Neoadjuvant Immunotherapy Combined with Chemotherapy for Local Advanced Non-Small-Cell Lung Cancer in a Patient with a History of Breast Cancer: A Case Report
Abstract
1. Introduction
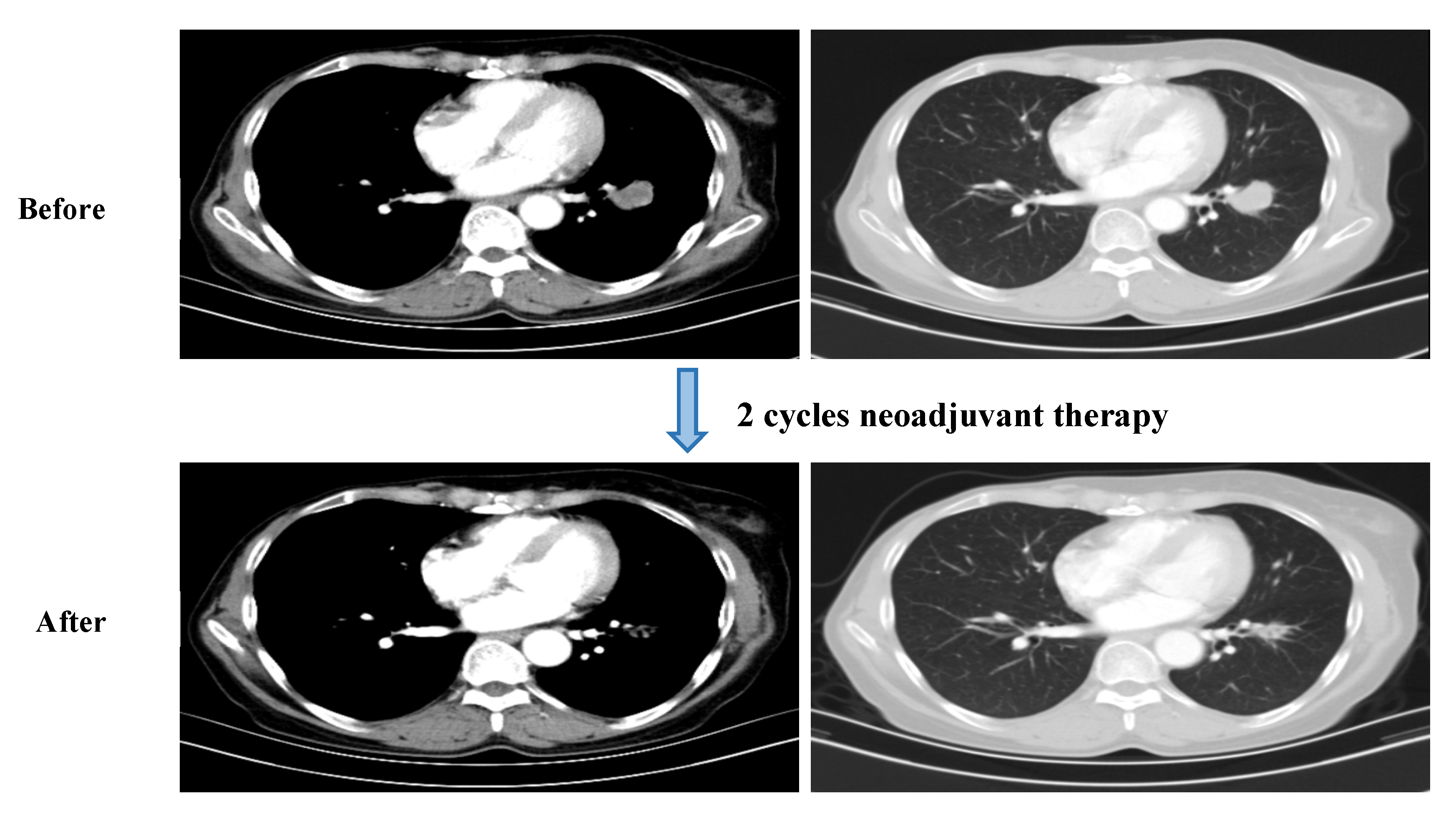
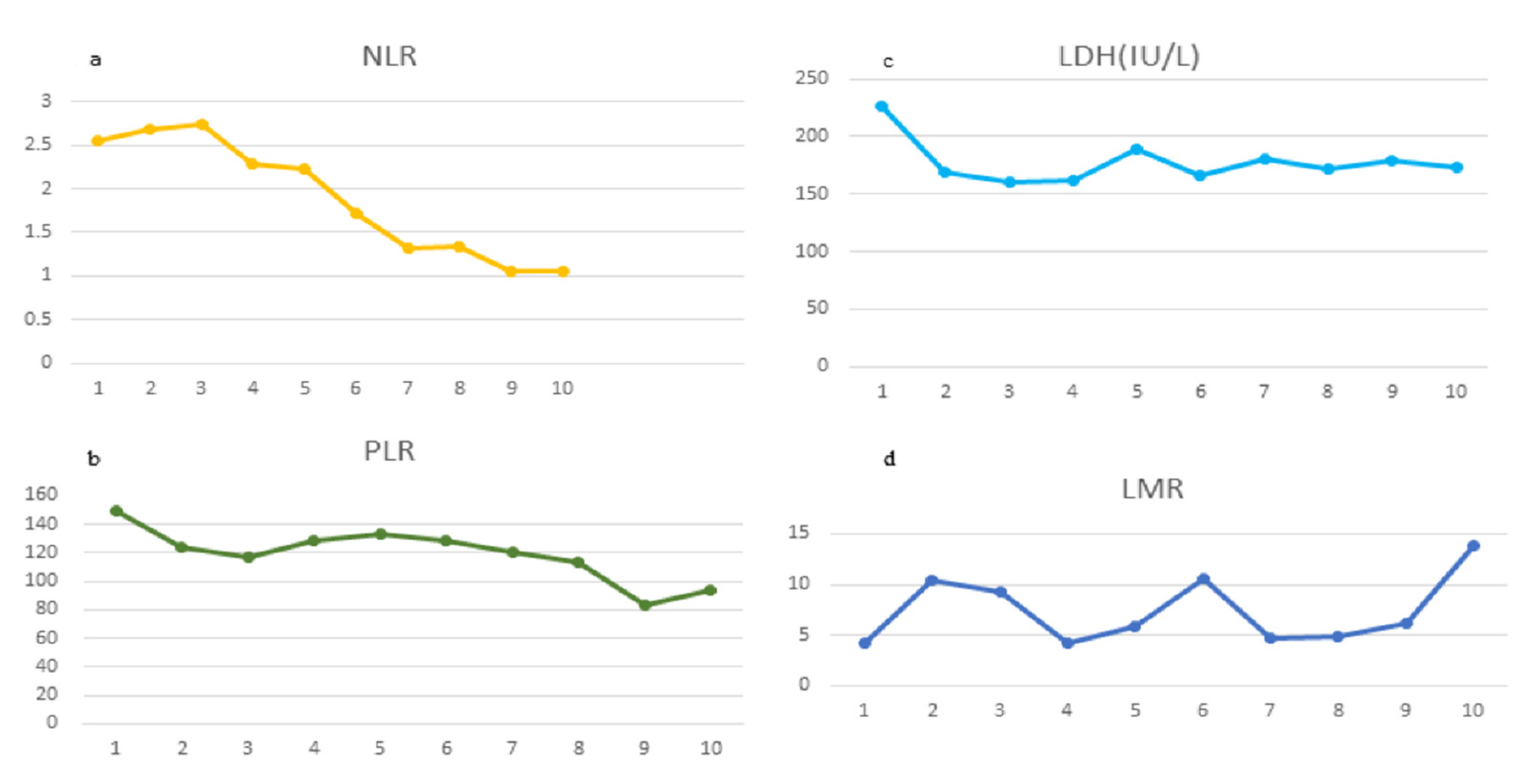
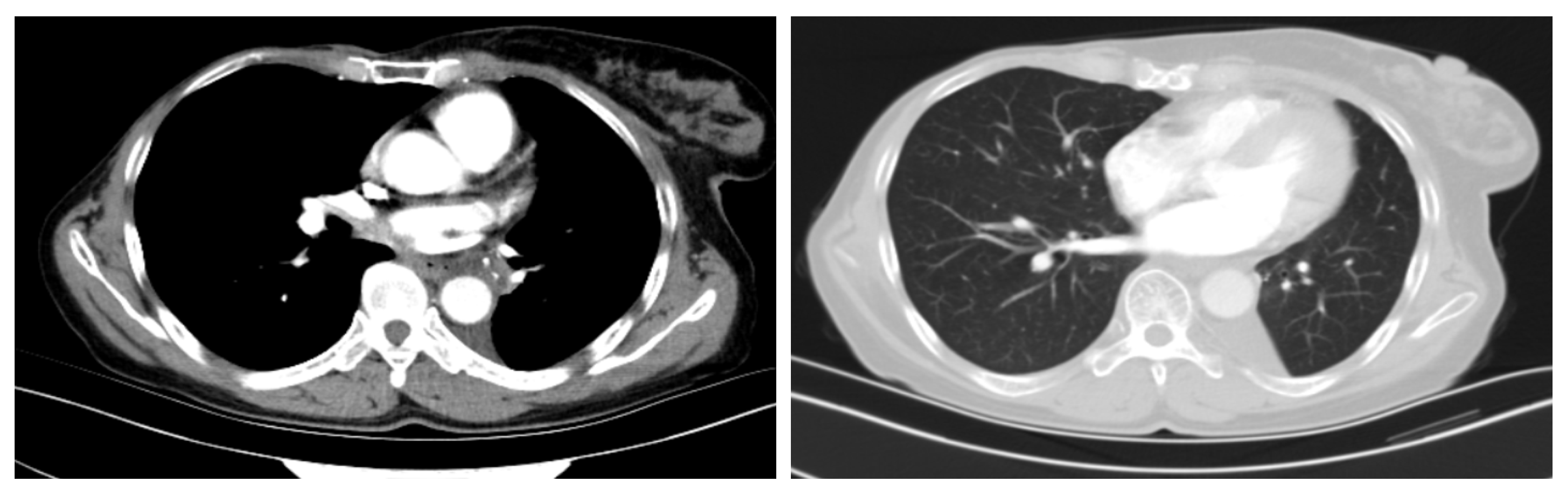
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424, Erratum in CA Cancer J. Clin. 2020, 70, 313. [Google Scholar] [CrossRef] [PubMed]
- Aupérin, A.; Le Péchoux, C.; Rolland, E.; Curran, W.J.; Furuse, K.; Fournel, P.; Belderbos, J.; Clamon, G.; Ulutin, H.C.; Paulus, R.; et al. Meta-Analysis of Concomitant Versus Sequential Radiochemotherapy in Locally Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2010, 28, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: A systematic review and meta-analysis of individual participant data. Lancet 2014, 383, 1561–1571. [Google Scholar] [CrossRef]
- de Luca, A.; Frusone, F.; Vergine, M.; Cocchiara, R.A.; La Torre, G.; Ballesio, L.; Monti, M.; Amabile, M.I. Breast Cancer and Multiple Primary Malignant Tumors: Case Report and Review of the Literature. Vivo 2019, 33, 1313–1324. [Google Scholar] [CrossRef]
- Wang, H.; Hou, J.; Zhang, G.; Zhang, M.; Li, P.; Yan, X.; Ma, Z. Clinical characteristics and prognostic analysis of multiple primary malignant neoplasms in patients with lung cancer. Cancer Gene Ther. 2019, 26, 419–426. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.; Kim, S.; Kim, J.; Ryu, J.; Park, H.S.; Kim, S.I.; Park, B.-W. Characteristics and Survival of Breast Cancer Patients with Multiple Synchronous or Metachronous Primary Cancers. Yonsei Med. J. 2015, 56, 1213–1220. [Google Scholar] [CrossRef][Green Version]
- Hoekstra, N.; Fleury, E.; Lara, T.R.M.; van der Baan, P.; Bahnerth, A.; Struik, G.; Hoogeman, M.; Pignol, J.-P. Long-term risks of secondary cancer for various whole and partial breast irradiation techniques. Radiother. Oncol. 2018, 128, 428–433. [Google Scholar] [CrossRef]
- Taylor, C.; Correa, C.; Duane, F.K.; Aznar, M.C.; Anderson, S.; Bergh, J.; Dodwell, D.; Ewertz, M.; Gray, R.; Jagsi, R.; et al. Estimating the Risks of Breast Cancer Radiotherapy: Evidence From Modern Radiation Doses to the Lungs and Heart and From Previous Randomized Trials. J. Clin. Oncol. 2017, 35, 1641–1649. [Google Scholar] [CrossRef]
- Pignol, J.-P.; Hoekstra, N.; Wilke, D.; Dahn, H.; Nolan, M.; Vicini, F. Estimation of Annual Secondary Lung Cancer Deaths Using Various Adjuvant Breast Radiotherapy Techniques for Early-Stage Cancers. Front. Oncol. 2021, 11, 713328. [Google Scholar] [CrossRef]
- Mielgo-Rubio, X.; Montemuiño, S.; Jiménez, U.; Luna, J.; Cardeña, A.; Mezquita, L.; Martín, M.; Couñago, F. Management of Resectable Stage III-N2 Non-Small-Cell Lung Cancer (NSCLC) in the Age of Immunotherapy. Cancers 2021, 13, 4811. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.L.; Chua, K.L.; Lin, C.-C.; Lee, V.H.; Tho, L.M.; Chan, A.W.; Ho, G.F.; Reungwetwattana, T.; Yang, J.C.; Kim, D.-W.; et al. Asian Thoracic Oncology Research Group Expert Consensus Statement on Optimal Management of Stage III NSCLC. J. Thorac. Oncol. 2020, 15, 324–343. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Pless, M.; Stupp, R.; Ris, H.-B.; Stahel, R.A.; Weder, W.; Thierstein, S.; Gerard, M.-A.; Xyrafas, A.; Früh, M.; Cathomas, R.; et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: A phase 3 randomised trial. Lancet 2015, 386, 1049–1056. [Google Scholar] [CrossRef]
- Felip, E.; Rosell, R.; Maestre, J.A.; Rodríguez-Paniagua, J.M.; Morán, T.; Astudillo, J.; Alonso, G.; Borro, J.M.; González-Larriba, J.L.; Torres, A.; et al. Preoperative Chemotherapy Plus Surgery Versus Surgery Plus Adjuvant Chemotherapy Versus Surgery Alone in Early-Stage Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2010, 28, 3138–3145. [Google Scholar] [CrossRef]
- Scagliotti, G.V.; Pastorino, U.; Vansteenkiste, J.F.; Spaggiari, L.; Facciolo, F.; Orlowski, T.M.; Maiorino, L.; Hetzel, M.; Leschinger, M.; Visseren-Grul, C.; et al. Randomized Phase III Study of Surgery Alone or Surgery Plus Preoperative Cisplatin and Gemcitabine in Stages IB to IIIA Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2012, 30, 172–178. [Google Scholar] [CrossRef]
- Jia, H.; Truica, C.I.; Wang, B.; Wang, Y.; Ren, X.; Harvey, H.A.; Song, J.; Yang, J.-M. Immunotherapy for triple-negative breast cancer: Existing challenges and exciting prospects. Drug Resist. Updat. 2017, 32, 1–15. [Google Scholar] [CrossRef]
- Blumenthal, G.M.; Bunn, P.A., Jr.; Chaft, J.E.; McCoach, C.E.; Perez, E.A.; Scagliotti, G.V.; Carbone, D.P.; Aerts, H.J.; Aisner, D.L.; Bergh, J.; et al. Current Status and Future Perspectives on Neoadjuvant Therapy in Lung Cancer. J. Thorac. Oncol. 2018, 13, 1818–1831. [Google Scholar] [CrossRef]
- Spicer, J.; Wang, C.; Tanaka, F.; Saylors, G.B.; Chen, K.-N.; Liberman, M.; Vokes, E.E.; Girard, N.; Lu, S.; Provencio, M.; et al. Surgical outcomes from the phase 3 CheckMate 816 trial: Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo alone as neoadjuvant treatment for patients with resectable non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2021, 39, 8503. [Google Scholar] [CrossRef]
- Gao, S.; Li, N.; Gao, S.; Xue, Q.; Ying, J.; Wang, S.; Tao, X.; Zhao, J.; Mao, Y.; Wang, B.; et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J. Thorac. Oncol. 2020, 15, 816–826. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.-C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.-Y.; Zhong, W.-Z.; Zhang, X.-C.; Su, J.; Xie, Z.; Liu, S.-Y.; Tu, H.-Y.; Chen, H.-J.; Sun, Y.-L.; Zhou, Q.; et al. Potential Predictive Value of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma. Clin. Cancer Res. 2017, 23, 3012–3024. [Google Scholar] [CrossRef]
- Biton, J.; Mansuet-Lupo, A.; Pécuchet, N.; Alifano, M.; Ouakrim, H.; Arrondeau, J.; Boudou-Rouquette, P.; Goldwasser, F.; Leroy, K.; Goc, J.; et al. TP53, STK11, and EGFR Mutations Predict Tumor Immune Profile and the Response to Anti–PD-1 in Lung Adenocarcinoma. Clin. Cancer Res. 2018, 24, 5710–5723. [Google Scholar] [CrossRef] [PubMed]
- Frelaut, M.; Du Rusquec, P.; De Moura, A.; Le Tourneau, C.; Borcoman, E. Pseudoprogression and Hyperprogression as New Forms of Response to Immunotherapy. BioDrugs 2020, 34, 463–476. [Google Scholar] [CrossRef]
- Champiat, S.; Dercle, L.; Ammari, S.; Massard, C.; Hollebecque, A.; Postel-Vinay, S.; Chaput, N.; Eggermont, A.M.; Marabelle, A.; Soria, J.-C.; et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin. Cancer Res. 2017, 23, 1920–1928. [Google Scholar] [CrossRef]
- Sekine, K.; Kanda, S.; Goto, Y.; Horinouchi, H.; Fujiwara, Y.; Yamamoto, N.; Motoi, N.; Ohe, Y. Change in the lymphocyte-to-monocyte ratio is an early surrogate marker of the efficacy of nivolumab monotherapy in advanced non-small-cell lung cancer. Lung Cancer 2018, 124, 179–188. [Google Scholar] [CrossRef]
- Wang, X.; Cao, K.; Guo, E.; Mao, X.; An, C.; Guo, L.; Zhang, C.; Guo, J.; Yang, X.; Sun, J.; et al. Assessment of immune status of laryngeal squamous cell carcinoma can predict prognosis and guide treatment. Cancer Immunol. Immunother. 2021, 71, 1199–1220. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.-X.; Hei, Y.; Zhu, W.-T.; Wang, Q.-R.; Zhang, H.-M.; Chen, Y. Neoadjuvant Immunotherapy Combined with Chemotherapy for Local Advanced Non-Small-Cell Lung Cancer in a Patient with a History of Breast Cancer: A Case Report. Curr. Oncol. 2022, 29, 6203-6210. https://doi.org/10.3390/curroncol29090487
Yang R-X, Hei Y, Zhu W-T, Wang Q-R, Zhang H-M, Chen Y. Neoadjuvant Immunotherapy Combined with Chemotherapy for Local Advanced Non-Small-Cell Lung Cancer in a Patient with a History of Breast Cancer: A Case Report. Current Oncology. 2022; 29(9):6203-6210. https://doi.org/10.3390/curroncol29090487
Chicago/Turabian StyleYang, Rui-Xia, Yue Hei, Wen-Ting Zhu, Qian-Rong Wang, Hong-Mei Zhang, and Yan Chen. 2022. "Neoadjuvant Immunotherapy Combined with Chemotherapy for Local Advanced Non-Small-Cell Lung Cancer in a Patient with a History of Breast Cancer: A Case Report" Current Oncology 29, no. 9: 6203-6210. https://doi.org/10.3390/curroncol29090487
APA StyleYang, R.-X., Hei, Y., Zhu, W.-T., Wang, Q.-R., Zhang, H.-M., & Chen, Y. (2022). Neoadjuvant Immunotherapy Combined with Chemotherapy for Local Advanced Non-Small-Cell Lung Cancer in a Patient with a History of Breast Cancer: A Case Report. Current Oncology, 29(9), 6203-6210. https://doi.org/10.3390/curroncol29090487




