Comparison of Efficacy and Safety of Taxanes Plus Platinum and Fluorouracil Plus Platinum in the First-Line Treatment of Esophageal Cancer: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction and Evidence Evaluation
2.5. Statistical Methods
3. Results
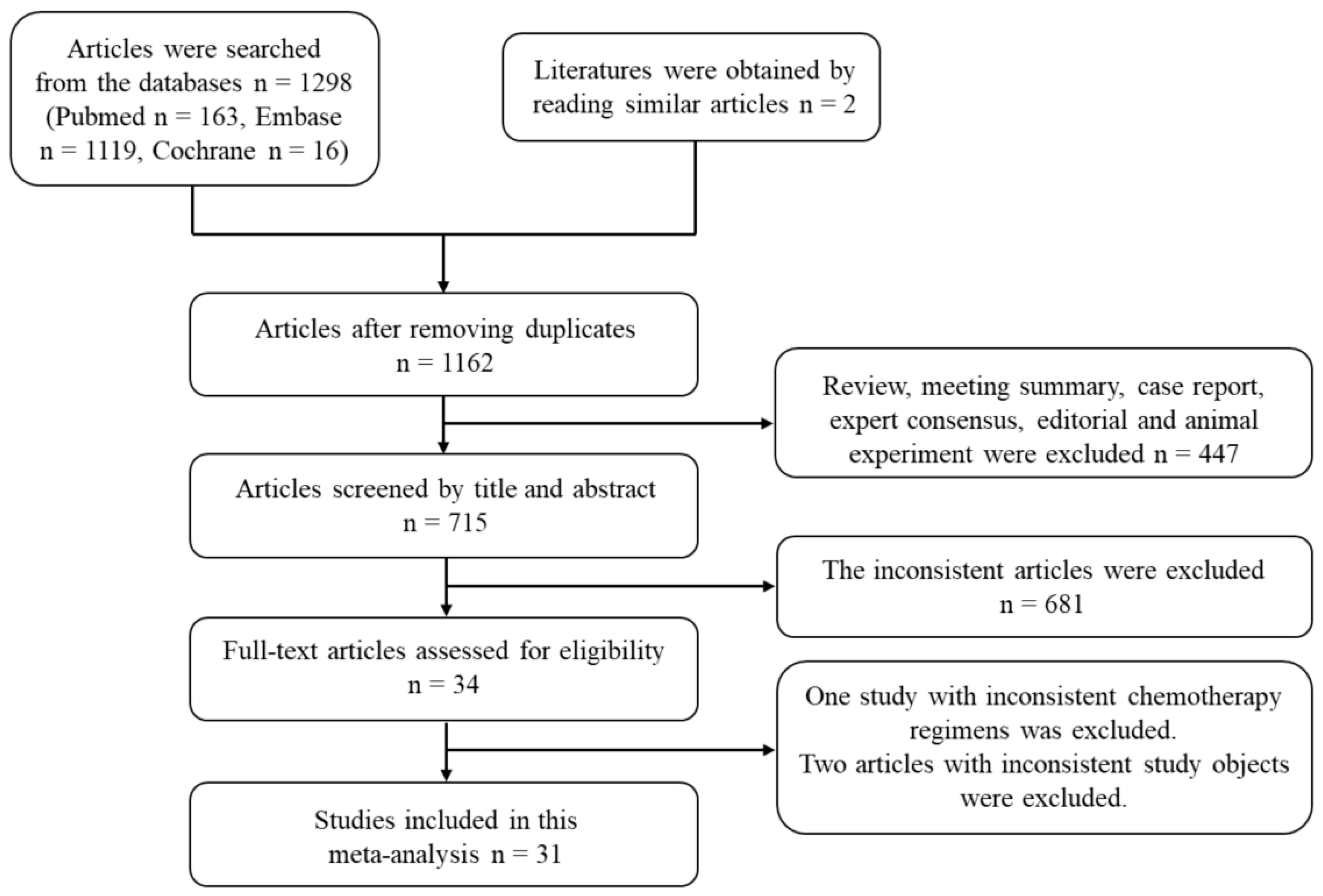
3.1. Selection of Studies
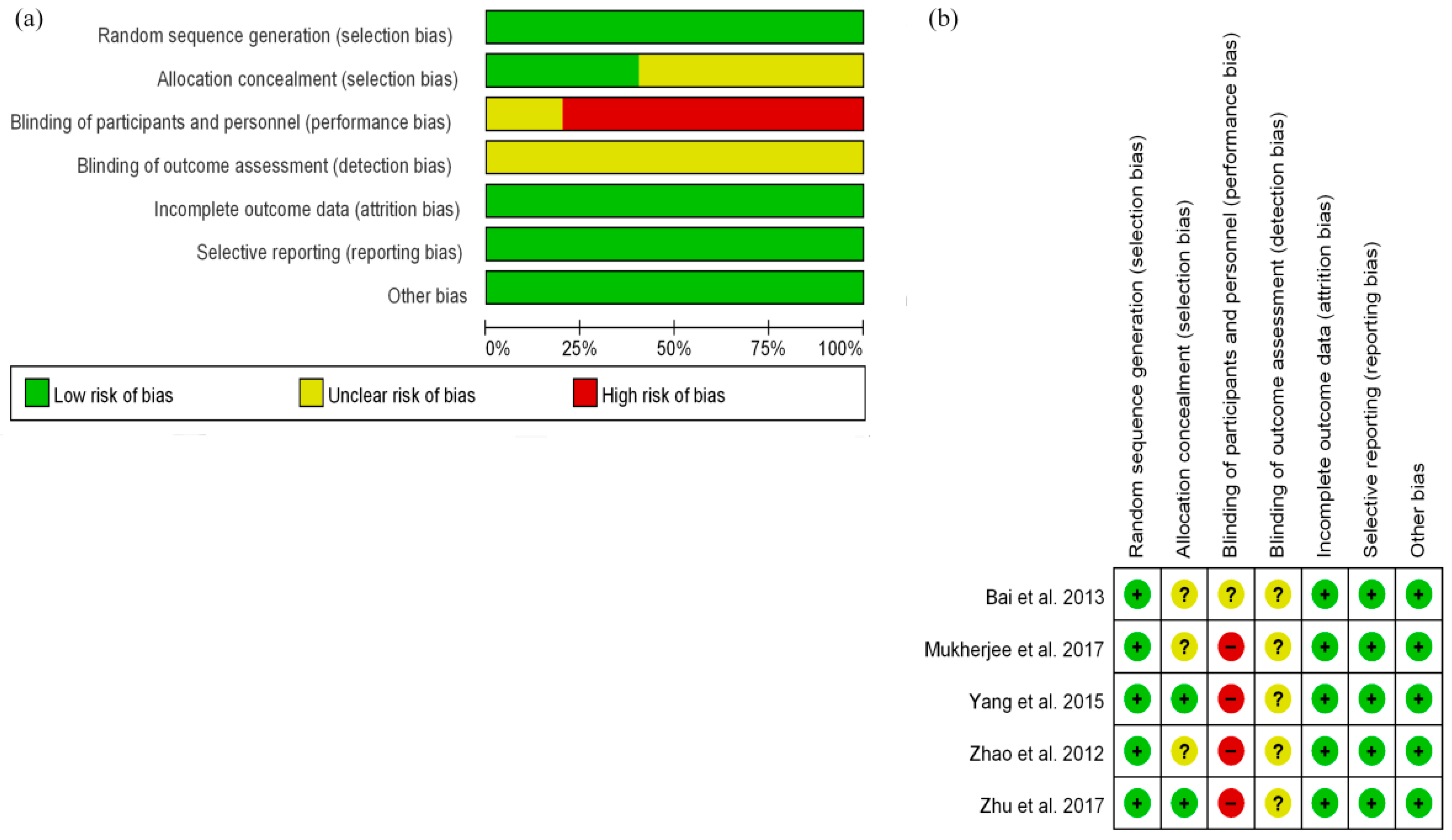
3.2. Quality Evaluation
3.3. Survival Outcome
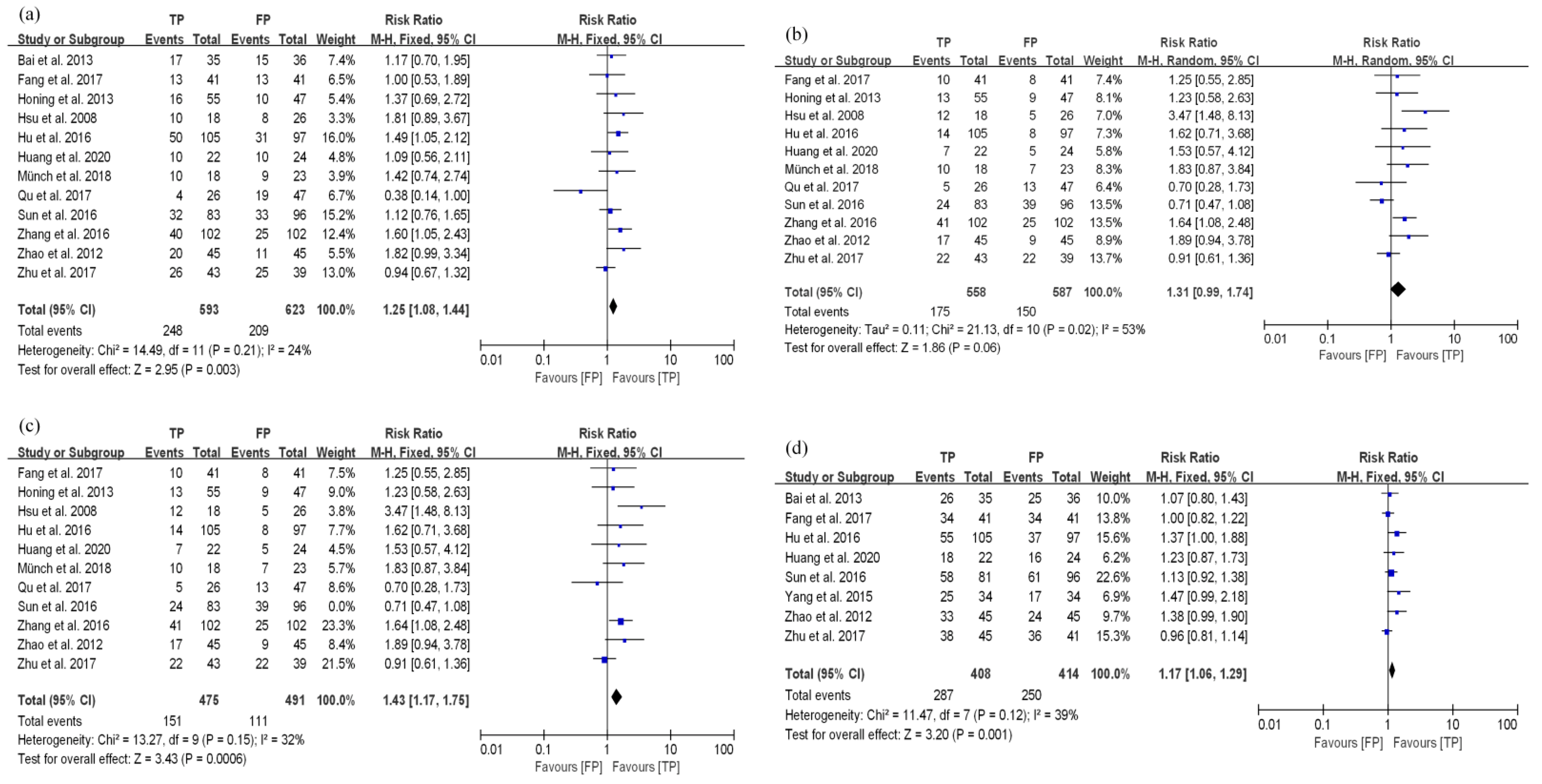
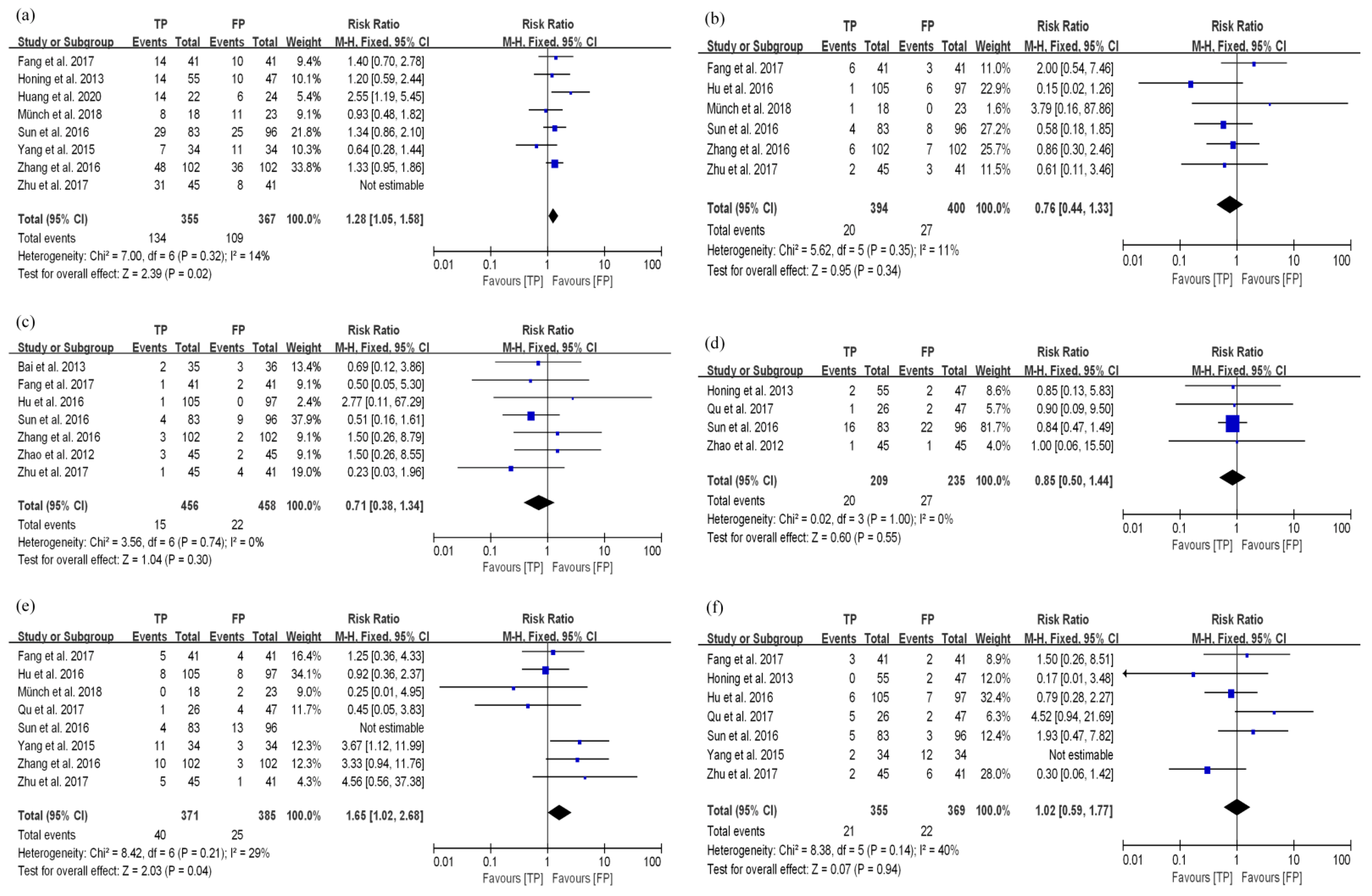
3.3.1. TP Results in Better Disease Control and Long-Term Survival Compared with FP in the dCRT Group
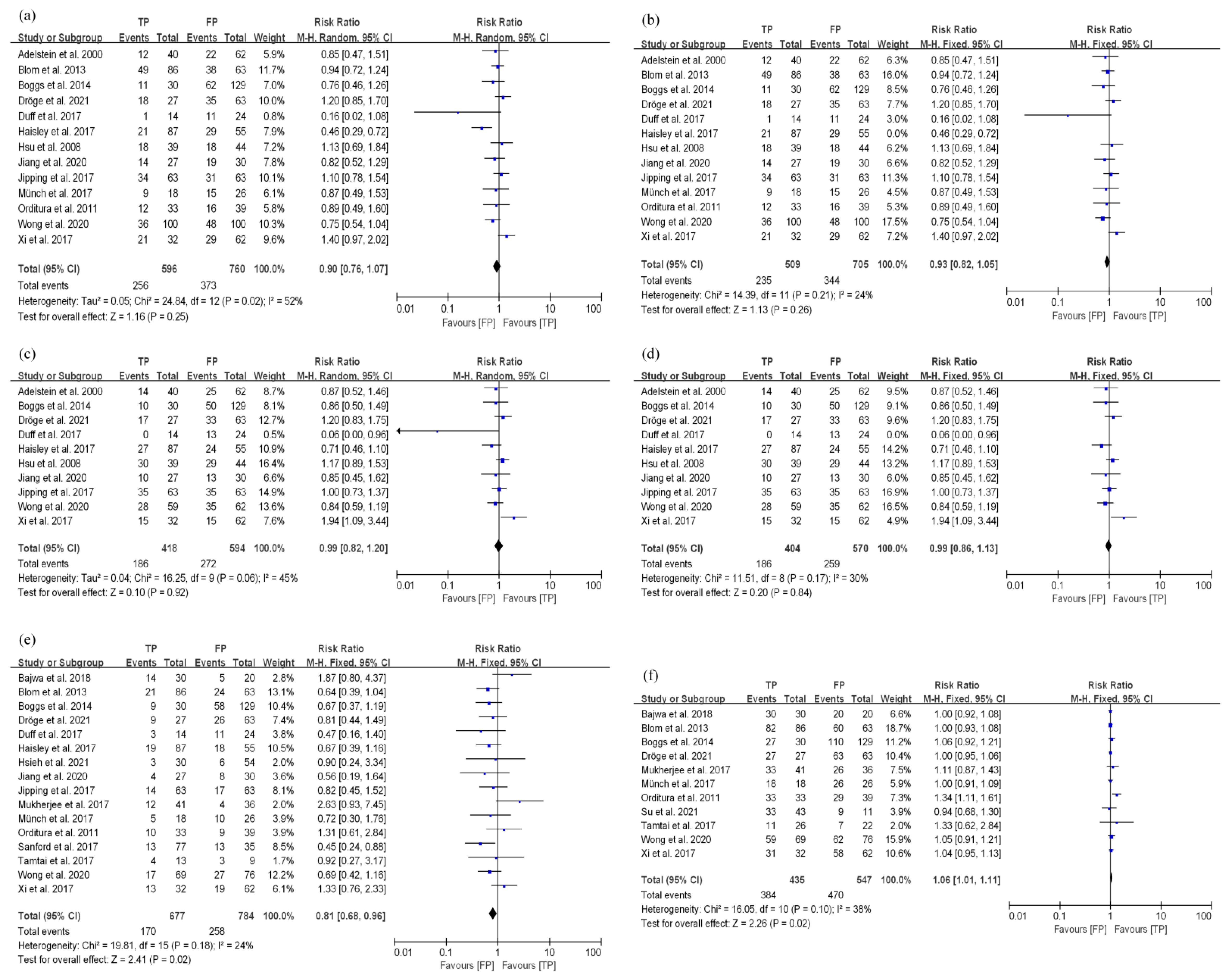
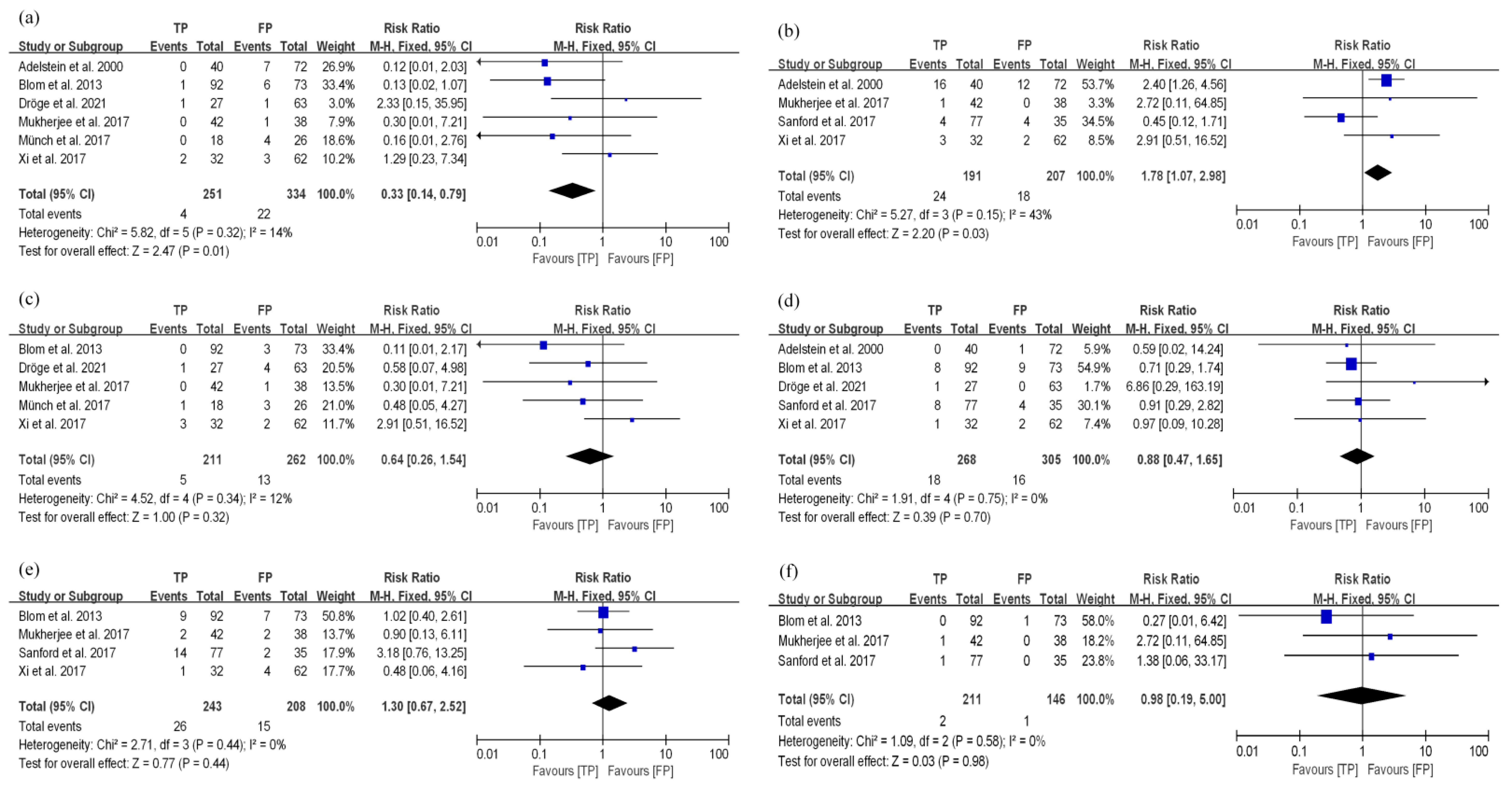
3.3.2. TP and FP Regimens Had Similar Survival Efficacy in EC Patients Treated with nCRT
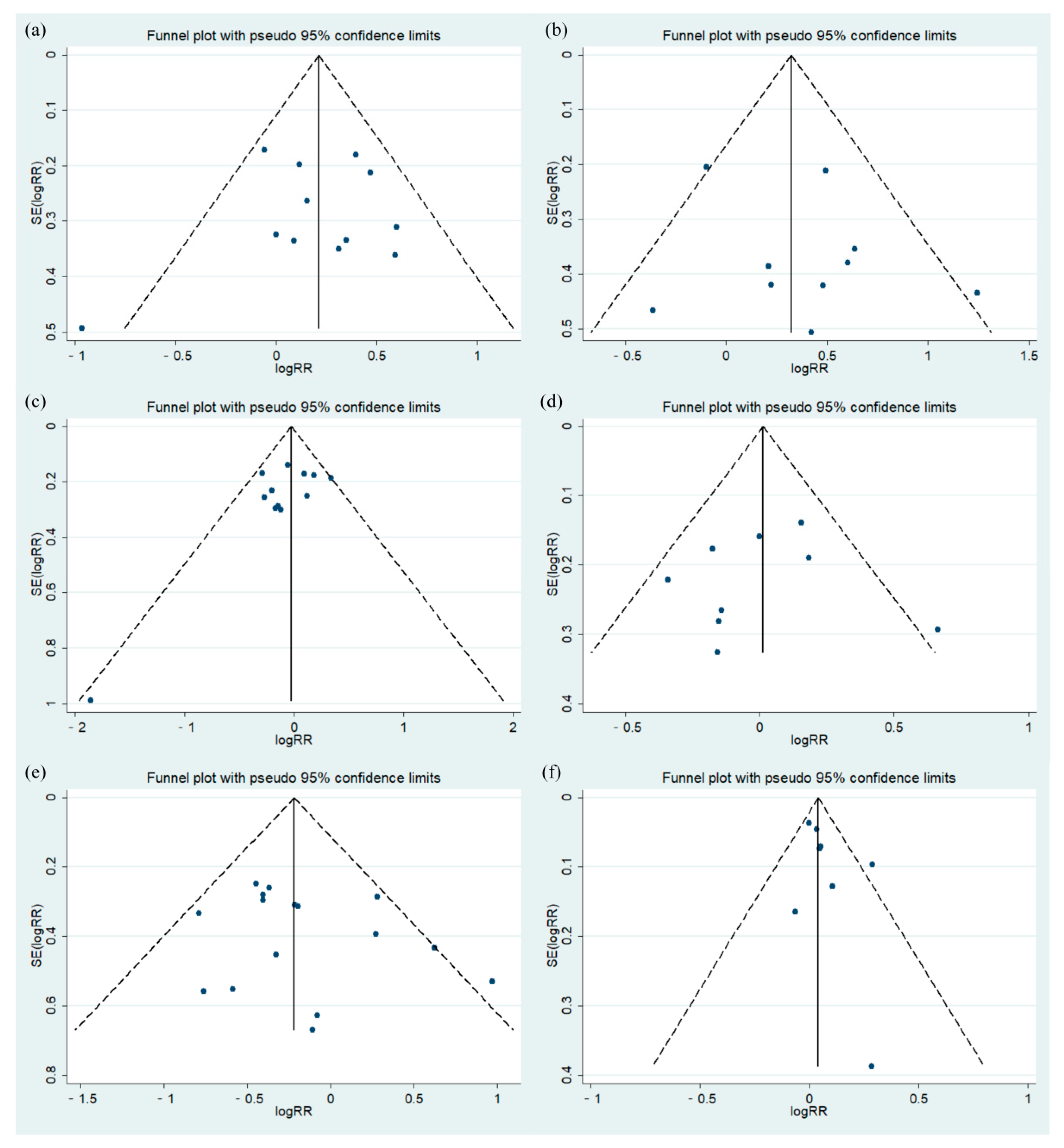
3.3.3. Bias Test
3.4. Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- D’Journo, X.B.; Thomas, P.A. Current management of esophageal cancer. J. Thorac. Dis. 2014, 6 (Suppl. 2), S253–S264. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Z.; Jin, G.F.; Shen, H.B. Epidemiologic differences in esophageal cancer between Asian and western populations. Chin. J. Cancer 2012, 31, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Seto, Y.; Takenaka, R.; Okuma, K.; Kiritooshi, T.; Mori, K.; Yamada, K.; Fukuda, T.; Kaminishi, M.; Abe, O.; et al. Survival comparison between radical surgery and definitive chemoradiation in 267 esophageal squamous cell carcinomas in a single institution: A propensity-matchedstudy. PLoS ONE 2017, 12, e0177133. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Budach, W.; Meyer, H.J.; Cervantes, A. Esophageal cancer: Clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010, 21 (Suppl. 5), v46–v49. [Google Scholar] [CrossRef]
- Allum, W.H.; Stenning, S.P.; Bancewicz, J.; Clark, P.I.; Langley, R.E. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J. Clin. Oncol. 2009, 27, 5062–5067. [Google Scholar] [CrossRef]
- Swisher, S.G.; Hofstetter, W.; Komaki, R.; Correa, A.M.; Erasmus, J.; Lee, J.H.; Liao, Z.X.; Maru, D.; Mehran, R.; Patel, S.; et al. Improved long-term outcome with chemoradiotherapy strategies in esophageal cancer. Ann. Thorac. Surg. 2010, 90, 892–898, discussion 898–899. [Google Scholar] [CrossRef]
- van Hagen, P.; Hulshof, M.C.C.M.; van Lanschot, J.J.B.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; Richel, D.J.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef]
- Huang, T.C.; Hsu, C.H.; Lin, C.C.; Tu, Y.K. Systematic review and network meta-analysis: Neoadjuvant chemoradiotherapy for locoregional esophageal cancer. Jpn. J. Clin. Oncol. 2015, 45, 1023–1028. [Google Scholar] [CrossRef]
- Choy, H. Combining taxanes with radiation for solid tumors. Int. J. Cancer 2000, 90, 113–127. [Google Scholar] [CrossRef]
- Tishler, R.B.; Schiff, P.B.; Geard, C.R.; Hall, E.J. Taxol: A novel radiation sensitizer. Int. J. Radiat. Oncol. Biol. Phys. 1992, 22, 613–617. [Google Scholar] [CrossRef]
- Milross, C.G.; Mason, K.A.; Hunter, N.R.; Chung, W.K.; Peters, L.J.; Milas, L. Relationship of mitotic arrest and apoptosis to antitumor effect of paclitaxel. J. Natl. Cancer Inst. 1996, 88, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, M.; Li, R.; Zhang, R. Melatonin sensitizes esophageal cancer cells to 5fluorouracil via promotion of apoptosis by regulating EZH2 expression. Oncol. Rep. 2021, 45, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yin, Y.; Xu, S.J.; Chen, W.S. 5-Fluorouracil: Mechanisms of resistance and reversal strategies. Molecules 2008, 13, 1551–1569. [Google Scholar] [CrossRef]
- Lv, J.; Cao, X.F.; Zhu, B.; Ji, L.; Tao, L.; Wang, D.D. Long-term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World J. Gastroenterol. 2010, 16, 1649–1654. [Google Scholar] [CrossRef]
- Wang, T.; Yu, J.; Liu, M.; Chen, Y.; Zhu, C.; Lu, L.; Wang, M.; Liu, X.; Zhang, X.; Chen, Y.; et al. The benefit of taxane-based therapies over fluoropyrimidine plus platinum (FP) in the treatment of esophageal cancer: A meta-analysis of clinical studies. Drug Des. Dev. Ther. 2019, 13, 539–553. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Hsu, F.M.; Lin, C.C.; Lee, J.M.; Chang, Y.L.; Hsu, C.H.; Tsai, Y.C.; Lee, Y.C.; Cheng, J.C.H. Improved local control by surgery and paclitaxel-based chemoradiation for esophageal squamous cell carcinoma: Results of a retrospective non-randomized study. J. Surg. Oncol. 2008, 98, 34–41. [Google Scholar] [CrossRef]
- Bai, M.; Wang, B.; Wang, X.; Ma, H.; Wang, Y.; Wang, Z. Randomized trial of weekly docetaxel and cisplatin combined with concurrent 3DCRT in patients with locally advanced esophageal cancer. Chin.-Ger. J. Clin. Oncol. 2013, 12, 361–364. [Google Scholar] [CrossRef]
- Huang, C.; Huang, D.; Zhu, Y.; Xie, G.; Wang, H.; Shi, J.; Jia, B.; Yuan, Y.; Zhang, W. Comparison of a concurrent fluorouracil-based regimen and a taxane-based regimen combined with radiotherapy in elderly patients with esophageal squamous cell carcinoma. Transl. Oncol. 2020, 13, 100736. [Google Scholar] [CrossRef]
- Hu, G.; Wang, Z.; Wang, Y.; Zhang, Q.; Tang, N.; Guo, J.; Liu, L.; Han, X. Comparison of cisplatinum/paclitaxel with cisplatinum/5-fluorouracil as first-line therapy for nonsurgical locally advanced esophageal squamous cell carcinoma patients. Drug Des. Dev. Ther. 2016, 10, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Münch, S.; Pigorsch, S.U.; Devečka, M.; Dapper, H.; Weichert, W.; Friess, H.; Braren, R.; Combs, S.E.; Habermehl, D. Comparison of definite chemoradiation therapy with carboplatin/paclitaxel or cisplatin/5-fluoruracil in patients with squamous cell carcinoma of the esophagus. Radiat. Oncol. 2018, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.M.; Biagi, J.J.; Hopman, W.M.; Mahmud, A. Shifting practice in definitive chemoradiation for localized esophageal cancer. Curr. Oncol. 2017, 24, e379–e387. [Google Scholar] [CrossRef]
- Sun, X.; Han, S.; Gu, F.; Lin, G.; Wang, Z.; Wang, Y.; Xu, Y. A retrospective comparison of taxane and fluorouracil-based chemoradiotherapy in patients with Inoperable esophageal squamous cell carcinoma. J. Cancer 2016, 7, 1066–1073. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Honing, J.; Smit, J.K.; Muijs, K.T.; Burgerhof, H.G.; Plukker, J.T.; Beukema, J.C.; Hospers, G.A. A comparison of carboplatin with paclitaxel and cisplatinum with 5-fluorouracil in definitive chemoradiotherapy in esophageal cancer patients. Ann. Oncol. 2014, 25, 638–643. [Google Scholar] [CrossRef]
- Fang, M.; Song, T.; Liang, X.; Lv, S.; Li, J.; Xu, H.; Luo, L.; Jia, Y. Comparative study of cisplatin-based definitive concurrent chemoradiotherapy with S-1 versus paclitaxel for unresectable locally advanced esophageal squamous cell carcinoma. Oncotarget 2017, 8, 37080–37090. [Google Scholar] [CrossRef]
- Yang, J.S.; Wang, T.; Qiu, M.Q.; Li, Q.L. Comparison of efficacy and toxicity profiles between paclitaxel/lobapoatin- and cisplatin/5-fluorouracil-based concurrent chemoradiotherapy of advanced inoperable oesophageal cancer. Intern. Med. J. 2015, 45, 757–761. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, H.; Zhang, T. Docetaxel and cisplatin concurrent with radiotherapy versus 5-fluorouracil and cisplatin concurrent with radiotherapy in treatment for locally advanced oesophageal squamous cell carcinoma: A randomized clinical study. Med. Oncol. 2012, 29, 3017–3023. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, W.; Li, Q.; Li, Q.; Qiu, B.; Liu, H.; Liu, M.; Hu, Y. A phase II randomized controlled trial: Definitive concurrent chemoradiotherapy with docetaxel plus cisplatin versus 5-fluorouracil plus cisplatin in patients with oesophageal squamous cell carcinoma. J. Cancer 2017, 8, 3657–3666. [Google Scholar] [CrossRef]
- Zhang, P.; Xi, M.; Li, Q.Q.; Hu, Y.H.; Guo, X.; Zhao, L.; Liu, H.; Liu, S.L.; Luo, L.L.; Liu, Q.; et al. Concurrent cisplatin and 5-fluorouracil versus concurrent cisplatin and docetaxel with radiotherapy for esophageal squamous cell carcinoma: A propensity score-matched analysis. Oncotarget 2016, 7, 44686–44694. [Google Scholar] [CrossRef]
- Su, P.H.; Hsueh, S.W.; Tseng, C.K.; Ho, M.M.; Su, P.J.; Hung, C.Y.; Yeh, K.Y.; Chang, P.H.; Hung, Y.S.; Ho, Y.W.; et al. Paclitaxel and carboplatin versus cisplatin and 5-fluorouracil in concurrent chemoradiotherapy in patients with esophageal cancer. In Vivo 2021, 35, 3391–3399. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.M.; Sim, H.W.; Espin-Garcia, O.; Chan, B.A.; Natori, A.; Lim, C.H.; Moignard, S.; Chen, E.X.; Liu, G.; Darling, G.; et al. Chemoradiotherapy using carboplatin plus paclitaxel versus cisplatin plus fluorouracil for esophageal or gastroesophageal junction cancer. Oncology 2021, 99, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.C.H.; Chiang, P.C.; Hung, T.M.; Chao, Y.K.; Kuo, Y.C.; Wen, C.T.; Su, P.J.; Peng, M.T.; Chen, H.W.; Liu, H.L.; et al. Definitive concurrent chemoradiotherapy with paclitaxel plus carboplatin is superior to cisplatin plus 5-fluorouracil in patients with inoperable esophageal squamous cell carcinoma using retrospective, real-world evidence. Cancer Med. 2021, 10, 8300–8309. [Google Scholar] [CrossRef] [PubMed]
- Dröge, L.H.; Karras, P.J.; Guhlich, M.; Schirmer, M.A.; Ghadimi, M.; Rieken, S.; Conradi, L.C.; Leu, M. Preoperative radiochemotherapy in esophageal squamous cell cancer with 5-fluorouracil/cisplatin or carboplatin/paclitaxel: Treatment practice over a 20-year period and implications for the individual treatment modalities. Cancers 2021, 13, 1813. [Google Scholar] [CrossRef]
- Wong, I.Y.H.; Lam, K.O.; Zhang, R.Q.; Chan, W.W.L.; Wong, C.L.Y.; Chan, F.S.Y.; Kwong, D.L.W.; Law, S.Y.K. Neoadjuvant chemoradiotherapy using cisplatin and 5-fluorouracil (PF) versus carboplatin and paclitaxel (cross regimen) for esophageal equamous cell carcinoma (ESCC): A propensity score-matched study. Ann. Surg. 2020, 272, 779–785. [Google Scholar] [CrossRef]
- Bajwa, H.K.; Singareddy, R.; Reddy, M.M.; Raju, K.A.; Rao, S.T.; Rajappa, S.J. Preoperative chemoradiation in carcinoma esophagus: Experience from a tertiary cancer center in India. Indian J. Med. Paediatr. Oncol. 2018, 39, 272–275. [Google Scholar] [CrossRef]
- Xi, M.; Zhang, P.; Zhang, L.; Yang, Y.D.; Liu, S.L.; Li, Y.; Fu, J.H.; Liu, M.Z. Comparing docetaxel plus cisplatin versus fluorouracil plus cisplatin in esophageal squamous cell carcinoma treated with neoadjuvant chemoradiotherapy. Jpn. J. Clin. Oncol. 2017, 47, 683–689. [Google Scholar] [CrossRef]
- Sanford, N.N.; Catalano, P.J.; Enzinger, P.C.; King, B.L.; Bueno, R.; Martin, N.E.; Hong, T.S.; Wo, J.Y.; Mamon, H.J. A retrospective comparison of neoadjuvant chemoradiotherapy regimens for locally advanced esophageal cancer. Dis. Esophagus 2017, 30, 1–8. [Google Scholar] [CrossRef]
- Jipping, K.M.; Hulshoff, J.B.; van Amerongen, E.A.; Bright, T.I.; Watson, D.I.; Plukker, J.T.M. Influence of tumor response and treatment schedule on the distribution of tumor recurrence in esophageal cancer patients treated with neoadjuvant chemoradiotherapy. J. Surg. Oncol. 2017, 116, 1096–1102. [Google Scholar] [CrossRef]
- Haisley, K.R.; Hart, K.D.; Nabavizadeh, N.; Bensch, K.G.; Vaccaro, G.M.; Thomas, C.R.; Schipper, P.H.; Hunter, J.G.; Dolan, J.P. Neoadjuvant chemoradiotherapy with concurrent cisplatin/5-fluorouracil is associated with increased pathologic complete response and improved survival compared to carboplatin/paclitaxel in patients with locally advanced esophageal cancer. Dis. Esophagus 2017, 30, 1–7. [Google Scholar] [CrossRef]
- Duff, J.M.; Peters, H.C.; Zingarelli, W.; Ben-David, K.; Sarosi, G.; Thomas, R.M. Comparative effectiveness of preoperative treatment regimens in patientswith potentially resectable esophageal cancer. JAMA Surg. 2017, 152, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Boggs, D.H.; Tarabolous, C.; Morris, C.G.; Hanna, A.; Burrows, W.; Horiba, N.; Suntharalingam, M. Analysis of pathological complete response rates with paclitaxel-based regimens in trimodality therapy for esophageal cancer. Dis. Esophagus 2015, 28, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Blom, R.L.G.M.; Sosef, M.N.; Nap, M.; Lammering, G.; van den Berkmortel, F.; Hulshof, M.C.C.M.; Meijer, S.L.; Wilmink, H.W.; van Berge Henegouwen, M.I. Comparison of two neoadjuvant chemoradiotherapy regimens in patients with potentially curable esophageal carcinoma. Dis. Esophagus 2014, 27, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Orditura, M.; Galizia, G.; Morgillo, F.; Martinelli, E.; Lieto, E.; Vitiello, F.; Di Martino, N.; Pacelli, R.; Renda, A.; Ciardiello, F.; et al. Complete response to preoperative chemoradiation and survival in esophageal cancer: A pooled analysis of three single-institution phase II trials. Dis. Esophagus 2012, 25, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Adelstein, D.J.; Rice, T.W.; Rybicki, L.A.; Larto, M.A.; Ciezki, J.; Saxton, J.; DeCamp, M.; Vargo, J.J.; Dumot, J.A.; Zuccaro, G. Does paclitaxel improve the chemoradiotherapy of locoregionally advanced esophageal cancer? A nonrandomized comparison with fluorouracil-based therapy. J. Clin. Oncol. 2000, 18, 2032–2039. [Google Scholar] [CrossRef] [PubMed]
- Münch, S.; Pigorsch, S.U.; Feith, M.; Slotta-Huspenina, J.; Weichert, W.; Friess, H.; Combs, S.E.; Habermehl, D. Comparison of neoadjuvant chemoradiation with carboplatin/paclitaxel or cisplatin/5-fluoruracil in patients with squamous cell carcinoma of the esophagus. Radiat. Oncol. 2017, 12, 182. [Google Scholar] [CrossRef]
- Tamtai, A.; Jiarpinitnun, C.; Hiranyatheb, P.; Unwanatham, N.; Sirachainun, E.; Supsamutchai, C.; Pattaranutaporn, P.; Ngamphaiboon, N. Tolerability and efficacy of concurrent chemoradiotherapy comparing carboplatin/paclitaxel versus platinum/5-FU regimen for locally advanced esophageal and esophagogastric junction cancers. Med. Oncol. 2017, 34, 157. [Google Scholar] [CrossRef]
- Mukherjee, S.; Hurt, C.N.; Gwynne, S.; Sebag-Montefiore, D.; Radhakrishna, G.; Gollins, S.; Hawkins, M.; Grabsch, H.I.; Jones, G.; Falk, S.; et al. NEOSCOPE: A randomised phase II study of induction chemotherapy followed by oxaliplatin/capecitabine or carboplatin/paclitaxel based pre-operative chemoradiation for resectable oesophageal adenocarcinoma. Eur. J. Cancer 2017, 74, 38–46. [Google Scholar] [CrossRef]
- Li, C.Y.; Huang, P.M.; Chu, P.Y.; Chen, P.M.; Lin, M.W.; Kuo, S.W.; Lee, J.M. Predictors of survival in esophageal squamous cell carcinoma with pathologic major response after neoadjuvant chemoradiation therapy and surgery: The impact of chemotherapy protocols. BioMed Res. Int. 2016, 2016, 6423297. [Google Scholar] [CrossRef]
- Bao, Y.; Liu, S.L.; Zhou, Q.C.; Cai, P.Q.; Anfossi, S.; Li, Q.Q.; Hu, Y.H.; Liu, M.Z.; Fu, J.H.; Rong, T.H.; et al. Three-dimensional conformal radiotherapy with concurrent chemotherapy for postoperative recurrence of esophageal squamous cell carcinoma: Clinical efficacy and failure pattern. Radiat. Oncol. 2013, 8, 241. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, F.; Li, N.; Liu, Y.; Xu, Y.; Zhou, L.; Wang, J.; Zhu, J.; Huang, M.; Gong, Y. Salvage concurrent radio-chemotherapy for post-operative local recurrence of squamous-cell esophageal cancer. Radiat. Oncol. 2012, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.; van Lanschot, J.J.B.; Hulshof, M.C.C.M.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (cross): Long-term results of a randomised controlled trial. Lancet Oncol. 2015, 16, 1090–1098. [Google Scholar] [CrossRef]
- Noronha, V.; Prabhash, K.; Joshi, A.; Patil, V.M.; Talole, S.; Nakti, D.; Sahu, A.; Shah, S.; Ghosh-Laskar, S.; Patil, P.S.; et al. Clinical outcome in definitive concurrent chemoradiation with weekly paclitaxel and carboplatin for locally advanced esophageal and junctional cancer. Oncol. Res. 2016, 23, 183–195. [Google Scholar] [CrossRef]
- NCCN. Practise Guidelines in Oncology-Esophageal and Esophagogastric Junction Cancers. Available online: https://www.nccn.org/professionals/physician_gls/PDF/esophageal.pdf (accessed on 22 March 2022).
- Li, Q.Q.; Liu, M.Z.; Hu, Y.H.; Liu, H.; He, Z.Y.; Lin, H.X. Definitive concomitant chemoradiotherapy with docetaxel and cisplatin in squamous esopheageal carcinoma. Dis. Esophagus 2010, 23, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, S.; Zheng, Y.; Han, Y.; Chen, X.; Cheng, Z.; Wu, Y.; Feng, X.; Qi, W.; Chen, K.; et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur. J. Cancer 2021, 144, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Wang, W.; Li, T.; Li, J.; Zhao, K.; Wang, W.; Liang, L.; Wu, H.; Ai, T.; Huang, W.; et al. RATIONALE 311, Tislelizumab plus concurrent chemoradiotherapy for localized esophageal squamous cell carcinoma. Future Oncol. 2021, 17, 4081–4089. [Google Scholar] [CrossRef]
- Athauda, A.; Watkins, D.; Mohammed, K.; Chau, I.; Starling, N.; Rao, S.; Tait, D.; Aitken, K.; Cunningham, D. Cisplatin substitution with carboplatin during radical chemoradiotherapy for oesophagogastric carcinoma: Outcomes from a tertiary centre. Anticancer Res. 2018, 38, 5943–5949. [Google Scholar] [CrossRef]
- Bruce, D.M.; Thomas, F.P.; Robert, J.G.; Thomas, M.P.; James, M.; Ritsuko, K.; Gordon, O.; Seth, A.R.; David, P.K. INT 0123 (radiation therapy oncology group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J. Clin. Oncol. 2002, 20, 1167–1174. [Google Scholar] [CrossRef]
| Authors | Year | Geographical Area | Research Type | Pathological Type (No.) | Treatment Strategy | Chemotherapy Regimen | No. of Patients (TP/FP) |
|---|---|---|---|---|---|---|---|
| Hsu et al. [18] | 2008 | Taiwan, China | RCS | ESCC (127) | dCRT/nCRT | PTX + DDP vs. 5-FU + DDP | 57/70 |
| Bai et al. [19] | 2013 | China | RCT | ESCC (71) | CCRT | DTX + DDP vs. 5-FU + DDP | 35/36 |
| Huang et al. [20] | 2020 | China | RCS | ESCC (46) | CCRT | PTX/DTX + DDP/CBP vs. 5-FU + DDP | 22/24 |
| Hu et al. [21] | 2016 | China | RCS | ESCC (202) | dCRT | PTX + DDP vs. 5-FU + DDP | 105/97 |
| Münch et al. [22] | 2018 | Germany | RCS | ESCC (41) | dCRT | PTX + CBP vs. 5-FU + DDP | 18/23 |
| Qu et al. [23] | 2017 | Canada | RCS | ESCC/EAC (26/47) | dCRT | PTX + CBP vs. 5-FU + DDP/CBP | 26/47 |
| Sun et al. [24] | 2016 | China | RCS | ESCC (179) | dCRT | PTX/DTX + DDP/CBP vs. 5-FU/Tegafur/FT207 + DDP/NDP | 83/96 |
| Honing et al. [25] | 2013 | Multicenter | RCS | ESCC/EAC (51/51) | dCRT | PTX + CBP vs. 5-FU + DDP | 55/47 |
| Fang et al. [26] | 2017 | China | RCS | ESCC (82) | CCRT | PTX + DDP vs. S-1 + DDP | 41/41 |
| Yang et al. [27] | 2015 | China | RCT | ESCC (68) | CCRT | PTX + LBP vs. 5-FU + DDP | 34/34 |
| Zhao et al. [28] | 2012 | China | RCT | ESCC (90) | CCRT | DTX + DDP vs. 5-FU + DDP | 45/45 |
| Zhu et al. [29] | 2017 | China | RCT | ESCC (86) | CCRT | DTX + DDP vs. 5-FU + DDP | 45/41 |
| Zhang et al. [30] | 2016 | China | RCS | ESCC (204) | dCRT | DTX + DDP vs. 5-FU + DDP | 102/102 |
| Su et al. [31] | 2021 | Taiwan, China | PCS | ESCC/EAC (133/3) | CCRT/nCRT | PTX + CBP vs. 5-FU + DDP | 87/49 |
| Jiang et al. [32] | 2020 | Canada | RCS | ESCC/EAC (34/59) | dCRT/nCRT | PTX + CBP vs. 5-FU + DDP | 40/53 |
| Hsieh et al. [33] | 2021 | Taiwan, China | RCS | ESCC (229) | CCRT/nCRT | PTX + CBP vs. 5-FU + DDP | 83/146 |
| Dröge et al. [34] | 2021 | Germany | RCS | ESCC (90) | nCRT | PTX + CBP vs. 5-FU + DDP | 27/63 |
| Wong et al. [35] | 2020 | Hong Kong, China | RCS | ESCC (200) | nCRT | PTX + CBP vs. 5-FU + DDP | 100/100 |
| Bajwa et al. [36] | 2018 | India | RCS | ESCC/EAC (38/12) | nCRT | PTX + CBP vs. 5-FU/Cape + DDP | 30/20 |
| Xi et al. [37] | 2017 | China | RCS | ESCC (94) | nCRT | DTX + DDP vs. 5-FU + DDP | 32/62 |
| Sanford et al. [38] | 2017 | America | RCS | ESCC/EAC (18/94) | nCRT | PTX + CBP vs. 5-FU + DDP | 77/35 |
| Jipping et al. [39] | 2017 | Netherlands | RCS | ESCC/EAC (22/104) | nCRT | PTX + CBP vs. 5-FU + DDP | 63/63 |
| Haisley et al. [40] | 2017 | Australia | RCS | ESCC/EAC (20/122) | nCRT | PTX + CBP vs. 5-FU + DDP | 87/55 |
| Duff et al. [41] | 2017 | America | RCS | ESCC/EAC (3/35) | nCRT | PTX + CBP vs. 5-FU + DDP | 14/24 |
| Boggs et al. [42] | 2014 | America | RCS | ESCC/EAC (44/115) | nCRT | PTX + DDP/CBP vs. 5-FU + DDP | 30/129 |
| Blom et al. [43] | 2013 | Netherlands | RCS | ESCC/EAC/other (39/124/2) | nCRT | PTX + CBP vs. 5-FU + DDP | 92/73 |
| Orditura et al. [44] | 2011 | Italy | RCS | ESCC/EAC (54/18) | nCRT | PTX + DDP vs. 5-FU + DDP | 33/39 |
| Adelstein et al. [45] | 2000 | America | RCS | ESCC/EAC/other (29/70/3) | nCRT | PTX + DDP vs. 5-FU + DDP | 40/62 |
| Münch et al. [46] | 2017 | Germany | RCS | ESCC (44) | nCRT | PTX + CBP vs. 5-FU + DDP | 18/26 |
| Tamtai et al. [47] | 2017 | Thailand | RCS | ESCC/EAC/other (113/6/5) | CCRT/nCRT | PTX + CBP vs. 5-FU + Platinum | 60/64 |
| Mukherjee et al. [48] | 2017 | Multicenter | RCT | EAC (85) | nCRT | PTX + CBP vs. Cape + OXA | 43/42 |
| 3-Year OS of dCRT | 3-Year PFS of dCRT | 3-Year OS of nCRT | 3-Year PFS of nCRT | pCR | R0 Resection | |
|---|---|---|---|---|---|---|
| Begg’s test | 0.837 | 0.858 | 0.304 | 1.000 | 0.300 | 0.174 |
| Egger’s test | 0.727 | 0.410 | 0.110 | 0.788 | 0.342 | 0.210 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Song, R.; Jia, Y.; Zhang, X.; Zhang, S.; Wu, C.; Zhang, R.; Guo, Z. Comparison of Efficacy and Safety of Taxanes Plus Platinum and Fluorouracil Plus Platinum in the First-Line Treatment of Esophageal Cancer: A Systematic Review and Meta-Analysis. Curr. Oncol. 2022, 29, 6610-6627. https://doi.org/10.3390/curroncol29090519
Zhao Y, Song R, Jia Y, Zhang X, Zhang S, Wu C, Zhang R, Guo Z. Comparison of Efficacy and Safety of Taxanes Plus Platinum and Fluorouracil Plus Platinum in the First-Line Treatment of Esophageal Cancer: A Systematic Review and Meta-Analysis. Current Oncology. 2022; 29(9):6610-6627. https://doi.org/10.3390/curroncol29090519
Chicago/Turabian StyleZhao, Yue, Rui Song, Yuanyuan Jia, Xiaoyun Zhang, Shasha Zhang, Chensi Wu, Ruixing Zhang, and Zhanjun Guo. 2022. "Comparison of Efficacy and Safety of Taxanes Plus Platinum and Fluorouracil Plus Platinum in the First-Line Treatment of Esophageal Cancer: A Systematic Review and Meta-Analysis" Current Oncology 29, no. 9: 6610-6627. https://doi.org/10.3390/curroncol29090519
APA StyleZhao, Y., Song, R., Jia, Y., Zhang, X., Zhang, S., Wu, C., Zhang, R., & Guo, Z. (2022). Comparison of Efficacy and Safety of Taxanes Plus Platinum and Fluorouracil Plus Platinum in the First-Line Treatment of Esophageal Cancer: A Systematic Review and Meta-Analysis. Current Oncology, 29(9), 6610-6627. https://doi.org/10.3390/curroncol29090519





