Disparate Time-to-Treatment and Varied Incidence of Actionable Non-Small Cell Lung Cancer Molecular Alterations in British Columbia: A Historical Cohort Study
Abstract
1. Introduction
1.1. Background
1.2. Objective
2. Methods
2.1. Study Design and Setting
2.2. Participants and Data Sources
2.3. Primary Outcomes
2.4. Targeted NGS Panels for NSCLC Genetic Alterations
2.5. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AJCC | American Joint Commission on Cancer |
| ALK | Anaplastic lymphoma kinase |
| BC | British Columbia |
| EGFR | Epidermal growth factor receptor |
| FHA | Fraser Health Authority |
| FDG-PET | Fluorodeoxyglucose positron emission tomography |
| FFPE | Formalin fixed paraffin embedded |
| HA | Health authority |
| ICR | Interquartile range |
| IHA | Interior Health Authority |
| IsHA | Island Health Authority |
| MET | Met proto-oncogene |
| NSCLC | Non-small cell lung cancer |
| RET | Ret proto-oncogene |
| ROS1 | c-ROS proto- oncogene 1 |
| SCLC | Small cell lung cancer (SCLC) |
| TKI | Tyrosine kinase inhibitor (TKI) |
| VCC | Vancouver Cancer Centre |
| VCHA | Vancouver Coastal Health Authority |
| WHO | World Health Organization |
References
- Brenner, D.R.; Poirier, A.; Woods, R.R.; Ellison, L.F.; Billette, J.; Demers, A.A.; Zhang, S.X.; Yao, C.; Finley, C.; Fitzgerald, N.; et al. Projected estimates of cancer in Canada in 2022. Cmaj 2022, 194, E601–E607. [Google Scholar] [CrossRef] [PubMed]
- Aberle, D.R.; DeMellow, S.; Berg, C.D.; Black, W.C.; Brewer, B.; Church, T.R.; Clingan, K.L.; Duan, F.; Fagerstom, R.M.; Green, I.F.; et al. Results of the two incidence screenings in the National Lung Screening Trial. N. Engl. J. Med. 2013, 369, 920–931. [Google Scholar] [CrossRef] [PubMed]
- McGuire, A.L.; McConecht, M.K.; Melosky, B.L.; English, J.C.; Choi, J.J.; Peng, D.; Yee, J.; Furman, B.L.S.; Hernandez, R.A.H.; Feijao, P.; et al. The Clinically Actionable Molecular Profile of Early versus Late-Stage Non-Small Cell Lung Cancer, an Individual Age and Sex Propensity-Matched Pair Analysis. Curr. Oncol. 2022, 29, 2630–2643. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Herbst, R.S.; Mann, H.; Rukazenkov, Y.; Marotti, M.; Tsuboi, M. ADAURA: Phase III, Double-blind, Randomized Study of Osimertinib Versus Placebo in EGFR Mutation-positive Early-stage NSCLC After Complete Surgical Resection. Clin. Lung. Cancer 2018, 19, e533–e536. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Provencio, M.; Serna-Blasco, R.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Casal Rubio, J.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; et al. Overall Survival and Biomarker Analysis of Neoadjuvant Nivolumab Plus Chemotherapy in Operable Stage IIIA Non-Small-Cell Lung Cancer (NADIM phase II trial). J. Clin. Oncol. 2022, 40, 2924–2933. [Google Scholar] [CrossRef]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef]
- Wu, Y.L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.W.; Kato, T.; et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef]
- Nishio, M.; Felip, E.; Orlov, S.; Park, K.; Yu, C.G.; Tsai, C.M.; Cobo, M.; McKeage, M.; Su, W.C.; Mok, T.; et al. Final Overall Survival and Other Efficacy and Safety Results From ASCEND-3: Phase II Study of Ceritinib in ALKi-Naive Patients With ALK-Rearranged NSCLC. J. Thorac. Oncol. 2020, 15, 609–617. [Google Scholar] [CrossRef]
- Shaw, A.T.; Kim, D.; Mehra, R.; Tan, D.S.W.; Felip, E.; Chow, L.Q.M.; Camidge, D.R.; Vansteenkiste, J.; Sharma, S.; De Pas, T.; et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N. Engl. J. Med. 2014, 370, 1189–1197. [Google Scholar] [CrossRef]
- Huang, C.; Zou, Q.; Liu, H.; Qiu, B.; Li, Q.; Lin, Y.; Liang, Y. Management of Non-small Cell Lung Cancer Patients with MET Exon 14 Skipping Mutations. Curr. Treat. Options Oncol. 2020, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- Paik, P.K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot, A.B.; Garassino, M.C.; Mazieres, J.; Viteri, S.; Senellart, H.; Van Meerbeeck, J.; et al. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020, 383, 931–943. [Google Scholar] [CrossRef]
- Ackermann, C.J.; Stock, G.; Tay, R.; Dawod, M.; Gomes, F.; Califano, R. Targeted Therapy For RET-Rearranged Non-Small Cell Lung Cancer: Clinical Development And Future Directions. Onco Targets Ther. 2019, 12, 7857–7864. [Google Scholar] [CrossRef] [PubMed]
- Molina-Arcas, M.; Moore, C.; Rana, S.; van Maldegem, F.; Mugarza, E.; Romero-Clavijo, P.; Herbert, E.; Horswell, S.; Li, L.; Janes, M.R.; et al. Development of combination therapies to maximize the impact of KRAS-G12C inhibitors in lung cancer. Sci. Transl. Med. 2019, 11, eaaw7999. [Google Scholar] [CrossRef] [PubMed]
- Mitsudomi, T.; Morita, S.; Yatabe, Y.; Negoro, S.; Okamoto, I.; Tsurutani, J.; Seto, T.; Satouchi, M.; Tada, H.; Hirashima, T.; et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010, 11, 121–128. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Suster, D.I.; Mino-Kenudson, M. Molecular Pathology of Primary Non-small Cell Lung Cancer. Arch Med. Res. 2020, 51, 784–798. [Google Scholar] [CrossRef]
- Network, N.C.C. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Non-small cell lung cancer. Cent. Nerv. Syst. Cancers Version 2011, 2, 19–21. [Google Scholar]
- Canon, J.; Rex, K.; Saiki, A.Y.; Mohr, C.; Cooke, K.; Bagal, D.; Gaida, K.; Holt, T.; Knutson, C.G.; Koppada, N.; et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019, 575, 217–223. [Google Scholar] [CrossRef]
- Nagasaka, M.; Li, Y.; Sukari, A.; Ou, S.I.; Al-Hallak, M.N.; Azmi, A.S. KRAS G12C Game of Thrones, which direct KRAS inhibitor will claim the iron throne? Cancer Treat. Rev. 2020, 84, 101974. [Google Scholar] [CrossRef]
- Ionescu, D.N.; Stockley, T.L.; Banerji, S.; Couture, C.; Mather, C.A.; Xu, Z.; Blais, N.; Cheema, P.K.; Chu, Q.S.; Melosky, B.; et al. Consensus Recommendations to Optimize Testing for New Targetable Alterations in Non-Small Cell Lung Cancer. Curr. Oncol. 2022, 29, 4981–4997. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.M.; Blais, N.; Soulieres, D.; Ionescu, D.N.; Kashyap, M.; Liu, G.; Melosky, B.; Reiman, T.; Romeo, P.; Shepherd, F.A.; et al. A systematic review and Canadian consensus recommendations on the use of biomarkers in the treatment of non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Blanc-Durand, F.; Florescu, M.; Tehfe, M.; Routy, B.; Alameddine, R.; Tran-Thanh, D.; Blais, N. Improvement of EGFR Testing over the Last Decade and Impact of Delaying TKI Initiation. Curr. Oncol. 2021, 28, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Sekhon, H.S.; Cutz, J.C.; Hwang, D.M.; Kamel-Reid, S.; Carter, R.F.; Santos, G.d.C.; Waddell, T.; Binnie, M.; Patel, M.; et al. Improving molecular testing and personalized medicine in non-small-cell lung cancer in Ontario. Curr. Oncol. 2017, 24, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Melosky, B.; Blais, N.; Cheema, P.; Couture, C.; Juergens, R.; Kamel-Reid, S.; Tsao, M.; Wheatley-Price, P.; Xu, Z.; Ionescu, D.N. Standardizing biomarker testing for Canadian patients with advanced lung cancer. Curr. Oncol. 2018, 25, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Kuang, S.; Fung, A.S.; Perdrizet, K.A.; Chen, K.; Li, J.J.N.; Le, L.W.; Cabanero, M.; Karsaneh, O.A.A.; Tsao, M.S.; Morganstein, J.; et al. Upfront Next Generation Sequencing in Non-Small Cell Lung Cancer. Curr. Oncol. 2022, 29, 4428–4437. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B. AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Pan, D.; Hu, A.Y.; Antonia, S.J.; Li, C. A Gene Mutation Signature Predicting Immunotherapy Benefits in Patients With NSCLC. J. Thorac. Oncol. 2021, 16, 419–427. [Google Scholar] [CrossRef]
- BC Cancer. BC Cancer launches lung-screening program. Available online: http://www.bccancer.bc.ca/about/news-stories/stories/bc-cancer-launches-lung-screening-program (accessed on 17 December 2022).
- Dogan, S.; Shen, R.; Ladanyi, M.; Ang, D.C.; Johnson, M.L.; D'Angelo, S.P.; Paik, P.K.; Brzostowski, E.B.; Riely, G.J.; Kris, M.G.; et al. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: Higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin. Cancer Res. 2012, 18, 6169–6177. [Google Scholar] [CrossRef]
- Graham, R.P.; Treece, A.L.; Lindeman, N.I.; Vasalos, P.; Shan, M.; Jennings, L.J.; Rimm, D.L. Worldwide Frequency of Commonly Detected EGFR Mutations. Arch Pathol. Lab. Med. 2018, 142, 163–167. [Google Scholar] [CrossRef]
- Boch, C.; Kollmeier, J.; Roth, A.; Stephan-Falkenau, S.; Misch, D.; Grüning, W.; Bauer, T.T.; Mairinger, T. The frequency of EGFR and KRAS mutations in non-small cell lung cancer (NSCLC): Routine screening data for central Europe from a cohort study. BMJ Open 2013, 3, e002560. [Google Scholar] [CrossRef]
- Cortes-Funes, H.; Gomez, C.; Rosell, R.; Valero, P.; Garcia-Giron, C.; Velasco, A.; Izquierdo, A.; Diz, P.; Camps, C.; Castellanos, D.; et al. Epidermal growth factor receptor activating mutations in Spanish gefitinib-treated non-small-cell lung cancer patients. Ann. Oncol. 2005, 16, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Reinersman, J.M.; Johnson, M.L.; Riely, G.J.; Chitale, D.A.; Nicastri, A.D.; Soff, G.A.; Schwartz, A.G.; Sima, C.S.; Ayalew, G.; Lau, C.; et al. Frequency of EGFR and KRAS mutations in lung adenocarcinomas in African Americans. J. Thorac. Oncol. 2011, 6, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhong, W.; Li, L.; Zhang, X.; Zhang, L.; Zhou, C.; Liu, W.; Jiang, B.; Mu, X.; Lin, J.; et al. Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non-small cell lung cancer: A meta-analysis based on updated individual patient data from six medical centers in mainland China. J. Thorac. Oncol. 2007, 2, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Oh, S.Y.; Kim, W.S.; Kim, S.J.; Yoo, G.H.; Kim, W.D.; Lee, K.Y. Clinical investigation of EGFR mutation detection by pyrosequencing in lung cancer patients. Oncol. Lett. 2013, 5, 271–276. [Google Scholar] [CrossRef][Green Version]
- Shi, Y.; Au, J.S.; Thongprasert, S.; Srinivasan, S.; Tsai, C.; Khoa, M.T.; Heeroma, K.; Itoh, Y.; Cornelio, G.; Yang, P.A. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J. Thorac. Oncol. 2014, 9, 154–162. [Google Scholar] [CrossRef]
- Pao, W.; Miller, V.A. Epidermal growth factor receptor mutations, small-molecule kinase inhibitors, and non-small-cell lung cancer: Current knowledge and future directions. J. Clin. Oncol. 2005, 23, 2556–2568. [Google Scholar] [CrossRef]
- Shigematsu, H.; Lin, L.; Takahashi, T.; Nomura, M.; Suzuki, M.; Wistuba, I.I.; Fong, K.M.; Lee, H.; Toyooka, S.; Shimizu, N.; et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J. Natl. Cancer Inst. 2005, 97, 339–346. [Google Scholar] [CrossRef]
- Statistics Canada. 2016 Census of Population. Vancouver [Census Metropolitan Area], British Columbia and British Columbia [Province]. Available online: https://www12.statcan.gc.ca/census-recensement/2016/ (accessed on 17 December 2022).
- Interior Health. Health Authority Profile 2020. Available online: https://www.interiorhealth.ca/sites/default/files/PDFS/interior-health-authority-profile.pdf (accessed on 17 December 2022).
- Kasymjanova, G.; Small, D.; Cohen, V.; Jagoe, R.T.; Batist, G.; Sateren, W.; Ernst, P.; Pepe, C.; Sakr, L.; Agulnik, J. Lung cancer care trajectory at a Canadian centre: An evaluation of how wait times affect clinical outcomes. Curr. Oncol. 2017, 24, 302–309. [Google Scholar] [CrossRef]
- BTS recommendations to respiratory physicians for organising the care of patients with lung cancer. The Lung Cancer Working Party of the British Thoracic Society Standards of Care Committee. Thorax 1998, 53 (Suppl. 1), S1–S8.
- Kmietowicz, Z. Cancer guidelines from NICE aim to reduce variation in referral times. BMJ 2005, 331, 10. [Google Scholar] [CrossRef][Green Version]
- Allgar, V.L.; Neal, R.D. Delays in the diagnosis of six cancers: Analysis of data from the National Survey of NHS Patients: Cancer. Br. J. Cancer 2005, 92, 1959–1970. [Google Scholar] [CrossRef]
- National Audit Office. NHS Waiting Times for Elective Care in England. Available online: https://www.nao.org.uk/reports/nhs-waiting-times-elective-care-england-2/ (accessed on 17 December 2022).
- Malin, J.L.; Asch, S.M.; Kerr, E.A.; McGlynn, E.A. Evaluating the quality of cancer care: Development of cancer quality indicators for a global quality assessment tool. Cancer 2000, 88, 701–707. [Google Scholar] [CrossRef]
- Olsson, J.K.; Schultz, E.M.; Gould, M.K. Timeliness of care in patients with lung cancer: A systematic review. Thorax 2009, 64, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Lennes, I.T.; Lynch, T.J. Quality indicators in cancer care: Development and implementation for improved health outcomes in non-small-cell lung cancer. Clin. Lung. Cancer 2009, 10, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Cancer Care Ontario. Target Wait Times for Cancer Surgery in Ontario. 2006. Available online: https://www.cancercareontario.ca/sites/ccocancercare/files/guidelines/full/SurgWTTargetsRpt_0.pdf (accessed on 17 December 2022).
- Van de Vosse, D.; Chowdhury, R.; Boyce, A.; Halperin, R. Wait Times Experienced by Lung Cancer Patients in the BC Southern Interior to Obtain Oncologic Care: Exploration of the Intervals from First Abnormal Imaging to Oncologic Treatment. Cureus 2015, 7, e330. [Google Scholar] [CrossRef]
- Lo, D.S.; Zeldin, R.A.; Skrastins, R.; Fraser, I.M.; Newman, H.; Monavvari, A.; Ung, Y.C.; Joseph, H.; Downton, T.; Maxwell, L.; et al. Time to treat: A system redesign focusing on decreasing the time from suspicion of lung cancer to diagnosis. J. Thorac. Oncol. 2007, 2, 1001–1006. [Google Scholar] [CrossRef]
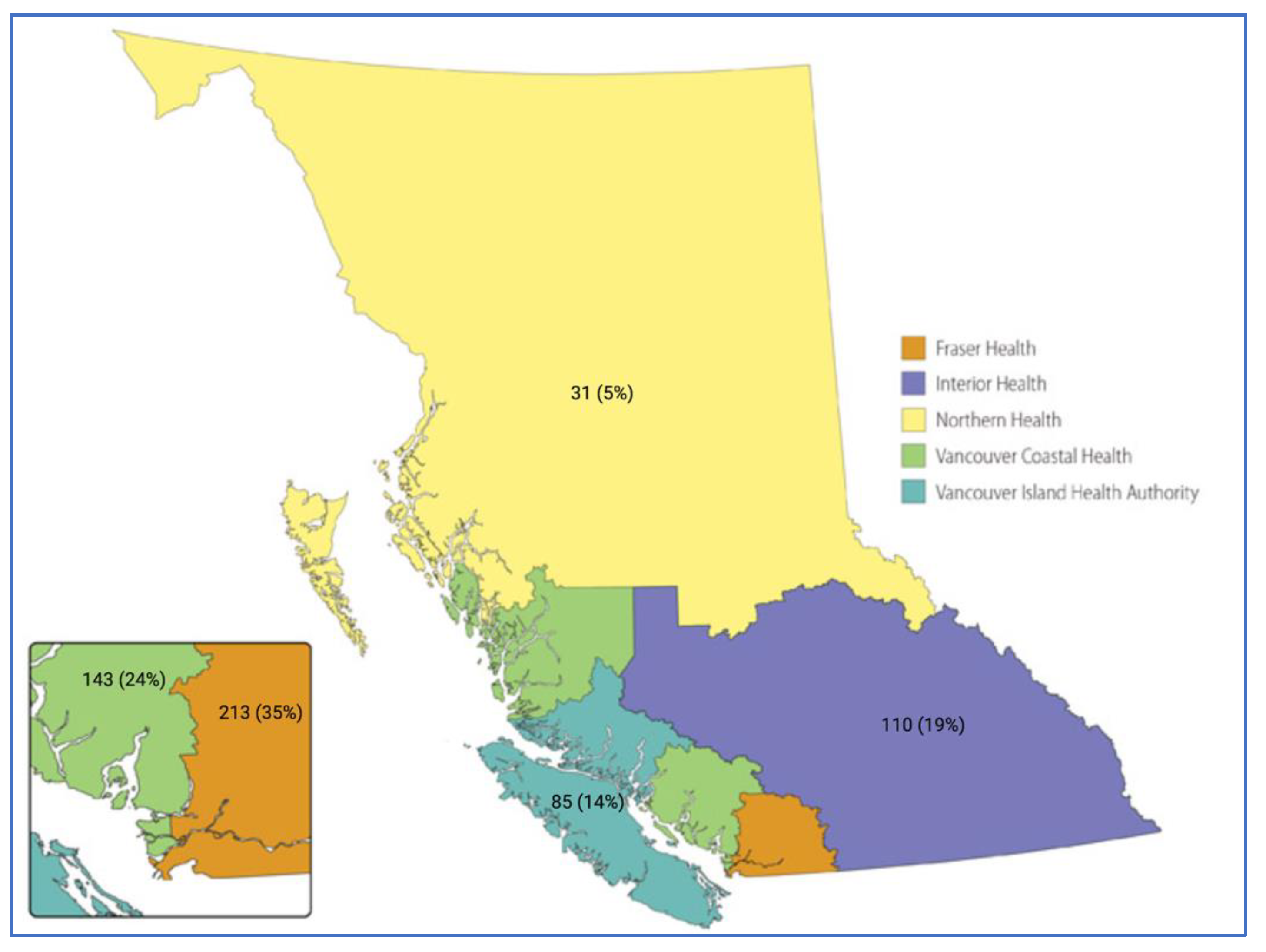
| Characteristic | Total (n = 582) | VCHA | FHA | IHA | NHA | isHA | p-Value * |
|---|---|---|---|---|---|---|---|
| (n = 143) | (n = 213) | (n = 110) | (n = 31) | (n = 85) | |||
| Female, n (%) | 326 (56) | 87 (60.8) | 107 (50.2) | 70 (63.6) | 17 (54.8) | 45 (52.9) | 0.19 |
| Male, n (%) | 256 (44) | 56 (39.2) | 106 (49.8) | 40 (36.4) | 14 (45.2) | 40 (47.1) | |
| Age at presentation, mean (SD) | 71 (10.1) | 70 (11.7) | 72 (10.3) | 71 (9.0) | 72 (8.2) | 72 (8.7) | 0.29 ** |
| Smoking status, n (%) | <0.001 | ||||||
| Never smoker | 121 (20.4) | 49 (34.3) | 45 (21.1) | 11 (10.0) | 2 (6.5) | 14 (16.5) | |
| Former smoker | 351 (59.2) | 71 (49.6) | 130 (61.0) | 73 (66.4) | 19 (61.3) | 54 (63.5) | |
| Current smoker | 104 (17.5) | 21 (14.7) | 37 (17.4) | 20 (18.2) | 10 (32.3) | 14 (16.5) | |
| No record | 17 (2.9) | 2 (1.4) | 2 (1.4) | 6 (5.5) | - | 3 (3.5) | |
| NSCLC histology, n (%) | 0.03 | ||||||
| Adenocarcinoma | 482 (82.8) | 119 (83.2) | 176 (82.6) | 87 (79.1) | 28 (90.3) | 72 (84.7) | |
| Squamous cell | 10 (1.7) | 5 (3.5) | 3 (1.41) | - | - | 2 (2.4) | |
| Adenosquamous carcinoma | 4 (<1) | 1 (0.7) | 2 (0.9) | 1 (0.9) | - | - | |
| NOS | 81 (13.9) | 18 (12.6) | 32 (15.0) | 21 (19.1) | 3 (9.7) | 7 (8.2) | |
| Large cell carcinoma | 2 (<1) | - | - | 1 (0.9) | - | 1 (1.2) | |
| Sarcomatous carcinoma | 3 (<1) | - | - | - | - | 3 (3.5) | |
| Any molecular alteration detected, n (%) | 540 (92.8) | 134 (93.7) | 197 (92.5) | 104 (94.6) | 26 (83.9) | 79 (93) | 0.36 |
| Actionable NSCLC molecular alteration, n (%) | 264 (45.4) | 73 (51.1) | 100 (47) | 42 (38.2) | 17 54.8) | 35 (41.2) | 0.29 |
| PDL1 expression, n (%) | 0.49 ** | ||||||
| <1% | 212 (36.4) | 46 (32.2) | 92 (43.2) | 36 (32.7) | 12 (38.7) | 26 (30.6) | |
| 1–49% | 128 (22.0) | 34 (23.8) | 43 (20.2) | 23 (20.9) | 6 (19.4) | 22 (25.9) | |
| >50% | 233 (40.0) | 59 (41.2) | 75 (35.2) | 49 (44.6) | 13 (41.9) | 37 (43.5) | |
| Not performed/no record | 9 (1.6) | 4 (2.8) | 3 (1.4) | 2 (1.8) | - | - |
| Total (n = 582) | VCHA | FHA | IHA | NHA | isHA | p-Value * | |
|---|---|---|---|---|---|---|---|
| (n = 143) | (n = 213) | (n = 110) | (n = 31) | (n = 85) | |||
| Patient with at least one actionable NSCLC | 264 (45.4) | 73 (51.1) | 100 (47) | 42 (38.2) | 17 (54.8) | 35 (41.2) | 0.29 |
| molecular alteration | |||||||
| n (%) | |||||||
| Any EGFRm + | 105 (18%) EGFRm+ in 582 | 42 (70.6) | 42 (19.7) | 11 (10) | - | 10 (11.8) | <0.001 |
| n (%) | patients | ||||||
| Description of EGFRm subtype detected overall and by a health authority (one patient may have >1 EGFRm detected), n (%) | |||||||
| Common sensitizing EGFRm+ | 84 (14.4) mutations | 35 (24.5) | 34 (16) | 10 (9.1) | - | 5 (5.9) | <0.001 |
| EGFR exon 19 deletion | |||||||
| EGFR L858R | 41 (7) | ||||||
| 43 (7.4) | 19 (13.2) | 17 (8) | 4 (3.6) | - | 1 (1.2) | ||
| 16 (11.2) | 17 (8) | 6 (5.45) | - | 4 (4.7) | |||
| Uncommon sensitizing EGFRm+ | 16 (2.8) mutations | 3 (2.1) | 8 (3.8) | 2 (1.8) | - | 3 (3.53) | 0.35 |
| EGFR G709X | 4 | ||||||
| EGFR G719X | 9 | 1 | 1 | 1 | - | 1 | |
| EGFR S768I | 7 | 1 | 4 | 2 | - | 3 | |
| EGFR L861Q | 3 | - | 5 | 1 | - | 1 | |
| EGFR co-mutation ** | 12 ** | 1 | 2 | - | - | - | |
| 2 ** | 6 ** | 2 ** | - | 2 ** | |||
| Uncommon non-sensitizing EGFRm+ | 11 (1.9) mutations | 6 (4.2) | 3 (1.4) | - | - | 2 (2.4) | 0.13 |
| EGFR exon 20 insertion | |||||||
| Denovo T790M | 9 (1.6) | ||||||
| 6 (4.2) | 2 (0.9) | - | - | 1 (1.2) | |||
| 2 (0.3) | |||||||
| - | 1 (0.5) | - | - | 1 (0.5) | |||
| Non-EGFR molecular alterations detected overall and by a health authority, n (%) | |||||||
| Any KRASm+ | 236 (40.6) | 49 (34.3) | 78 (36.6) | 55 (50) | 17 (54.8) | 37 (43.5) | 0.03 |
| KRAS G12C | 108 (18.6) | 17 (11.9) | 40 (18.8) | 24 (21.8) | 10 (32.3) | 17 (20) | 0.06 |
| MET exon 14 skip | 17 (2.9) | 6 (4.2) | 5 (2.4) | 1 (0.91) | 2 (6.5) | 3 (3.5) | 0.39 |
| ERRB2 (HER2) | 13 (2.2) | 6 (4.2) | 3 (1.4) | 2 (1.8) | 1 (3.2) | 1 (1.2) | 0.43 |
| BRAF V600E | 14 (2.4) | 1 (0.7) | 8 (3.8) | 1 (0.9) | 1 (3.2) | 3 (3.5) | 0.29 |
| Fusion + | 14 (2.4) | 3 (2.1) | 4 (1.9) | 3 (2.73) | 1 (3.2) | 3 (3.5) | 0.92 |
| ALK | 13 (2.2) | 3 (2.1) | 4 (1.9) | 3 (2.73) | 1 (3.2) | 2.35 | |
| ROS1 | 1 (0.2) | - | - | - | - | 1 (1.2) | |
| Health Authority | Time to Treatment—Median (days) [IQR] |
|---|---|
| Overall | 39 (27, 63) |
| VCHA | 40.5 (28.5, 61.75) |
| FHA | 36 (25, 49) |
| IHA | 55 (30, 75.5) |
| NHA | 64 (48, 71) |
| isHA | 36 (22, 68) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilzenrat, R.A.; Yip, S.; Melosky, B.; Ho, C.; Laskin, J.; Sun, S.; Choi, J.J.; McGuire, A.L. Disparate Time-to-Treatment and Varied Incidence of Actionable Non-Small Cell Lung Cancer Molecular Alterations in British Columbia: A Historical Cohort Study. Curr. Oncol. 2023, 30, 145-156. https://doi.org/10.3390/curroncol30010012
Hilzenrat RA, Yip S, Melosky B, Ho C, Laskin J, Sun S, Choi JJ, McGuire AL. Disparate Time-to-Treatment and Varied Incidence of Actionable Non-Small Cell Lung Cancer Molecular Alterations in British Columbia: A Historical Cohort Study. Current Oncology. 2023; 30(1):145-156. https://doi.org/10.3390/curroncol30010012
Chicago/Turabian StyleHilzenrat, Roy Avraham, Stephen Yip, Barbara Melosky, Cheryl Ho, Janessa Laskin, Sophie Sun, James J. Choi, and Anna L. McGuire. 2023. "Disparate Time-to-Treatment and Varied Incidence of Actionable Non-Small Cell Lung Cancer Molecular Alterations in British Columbia: A Historical Cohort Study" Current Oncology 30, no. 1: 145-156. https://doi.org/10.3390/curroncol30010012
APA StyleHilzenrat, R. A., Yip, S., Melosky, B., Ho, C., Laskin, J., Sun, S., Choi, J. J., & McGuire, A. L. (2023). Disparate Time-to-Treatment and Varied Incidence of Actionable Non-Small Cell Lung Cancer Molecular Alterations in British Columbia: A Historical Cohort Study. Current Oncology, 30(1), 145-156. https://doi.org/10.3390/curroncol30010012






