Acquired G2032R Resistance Mutation in ROS1 to Lorlatinib Therapy Detected with Liquid Biopsy
Abstract
:1. Introduction
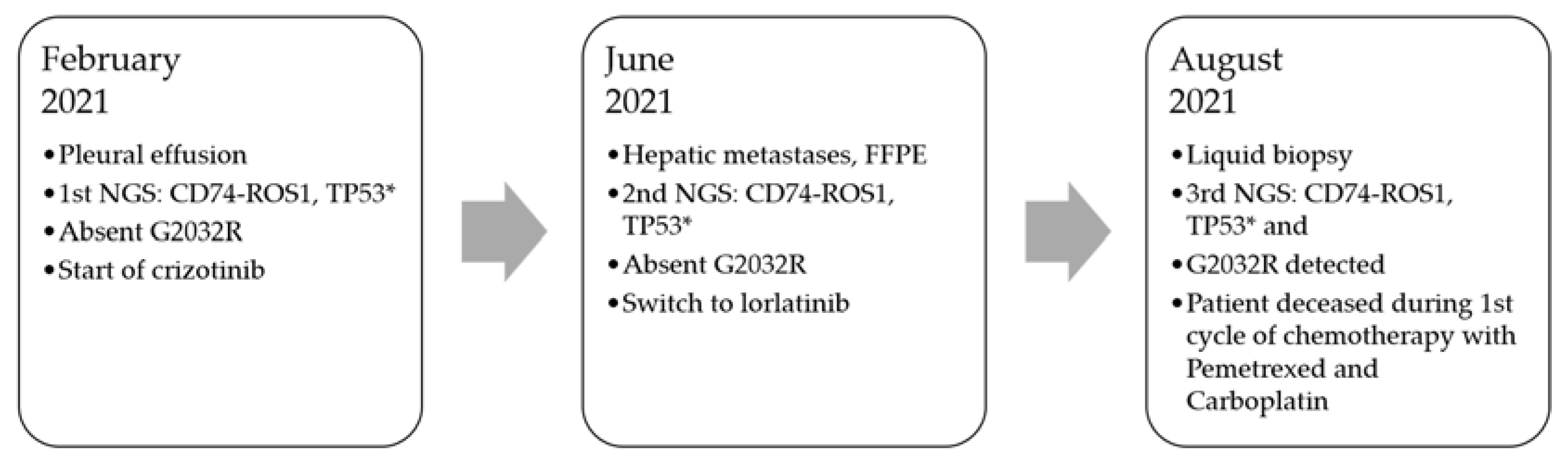
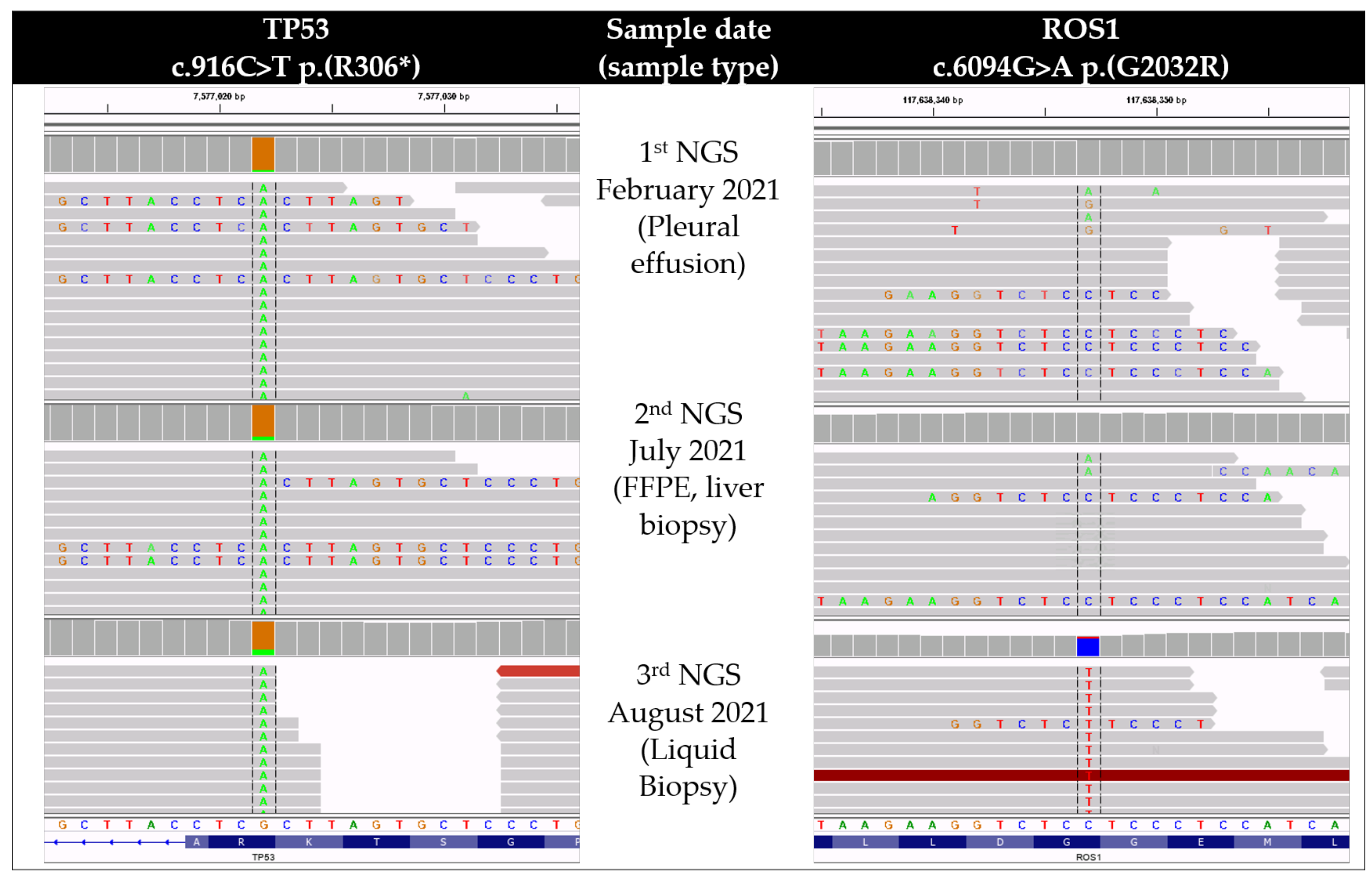
2. Case Presentation
3. Discussion
3.1. Thrombotic Diathesis and Clinical Course
3.2. G2032R Resistance Mutation Development under Lorlatinib
3.3. The Clinical Utility of Liquid Biopsy
3.4. TP53 as a Poor Prognosis Biomarker
3.5. Therapeutic Options after G2032R
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guaitoli, G.; Bertolini, F.; Bettelli, S.; Manfredini, S.; Maur, M.; Trudu, L.; Aramini, B.; Masciale, V.; Grisendi, G.; Dominici, M.; et al. Deepening the Knowledge of ROS1 Rearrangements in Non-Small Cell Lung Cancer: Diagnosis, Treatment, Resistance and Concomitant Alterations. Int. J. Mol. Sci. 2021, 22, 12867. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Shaw, A.T. Recent Advances in Targeting ROS1 in Lung Cancer. J. Thorac. Oncol. 2017, 12, 1611–1625. [Google Scholar] [CrossRef] [PubMed]
- Patil, T.; Smith, D.E.; Bunn, P.A.; Aisner, D.L.; Le, A.T.; Hancock, M.; Purcell, W.T.; Bowles, D.W.; Camidge, D.R.; Doebele, R.C. The Incidence of Brain Metastases in Stage IV ROS1-Rearranged Non-Small Cell Lung Cancer and Rate of Central Nervous System Progression on Crizotinib. J. Thorac. Oncol. 2018, 13, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Tseng, D.; Yoda, S.; Dagogo-Jack, I.; Friboulet, L.; Lin, J.J.; Hubbeling, H.G.; Dardaei, L.; Farago, A.F.; Schultz, K.R.; et al. Patterns of Metastatic Spread and Mechanisms of Resistance to Crizotinib in ROS1-Positive Non-Small-Cell Lung Cancer. JCO Precis. Oncol. 2017, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Choudhury, N.J.; Yoda, S.; Zhu, V.W.; Johnson, T.W.; Sakhtemani, R.; Dagogo-Jack, I.; Digumarthy, S.R.; Lee, C.; Do, A.; et al. Spectrum of Mechanisms of Resistance to Crizotinib and Lorlatinib in ROS1 Fusion-Positive Lung Cancer. Clin. Cancer Res. 2021, 27, 2899–2909. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.Y.; Li, Q.; Engstrom, L.D.; West, M.; Appleman, V.; Wong, K.A.; McTigue, M.; Deng, Y.L.; Liu, W.; Brooun, A.; et al. PF-06463922 is a potent and selective next-generation ROS1/ALK inhibitor capable of blocking crizotinib-resistant ROS1 mutations. Proc. Natl. Acad. Sci. USA 2015, 112, 3493–3498. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.M.; Shaw, A.T.; Johnson, M.L.; Navarro, A.; Gainor, J.F.; Thurm, H.; Pithavala, Y.K.; Abbattista, A.; Peltz, G.; Felip, E. Brain Penetration of Lorlatinib: Cumulative Incidences of CNS and Non-CNS Progression with Lorlatinib in Patients with Previously Treated ALK-Positive Non-Small-Cell Lung Cancer. Target. Oncol. 2020, 15, 55–65. [Google Scholar] [CrossRef]
- Hochmair, M.J.; Fabikan, H.; Illini, O.; Weinlinger, C.; Setinek, U.; Krenbek, D.; Prosch, H.; Rauter, M.; Schumacher, M.; Wöll, E.; et al. Later-Line Treatment with Lorlatinib in ALK- and ROS1-Rearrangement-Positive NSCLC: A Retrospective, Multicenter Analysis. Pharmaceuticals 2020, 13, 371. [Google Scholar] [CrossRef]
- Landi, L.; Tiseo, M.; Heukamp, L.C.; Menon, R.; Spitaleri, G.; Cortinovis, D.L.; Delmonte, A.; Galetta, D.; D’Arcangelo, M.; D’Incà, F.; et al. Secondary ROS1 mutations and lorlatinib sensitivity in crizotinib-refractory ROS1 positive NSCLC: Results of the prospective PFROST trial. Ann. Oncol. 2019, 30 (Suppl. 5), v602–v660. [Google Scholar] [CrossRef]
- Keddy, C.; Shinde, P.; Jones, K.; Kaech, S.; Somwar, R.; Shinde, U.; Davare, M.A. Resistance Profile and Structural Modeling of Next-Generation ROS1 Tyrosine Kinase Inhibitors. Mol. Cancer Ther. 2022, 21, 336–346. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, W.; Zeng, L.; Mi, J.; Song, L.; Lizaso, A.; Mao, X.; Yang, N.; Zhang, Y. A novel ROS1 G2032 K missense mutation mediates lorlatinib resistance in a patient with ROS1-rearranged lung adenocarcinoma but responds to nab-paclitaxel plus pembrolizumab. Lung Cancer 2020, 143, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Frost, N.; Christopoulos, P.; Kauffmann-Guerrero, D.; Stratmann, J.; Riedel, R.; Schaefer, M.; Alt, J.; Gütz, S.; Christoph, D.C.; Laack, E.; et al. Lorlatinib in pretreated ALK- or ROS1-positive lung cancer and impact of TP53 co-mutations: Results from the German early access program. Ther. Adv. Med. Oncol. 2021, 13, 1758835920980558. [Google Scholar] [CrossRef] [PubMed]
- Roeper, J.; Falk, M.; Chalaris-Rißmann, A.; Lueers, A.C.; Ramdani, H.; Wedeken, K.; Stropiep, U.; Diehl, L.; Tiemann, M.; Heukamp, L.C.; et al. TP53 co-mutations in EGFR mutated patients in NSCLC stage IV: A strong predictive factor of ORR, PFS and OS in EGFR mt+ NSCLC. Oncotarget 2020, 11, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Roeper, J.; Christopoulos, P.; Falk, M.; Heukamp, L.C.; Tiemann, M.; Stenzinger, A.; Thomas, M.; Griesinger, F. TP53 co-mutations as an independent prognostic factor in 2nd and further line therapy-EGFR mutated non-small cell lung cancer IV patients treated with osimertinib. Transl. Lung Cancer Res. 2022, 11, 4–13. [Google Scholar] [CrossRef]
- Yu, Y.; Ou, Q.; Wu, X.; Bao, H.; Ding, Y.; Shao, Y.W.; Lu, S. Concomitant resistance mechanisms to multiple tyrosine kinase inhibitors in ALK-positive non-small cell lung cancer. Lung Cancer 2019, 127, 19–24. [Google Scholar] [CrossRef]
- Song, P.; Zhang, F.; Li, Y.; Yang, G.; Li, W.; Ying, J.; Gao, S. Concomitant TP53 mutations with response to crizotinib treatment in patients with ALK-rearranged non-small-cell lung cancer. Cancer Med. 2019, 8, 1551–1557. [Google Scholar] [CrossRef]
- Wang, W.X.; Xu, C.W.; Chen, Y.P.; Liu, W.; Zhong, L.H.; Chen, F.F.; Zhuang, W.; Huang, Y.J.; Huang, Z.Z.; Chen, R.R.; et al. TP53 mutations predict for poor survival in ALK rearrangement lung adenocarcinoma patients treated with crizotinib. J. Thorac. Dis. 2018, 10, 2991–2998. [Google Scholar] [CrossRef]
- Kron, A.; Alidousty, C.; Scheffler, M.; Merkelbach-Bruse, S.; Seidel, D.; Riedel, R.; Ihle, M.A.; Michels, S.; Nogova, L.; Fassunke, J.; et al. Impact of TP53 mutation status on systemic treatment outcome in ALK-rearranged non-small-cell lung cancer. Ann. Oncol. 2018, 29, 2068–2075. [Google Scholar] [CrossRef]
- Costa, D.B. TP53 mutations are predictive and prognostic when co-occurring with ALK rearrangements in lung cancer. Ann. Oncol. 2018, 29, 2028–2030. [Google Scholar] [CrossRef]
- Gen, L.; Xu, H.; Zhao, J.; Kong, J.; Ai, X.; Yu, F.; Du, K.; Zhu, L.; Li, L.; Ma, H.; et al. Concurrent TP53 mutation adversely impact the efficacy of crizotinib in ROS1-rearranged lung cancer patients. J. Clin. Oncol. 2019, 37, e20535. [Google Scholar] [CrossRef]
- Rolfo, C.; Mack, P.C.; Scagliotti, G.V.; Baas, P.; Barlesi, F.; Bivona, T.G.; Herbst, R.S.; Mok, T.S.; Peled, N.; Pirker, R.; et al. Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC. J. Thorac. Oncol. 2018, 13, 1248–1268. [Google Scholar] [CrossRef] [PubMed]
- Zhu, V.W.; Zhao, J.J.; Gao, Y.; Syn, N.L.; Zhang, S.S.; Ou, S.I.; Bauer, K.A.; Nagasaka, M. Thromboembolism in ALK+ and ROS1+ NSCLC patients: A systematic review and meta-analysis. Lung Cancer 2021, 157, 147–155. [Google Scholar] [CrossRef]
- Zugazagoitia, J.; Biosca, M.; Oliveira, J.; Olmedo, M.E.; Dómine, M.; Nadal, E.; Ruffinelli, J.C.; Muñoz, N.; Luna, A.M.; Hernández, B.; et al. Incidence, predictors and prognostic significance of thromboembolic disease in patients with advanced ALK-rearranged non-small cell lung cancer. Eur. Respir. J. 2018, 51, 1702431. [Google Scholar] [CrossRef] [PubMed]
- Schatz, S.; Falk, M.; Jóri, B.; Ramdani, H.O.; Schmidt, S.; Willing, E.M.; Menon, R.; Groen, H.J.M.; Diehl, L.; Kröger, M.; et al. Integration of Tumor Mutation Burden and PD-L1 Testing in Routine Laboratory Diagnostics in Non-Small Cell Lung Cancer. Cancers 2020, 12, 1685. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.N.; Falk, M.; Talwar, J.; Neemann, N.; Mariotti, E.; Bertrand, M.; Zacherle, T.; Lakis, S.; Menon, R.; Gloeckner, C.; et al. Concordance between Comprehensive Cancer Genome Profiling in Plasma and Tumor Specimens. J. Thorac. Oncol. 2017, 12, 1503–1511. [Google Scholar] [CrossRef]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Math, M.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892, Erratum in N. Engl. J. Med. 2012, 367, 976. [Google Scholar] [CrossRef]
- Ramón, Y.; Cajal, S.; Sesé, M.; Capdevila, C.; Aasen, T.; De Mattos-Arruda, L.; Diaz-Cano, S.J.; Hernández-Losa, J.; Castellví, J. Clinical implications of intratumor heterogeneity: Challenges and opportunities. J. Mol. Med. 2020, 98, 161–177. [Google Scholar] [CrossRef]
- Lakis, S.; Heukamp, L.C.; Griesinger, F. Detection of Discrepant Driver Mutations in a Patient with Two Synchronous Primary Non-Small Cell Lung Cancers (NSCLCs) with Liquid Biopsy. J. Thorac. Oncol. 2017, 12, e186–e188. [Google Scholar] [CrossRef]
- Russano, M.; Napolitano, A.; Ribelli, G.; Iuliani, M.; Simonetti, S.; Citarella, F.; Pantano, F.; Dell’Aquila, E.; Anesi, C.; Silvestris, N.; et al. Liquid biopsy and tumor heterogeneity in metastatic solid tumors: The potentiality of blood samples. J. Exp. Clin. Cancer Res. 2020, 39, 95, Erratum in J. Exp. Clin. Cancer Res. 2020, 39, 120. [Google Scholar] [CrossRef]
- Shaw, A.T.; Riely, G.J.; Bang, Y.J.; Kim, D.W.; Camidge, D.R.; Solomon, B.J.; Varella-Garcia, M.; Iafrate, A.J.; Shapiro, G.I.; Usari, T.; et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): Updated results, including overall survival, from PROFILE 1001. Ann. Oncol. 2019, 30, 1121–1126. [Google Scholar] [CrossRef]
- Christopoulos, P.; Kirchner, M.; Bozorgmehr, F.; Endris, V.; Elsayed, M.; Budczies, J.; Ristau, J.; Penzel, R.; Herth, F.J.; Heussel, C.P.; et al. Identification of a highly lethal V3+ TP53+ subset in ALK+ lung adenocarcinoma. Int. J. Cancer 2019, 144, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Katayama, R.; Gong, B.; Togashi, N.; Miyamoto, M.; Kiga, M.; Iwasaki, S.; Kamai, Y.; Tominaga, Y.; Takeda, Y.; Kagoshima, Y.; et al. The new-generation selective ROS1/NTRK inhibitor DS-6051b overcomes crizotinib resistant ROS1-G2032R mutation in preclinical models. Nat. Commun. 2019, 10, 3604. [Google Scholar] [CrossRef] [PubMed]
- Yun, M.R.; Kim, D.H.; Kim, S.Y.; Joo, H.S.; Lee, Y.W.; Choi, H.M.; Park, C.W.; Heo, S.G.; Kang, H.N.; Lee, S.S.; et al. Repotrectinib Exhibits Potent Antitumor Activity in Treatment-Naïve and Solvent-Front-Mutant ROS1-Rearranged Non-Small Cell Lung Cancer. Clin. Cancer Res. 2020, 26, 3287–3295. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Borazanci, E.; Shaw, A.T.; Katayama, R.; Shimizu, Y.; Zhu, V.W.; Sun, T.Y.; Wakelee, H.A.; Madison, R.; Schrock, A.B.; et al. U.S. Phase I First-in-human Study of Taletrectinib (DS-6051b/AB-106), a ROS1/TRK Inhibitor, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2020, 26, 4785–4794. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jóri, B.; Falk, M.; Hövel, I.; Weist, P.; Tiemann, M.; Heukamp, L.C.; Griesinger, F. Acquired G2032R Resistance Mutation in ROS1 to Lorlatinib Therapy Detected with Liquid Biopsy. Curr. Oncol. 2022, 29, 6628-6634. https://doi.org/10.3390/curroncol29090520
Jóri B, Falk M, Hövel I, Weist P, Tiemann M, Heukamp LC, Griesinger F. Acquired G2032R Resistance Mutation in ROS1 to Lorlatinib Therapy Detected with Liquid Biopsy. Current Oncology. 2022; 29(9):6628-6634. https://doi.org/10.3390/curroncol29090520
Chicago/Turabian StyleJóri, Balázs, Markus Falk, Iris Hövel, Peggy Weist, Markus Tiemann, Lukas C. Heukamp, and Frank Griesinger. 2022. "Acquired G2032R Resistance Mutation in ROS1 to Lorlatinib Therapy Detected with Liquid Biopsy" Current Oncology 29, no. 9: 6628-6634. https://doi.org/10.3390/curroncol29090520
APA StyleJóri, B., Falk, M., Hövel, I., Weist, P., Tiemann, M., Heukamp, L. C., & Griesinger, F. (2022). Acquired G2032R Resistance Mutation in ROS1 to Lorlatinib Therapy Detected with Liquid Biopsy. Current Oncology, 29(9), 6628-6634. https://doi.org/10.3390/curroncol29090520





