN | Bevor Sie mit einer Krebsdiöt beginnen, sprechen Sie bitte mit Ihrer/m HausärztIn oder behandelnde/n OnkologIn | ||||
| Bevorzugen Sie bestimmte Nahrungsmittel? | JA | NEIN | |||||
| Nehmen Sie Nahrungsergänzungsmittel ein? | JA | NEIN | |||||
| Folgen Sie besonderen Ernährungshinweise/Diätplänen? | JA | NEIN | |||||
References
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef]
- Bossi, P.; Delrio, P.; Mascheroni, A.; Zanetti, M. The Spectrum of Malnutrition/Cachexia/Sarcopenia in Oncology According to Different Cancer Types and Settings: A Narrative Review. Nutrients 2021, 13, 1980. [Google Scholar] [CrossRef]
- Ravasco, P. Nutrition in Cancer Patients. J. Clin. Med. 2019, 8, 1211. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Aapro, M. From guidelines to clinical practice: A roadmap for oncologists for nutrition therapy for cancer patients. Ther. Adv. Med. Oncol. 2019, 11, 1758835919880084. [Google Scholar] [CrossRef]
- Kubrak, C.; Martin, L.; Gramlich, L.; Scrimger, R.; Jha, N.; Debenham, B.; Chua, N.; Walker, J.; Baracos, V.E. Prevalence and prognostic significance of malnutrition in patients with cancers of the head and neck. Clin. Nutr. 2019, 39, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Gillis, C.; Davies, S.J.; Carli, F.; Wischmeyer, P.E.; Wootton, S.A.; Jackson, A.A.; Riedel, B.; Marino, L.V.; Levett, D.Z.H.; West, M.A. Current Landscape of Nutrition Within Prehabilitation Oncology Research: A Scoping Review. Front. Nutr. 2021, 8, 644723. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Wells, L.; Nwulu, U.; Currow, D.; Johnson, M.J.; Skipworth, R.J.E. Validated screening tools for the assessment of cachexia, sarcopenia, and malnutrition: A systematic review. Am. J. Clin. Nutr. 2018, 108, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Capra, S.; Ferguson, M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur. J. Clin. Nutr. 2002, 56, 779–785. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Schmid, A.; Mathies, V.; Buentzel, J.; Keinki, C.; Huebner, J. Diet Changes and Underlying Motives in Cancer Patients. Nutr. Cancer 2021, 74, 2017–2028. [Google Scholar] [CrossRef]
- Büntzel, J.; Büntzel, J. Protocols Related to Nutritional Anamnesis in Head and Neck Cancer Patients. In Basic Protocols in Foods and Nutrition; Springer: Berlin, Germany, 2022; pp. 209–222. [Google Scholar]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.C.; Crozier, J.E.M.; Canna, K.; Angerson, W.J.; McArdle, C.S. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int. J. Color. Dis. 2007, 22, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Nutritionday Worldwide, Country Report Nutrition Day 2021 Germany. Available online: https://www.nutritionday.org/ (accessed on 15 January 2023).
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Ruan, X.; Nakyeyune, R.; Shao, Y.; Shen, Y.; Niu, C.; Zang, Z.; Miles, T.; Liu, F. Nutritional screening tools for adult cancer patients: A hierarchical Bayesian latent-class meta-analysis. Clin. Nutr. 2021, 40, 1733–1743. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef]
- Li, Z.; Chen, W.; Li, H.; Chinese Oncology Nutrition Survey Group. Nutrition support in hospitalized cancer patients with malnutrition in China. Asia. Pac. J. Clin. Nutr. 2018, 27, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Pressoir, M.; Desné, S.; Berchery, D.; Rossignol, G.; Poiree, B.; Meslier, M.; Traversier, S.; Vittot, M.; Simon, M.; Gekiere, J.P.; et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br. J. Cancer 2010, 102, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Lucia, S.; Farcomeni, A.; Lorusso, V.; Saracino, V.; Barone, C.; Plastino, F.; Gori, S.; Magarotto, R.; Carteni, G.; et al. Prevalence of malnutrition in patients at first medical oncology visit: The PreMiO study. Oncotarget 2017, 8, 79884–79896. [Google Scholar] [CrossRef]
- Sanz, E.Á.; Siles, M.G.; Fernández, L.R.; Roldán, R.V.; Domínguez, A.R.; Abilés, J. Nutritional risk and malnutrition rates at diagnosis of cancer in patients treated in outpatient settings: Early intervention protocol. Nutrition 2019, 57, 148–153. [Google Scholar] [CrossRef]
- Bozzetti, F.; The SCRINIO Working Group; Mariani, L.; Vullo, S.L.; Amerio, M.L.; Biffi, R.; Caccialanza, R.; Capuano, G.; Correja, I.; Cozzaglio, L.; et al. The nutritional risk in oncology: A study of 1,453 cancer outpatients. Support. Care Cancer 2012, 20, 1919–1928. [Google Scholar] [CrossRef]
- Büntzel, J.; Krauß, T.; Büntzel, H.; Küttner, K.; Fröhlich, D.; Oehler, W.; Glatzel, M.; Wackes, M.; Putziger, J.; Micke, O.; et al. Nutri-tional parameters for patients with head and neck cancer. Anticancer. Res. 2012, 32, 2119–2123. [Google Scholar]
- Hébuterne, X.; Lemarié, E.; Michallet, M.; De Montreuil, C.B.; Schneider, S.; Goldwasser, F. Prevalence of Malnutrition and Current Use of Nutrition Support in Patients With Cancer. J. Parenter. Enter. Nutr. 2014, 38, 196–204. [Google Scholar] [CrossRef]
- Marshall, K.M.; Loeliger, J.; Nolte, L.; Kelaart, A.; Kiss, N.K. Prevalence of malnutrition and impact on clinical outcomes in cancer services: A comparison of two time points. Clin. Nutr. 2018, 38, 644–651. [Google Scholar] [CrossRef]
- Büntzel, J.; Hübner, J.; Büntzel, J. Komplementärmedizinische Behandlungsansätze bei Inappetenz und Ösophagitis. Der Onkol. 2019, 25, 1110–1117. [Google Scholar] [CrossRef]
- Büntzel, J.; Hübner, J.; Büntzel, J. Komplementärmedizinische Behandlungsansätze bei oraler Mukositis und Xerostomie. Der Onkol. 2019, 25, 269–274. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Dev, R. Measuring cachexia—Diagnostic criteria. Ann. Palliat. Med. 2019, 8, 24–32. [Google Scholar] [CrossRef]
- Pin, F.; Barreto, R.; Couch, M.E.; Bonetto, A.; O’Connell, T.M. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J. Cachexia Sarcopenia Muscle 2019, 10, 140–154. [Google Scholar] [CrossRef] [PubMed]
- von Renesse, J.; von Bechtolsheim, F.; Jonas, S.; Seifert, L.; Alves, T.C.; Seifert, A.M.; Komorek, F.; Tritchkova, G.; Menschikowski, M.; Bork, U.; et al. Tumour catabolism independent of malnutrition and inflammation in upper GI cancer patients revealed by longitudinal metabolomics. J. Cachexia Sarcopenia Muscle 2022, 14, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Le-Rademacher, J.; Lopez, C.; Wolfe, E.; Foster, N.R.; Mandrekar, S.J.; Wang, X.; Kumar, R.; Adjei, A.; Jatoi, A. Weight loss over time and survival: A landmark analysis of 1000+ prospectively treated and monitored lung cancer patients. J. Cachexia Sarcopenia Muscle 2020, 11, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Bullock, A.F.; Greenley, S.L.; McKenzie, G.A.G.; Paton, L.W.; Johnson, M.J. Relationship between markers of malnutrition and clinical outcomes in older adults with cancer: Systematic review, narrative synthesis and meta-analysis. Eur. J. Clin. Nutr. 2020, 74, 1519–1535. [Google Scholar] [CrossRef] [PubMed]
- Erickson, N.; Buchholz, D.; Hübner, J. Aktualisierte Stellungnahme ketogene und kohlenhydratarme Diäten veröffentlicht. Forum 2017, 32, 429–430. [Google Scholar] [CrossRef]
- Zick, S.M.; Snyder, D.; Abrams, D.I. Pros and Cons of Dietary Strategies Popular Among Cancer Patients. Oncol. (Wil-List. Park) 2018, 32, 542–547. [Google Scholar]
- Tajan, M.; Vousden, K.H. Dietary Approaches to Cancer Therapy. Cancer Cell 2020, 37, 767–785. [Google Scholar] [CrossRef]
- Molassiotis, A.; Fernadez-Ortega, P.; Pud, D.; Ozden, G.; Scott, J.A.; Panteli, V.; Margulies, A.; Browall, M.; Magri, M.; Selvekerova, S.; et al. Use of complementary and alternative medicine in cancer patients: A European survey. Ann. Oncol. 2005, 16, 655–663. [Google Scholar] [CrossRef]
- Micke, O.; Bruns, F.; Glatzel, M.; Schönekaes, K.; Micke, P.; Mücke, R.; Büntzel, J. Predictive factors for the use of complementary and alternative medicine (CAM) in radiation oncology. Eur. J. Integr. Med. 2009, 1, 19–25. [Google Scholar] [CrossRef]
- Huebner, J.; Micke, O.; Muecke, R.; Buentzel, J.; Prott, F.J.; Kleeberg, U.; Senf, B.; Muenstedt, K.; PRIO (Working Group Prevention and Integrative Oncology of the German Cancer Society). User rate of complementary and alternative medicine (CAM) of patients visiting a counseling facility for CAM of a German comprehensive cancer center. Anticancer. Res. 2014, 34, 943–948. [Google Scholar]
- Wortmann, J.K.; Bremer, A.; Eich, H.; Wortmann, H.K.; Schuster, A.; Fühner, J.; Büntzel, J.; Muecke, R.; Prott, F.; Huebner, J. Use of complementary and alternative medicine by patients with cancer: A cross-sectional study at different points of cancer care. Med. Oncol. 2016, 33, 1–11. [Google Scholar] [CrossRef]
- Huebner, J.; Muenstedt, K.; Prott, F.-J.; Stoll, C.; Micke, O.; Buentzel, J.; Muecke, R.; Senf, B. Online Survey of Patients with Breast Cancer on Complementary and Alternative Medicine. Breast Care 2014, 9, 60–63. [Google Scholar] [CrossRef]
- Saghatchian, M.; Bihan, C.; Chenailler, C.; Mazouni, C.; Dauchy, S.; Delaloge, S. Exploring frontiers: Use of complementary and alternative medicine among patients with early-stage breast cancer. Breast 2014, 23, 279–285. [Google Scholar] [CrossRef]
- Tang, J.W.C.; Lam, W.W.T.; Ma, A.S.Y.; Law, W.L.; Wei, R.; Fielding, R. Dietary changes adopted by Chinese colorectal cancer patients: A qualitative study. Eur. J. Cancer Care 2019, 28, e13159. [Google Scholar] [CrossRef] [PubMed]
- Harvie, M. Nutritional Supplements and Cancer: Potential Benefits and Proven Harms. Am. Soc. Clin. Oncol. Educ. Book 2014, 34, e478–e486. [Google Scholar] [CrossRef]
- Caccialanza, R.; Lobascio, F.; Cereda, E.; Aprile, G.; Farina, G.; Traclò, F.; Borioli, V.; Caraccia, M.; Turri, A.; De Lorenzo, F.; et al. Cancer-related malnutrition management: A survey among Italian Oncology Units and Patients’ Associations. Curr. Probl. Cancer 2020, 44, 100554. [Google Scholar] [CrossRef]
| Total | 235 | |
|---|---|---|
| Gender | Male | 144 |
| Female | 91 | |
| Age | Median (range) [years] | 65.64 [29.43–88.35] |
| Cohort | Radiation | 97 |
| Hema/Onco | 102 | |
| Head–neck | 36 | |
| Entity | Breast | 17 |
| Other gynecological | 6 | |
| Urooncology | 17 | |
| Head–neck | 46 | |
| Lung | 39 | |
| Colorectal | 8 | |
| Upper gastrointestinal tract | 9 | |
| Cancer of unknown primary | 4 | |
| Hematology malignant | 64 | |
| Hematology benign | 10 | |
| Other | 15 |
| Entity | Pre-Screen Negative [N] | Pre-Screen Positive [N] | p-Value |
|---|---|---|---|
| Breast | 13 | 4 | 0.0093 |
| All | 91 | 127 | |
| Hematology malignant | 27 | 37 | 0.7685 |
| All | 77 | 94 | |
| Head–neck | 24 | 20 | 0.1338 |
| All | 80 | 111 | |
| Lung | 16 | 23 | 0.7257 |
| All | 88 | 108 | |
| Urooncology | 5 | 12 | 0.3106 |
| All | 99 | 119 | |
| Entity | NRS2002 < 3 [N] | NRS2002 ≥ 3 [N] | p-Value |
| Breast | 2 | 2 | 0.5829 |
| All | 35 | 84 | |
| Hematology malignant | 6 | 31 | 0.0326 |
| All | 31 | 55 | |
| Head–neck | 6 | 14 | 1.0000 |
| All | 31 | 72 | |
| Lung | 6 | 17 | 0.8023 |
| All | 31 | 69 | |
| Urooncology | 4 | 8 | 0.7516 |
| All | 33 | 78 |
| Entity | Diet Change: Yes | Diet Change: No | p-Value |
|---|---|---|---|
| Breast | 4 | 13 | 0.4352 |
| Others | 71 | 137 | |
| Hematology malignant | 25 | 38 | 0.2721 |
| Others | 51 | 112 | |
| Head–neck | 18 | 26 | 0.2874 |
| Others | 58 | 124 | |
| Lung | 10 | 29 | 0.2698 |
| Others | 66 | 121 | |
| Urooncology | 4 | 13 | 0.4352 |
| Others | 71 | 137 | |
| Do You Prefer Specific Food? | |||
| Entity | Prefer Foods: Yes | Prefer Foods: No | p-Value |
| Breast | 2 | 15 | 0.5354 |
| Others | 45 | 164 | |
| Hematology malignant | 20 | 43 | 0.0169 |
| Others | 27 | 136 | |
| Head–neck | 11 | 33 | 0.5345 |
| Others | 36 | 146 | |
| Lung | 7 | 32 | 0.8284 |
| Others | 40 | 147 | |
| Urooncology | 1 | 16 | 0.1328 |
| Others | 46 | 161 | |
| Do You Dispense or Avoid Specific Food? | |||
| Entity | Avoid Foods: Yes | Avoid Foods: No | p-Value |
| Breast | 4 | 13 | 1.000 |
| Others | 58 | 151 | |
| Hematology malignant | 17 | 46 | 1.000 |
| Others | 45 | 118 | |
| Head–neck | 17 | 27 | 0.0889 |
| Others | 45 | 137 | |
| Lung | 9 | 30 | 0.8307 |
| Others | 43 | 164 | |
| Urooncology | 4 | 13 | 1.000 |
| Others | 58 | 151 | |
| Do You Take Additional Supplements? | |||
| Entity | Supplement Use | No Supplement Use | p-Value |
| Breast | 12 | 5 | 0.0021 |
| Others | 61 | 138 | |
| Hematology malignant | 20 | 63 | 0.7527 |
| Others | 53 | 100 | |
| Head–neck | 5 | 38 | 0.0012 |
| Others | 67 | 105 | |
| Lung | 12 | 27 | 0.7121 |
| Others | 61 | 116 | |
| Urooncology | 3 | 14 | 0.1856 |
| Others | 70 | 129 | |
| Cohort | Total [N] | Any Diet Change [N] | Preference [N] | Avoidance [N] | Supplements [N] |
|---|---|---|---|---|---|
| All | 235 | 76 | 47 | 62 | 65 |
| Radiation | 97 | 14 | 6 | 11 | 25 |
| Hema/Onco | 102 | 45 | 32 | 35 | 36 |
| Head–Neck | 36 | 17 | 9 | 16 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Döring, K.; Wiechers, L.; Büntzel, J.; Büntzel, J. Why We Should Look at Dinner Plates: Diet Changes in Cancer Patients. Curr. Oncol. 2023, 30, 2715-2728. https://doi.org/10.3390/curroncol30030205
Döring K, Wiechers L, Büntzel J, Büntzel J. Why We Should Look at Dinner Plates: Diet Changes in Cancer Patients. Current Oncology. 2023; 30(3):2715-2728. https://doi.org/10.3390/curroncol30030205
Chicago/Turabian StyleDöring, Katja, Lara Wiechers, Jens Büntzel, and Judith Büntzel. 2023. "Why We Should Look at Dinner Plates: Diet Changes in Cancer Patients" Current Oncology 30, no. 3: 2715-2728. https://doi.org/10.3390/curroncol30030205
APA StyleDöring, K., Wiechers, L., Büntzel, J., & Büntzel, J. (2023). Why We Should Look at Dinner Plates: Diet Changes in Cancer Patients. Current Oncology, 30(3), 2715-2728. https://doi.org/10.3390/curroncol30030205





