Evaluation of a Navigated 3D Ultrasound Integration for Brain Tumor Surgery: First Results of an Ongoing Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
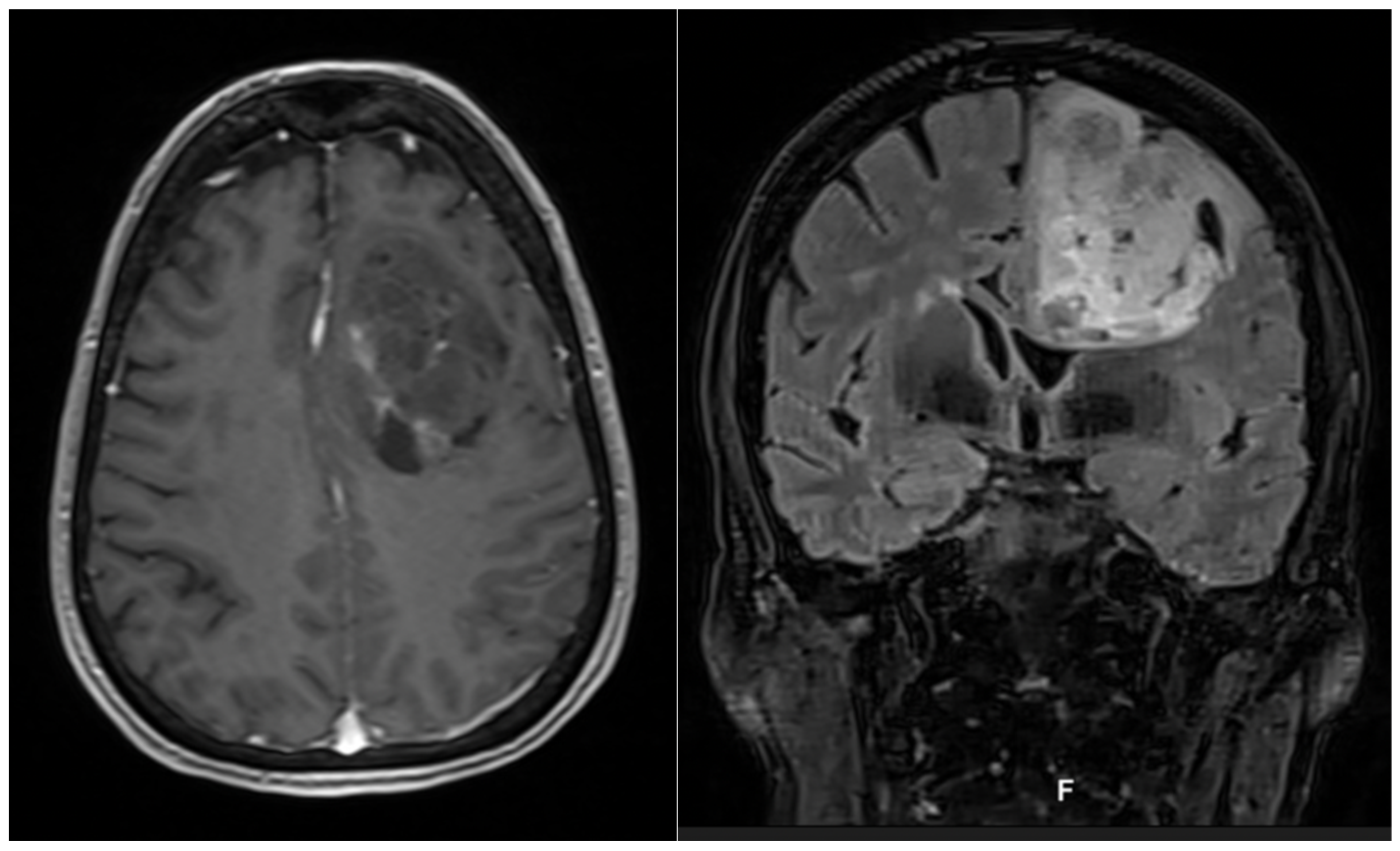
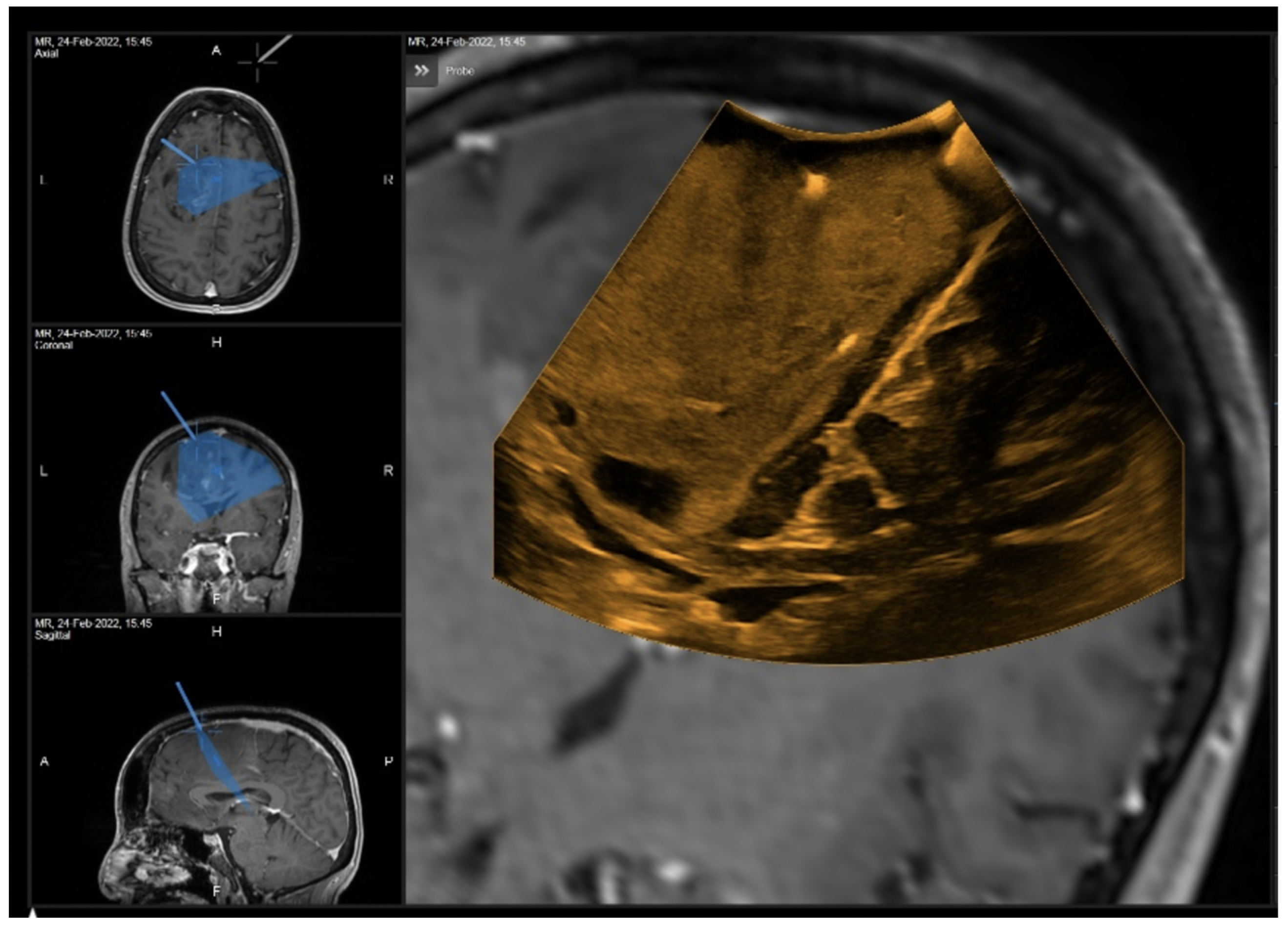
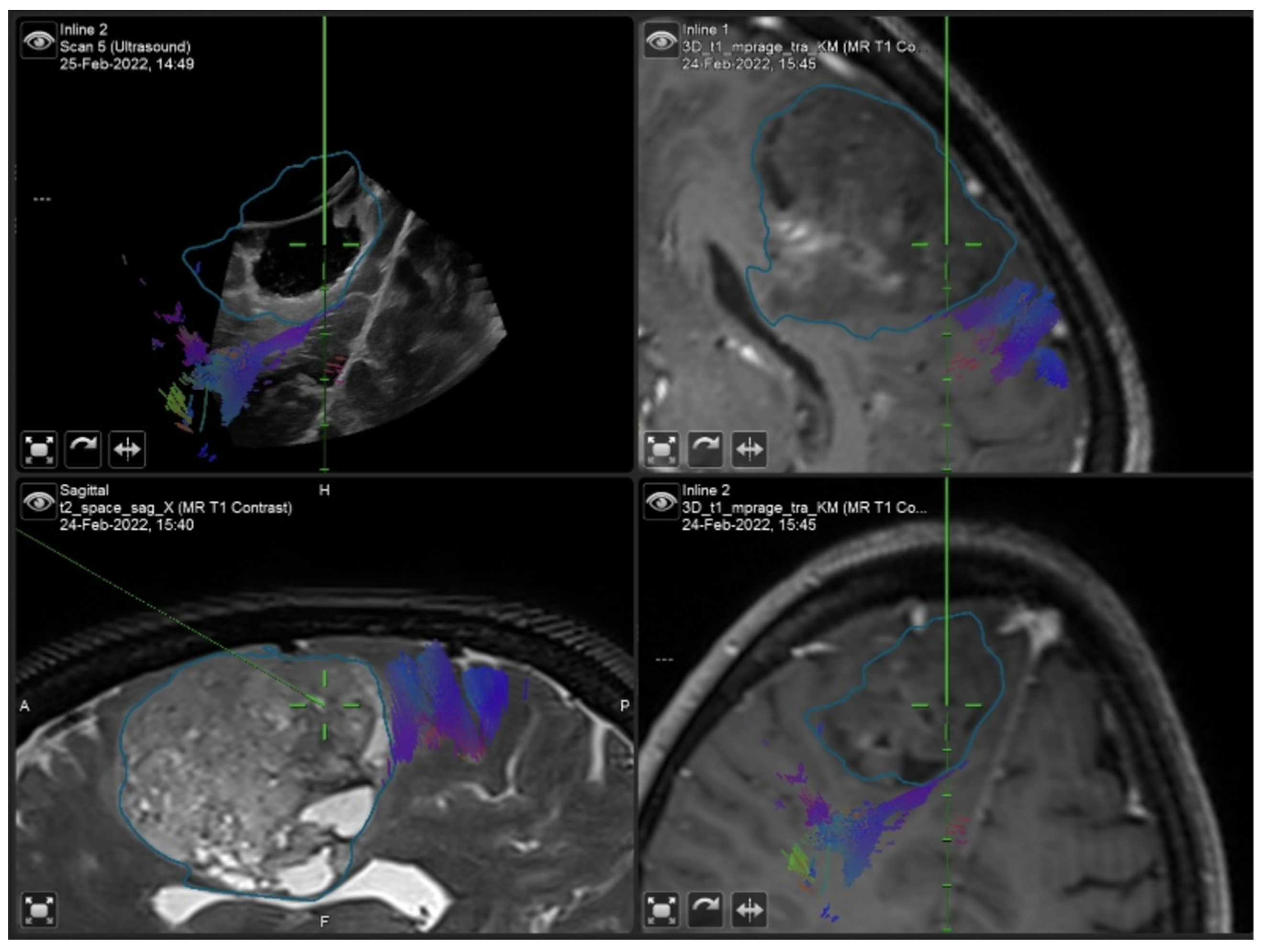
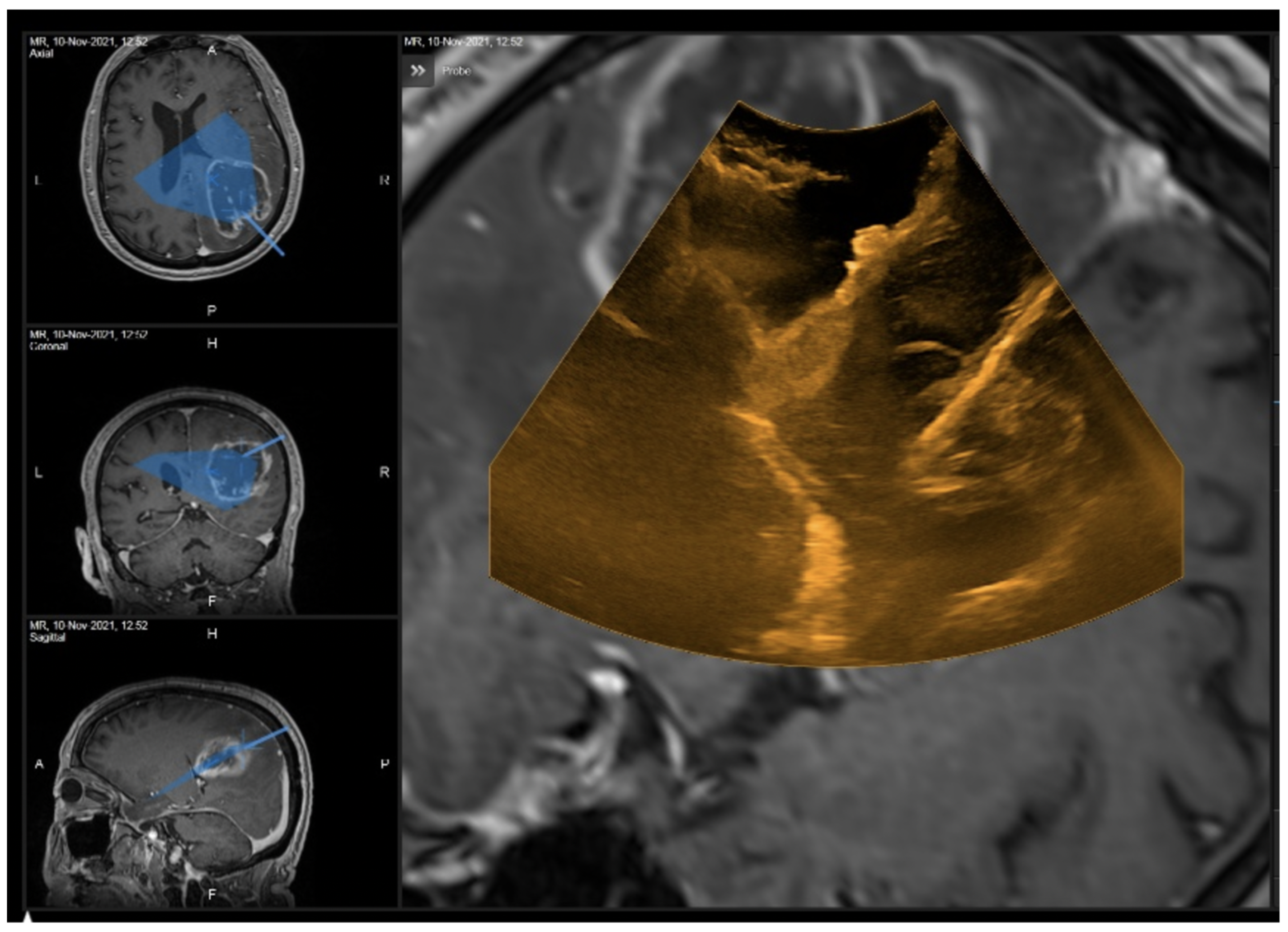
3.1. Illustrative Cases
3.1.1. Case 1
3.1.2. Case 2
3.1.3. Case 3
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| # | AGE | Volume (cm3) | Localization | Side | Eloquent Area | Recurrence | T OP (min) | T RM (min) | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | 35.6 | Temporal | Right | N | No | 255 | 45 | Metastasis |
| 2 | 53 | 2.11 | Temporal | Left | Y (speech) | Yes | 198 | 50 | Glioblastoma, IDH-wildtype |
| 3 | 33 | 1.57 | Frontal | Right | N | No | 247 | 60 | Astrocytoma IDH-mutant WHO II |
| 4 | 68 | 88.1 | Parietal | Right | Y (sight) | No | 386 | 70 | Glioblastoma, IDH-wildtype |
| 5 | 66 | 5 | Occipital | Left | Y (sight) | No | 226 | 70 | Metastasis |
| 6 | 82 | 40 | Temporal | Left | Y (speech) | No | 341 | 50 | Metastasis |
| 7 | 35 | 1 | Frontal | Right | N | Yes | 293 | 65 | Astrocytoma IDH-mutant WHO III |
| 8 | 62 | 33 | Temporal | Left | Y (speech) | No | 260 | 60 | Glioblastoma, IDH-wildtype |
| 9 | 67 | 35.3 | Frontal | Right | N | No | 243 | * | Glioblastoma, IDH-wildtype |
| 10 | 55 | 5 | Frontal | Right | Y (motor) | No | 358 | 60 | Astrocytoma IDH-mutant WHO IV |
| 11 | 77 | 35.5 | Frontal | Right | N | No | 300 | 60 | Glioblastoma, IDH-wildtype |
| 12 | 24 | 36.6 | Frontal | Right | N | No | 324 | 78 | Astrocytoma IDH-mutant WHO III |
| 13 | 66 | 56.1 | Temporal | Right | N | No | 392 | 60 | Glioblastoma, IDH-wildtype |
| 14 | 24 | 0.8 | Frontal | Right | N | No | 224 | 70 | Astrocytoma IDH-mutant WHO II |
| 15 | 56 | 59 | Frontal | Right | N | No | 405 | 87 | Oligodendroglioma WHO III |
| 16 | 64 | 78 | Frontal | Left | N | No | 474 | 77 | Oligodendroglioma WHO II |
| N | Mean | Std. Deviation | Minimum | Maximum | Percentiles | Wilcoxon Test | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 25th | 50th (Median) | 75th | HGG 2D-LGG 2D | MTX 2D-HGG 2D | MTX 2D-LGG 2D | ||||||
| LGG 2D | 9 | 2.22 | 0.833 | 1 | 4 | 2 | 2 | 2.5 | p = 1.000 | p = 0.375 | p = 0.500 |
| HGG 2D | 27 | 2.07 | 0.781 | 1 | 4 | 2 | 2 | 3 | |||
| MTX 2D | 9 | 2.56 | 0.726 | 2 | 4 | 2 | 2 | 3 | |||
| HGG 3D-LGG 3D | MTX 3D-HGG 3D | MTX 3D-LGG 3D | |||||||||
| LGG 3D | 9 | 2.33 | 0.866 | 1 | 4 | 2 | 2 | 3 | p = 0.313 | p = 0.938 | p = 0.078 |
| HGG 3D | 27 | 2.48 | 1.252 | 1 | 5 | 2 | 2 | 4 | |||
| MTX 3D | 9 | 3.22 | 1.202 | 2 | 6 | 2.5 | 3 | 3.5 | |||
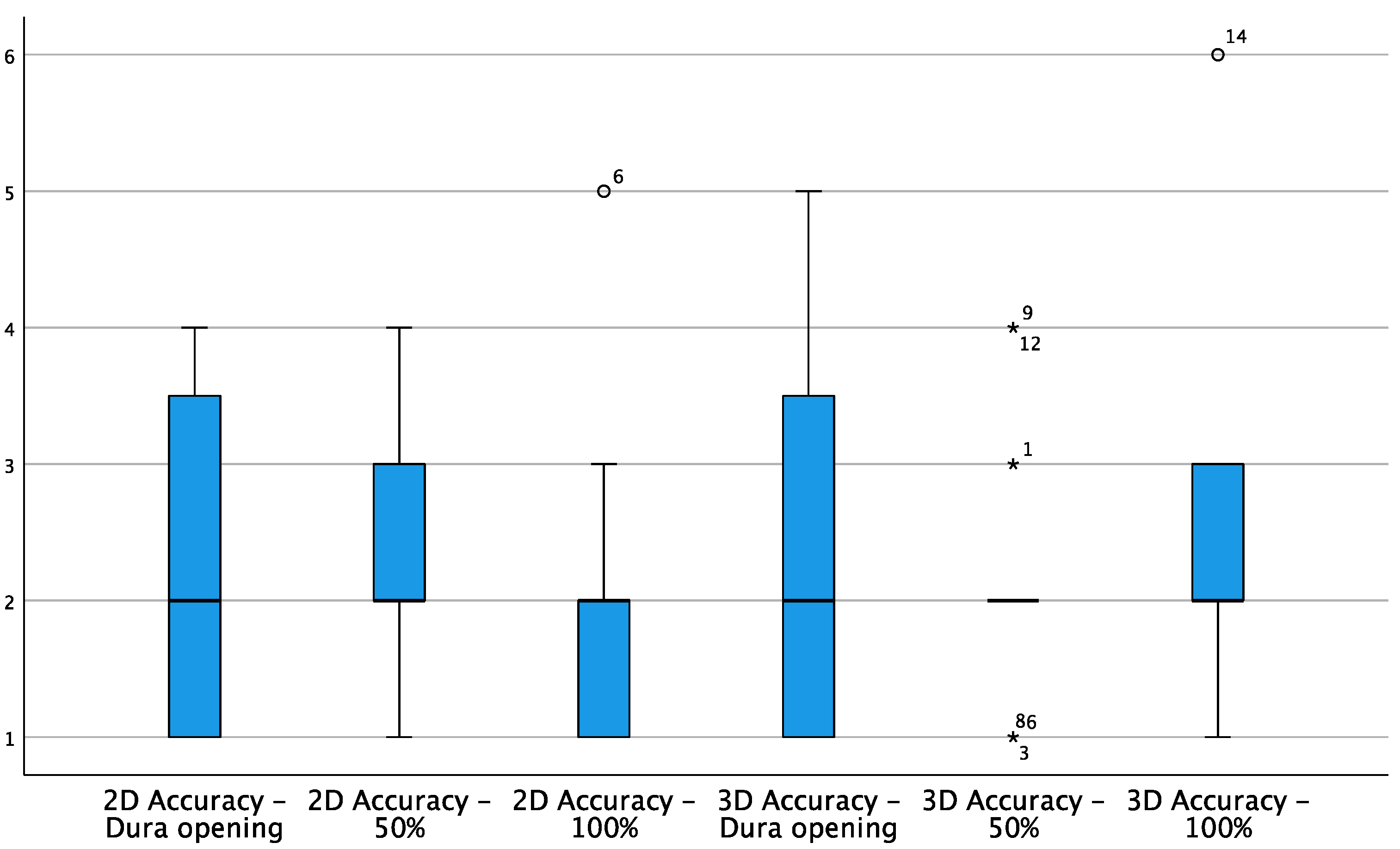
| # | 2D Accuracy—Dura Opening | 3D Accuracy—Dura Opening | 2D Accuracy—50% | 3D Accuracy—50% | 2D Accuracy—100% | 3D Accuracy—100% |
|---|---|---|---|---|---|---|
| 1 | 4 | 4 | 3 | 3 | 3 | 3 |
| 2 | 1 | 1 | 2 | 2 | * | * |
| 3 | 1 | 1 | 2 | 1 | 1 | 3 |
| 4 | 2 | 2 | 2 | 2 | 1 | 1 |
| 5 | 3 | 3 | 3 | 2 | 2 | 2 |
| 6 | 1 | 1 | 1 | 1 | 5 | 2 |
| 7 | 1 | 1 | * | * | 3 | 3 |
| 8 | 3 | 2 | 1 | 1 | 2 | 2 |
| 9 | 4 | 4 | 2 | 4 | 1 | 1 |
| 10 | 2 | 5 | * | * | 2 | 2 |
| 11 | 1 | 1 | 2 | 2 | 1 | 1 |
| 12 | 4 | 3 | 4 | 4 | * | 3 |
| 13 | 4 | 2 | 3 | 2 | 2 | 2 |
| 14 | 2 | 4 | * | * | 2 | 6 |
| 15 | 2 | 1 | 2 | 2 | 2 | 2 |
| 16 | 2 | 1 | 2 | 2 | 2 | 2 |
| Wilcoxon Test | p = 0.813 | p = 1.000 | p = 0.750 | |||
References
- Marko, N.F.; Weil, R.J.; Schroeder, J.L.; Lang, F.F.; Suki, D.; Sawaya, R.E. Extent of Resection of Glioblastoma Revisited: Personalized Survival Modeling Facilitates More Accurate Survival Prediction and Supports a Maximum-Safe-Resection Approach to Surgery. J. Clin. Oncol. 2014, 32, 774. [Google Scholar] [CrossRef]
- Wykes, V.; Zisakis, A.; Irimia, M.; Ughratdar, I.; Sawlani, V.; Watts, C. Importance and Evidence of Extent of Resection in Glioblastoma. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2021, 82, 75–86. [Google Scholar] [CrossRef]
- Jakola, A.S.; Myrmel, K.S.; Kloster, R.; Torp, S.H.; Lindal, S.; Unsgård, G.; Solheim, O. Comparison of a Strategy Favoring Early Surgical Resection vs a Strategy Favoring Watchful Waiting in Low-Grade Gliomas. JAMA 2012, 308, 1881. [Google Scholar] [CrossRef] [PubMed]
- Eyüpoglu, I.Y.; Buchfelder, M.; Savaskan, N.E. Surgical Resection of Malignant Gliomas—Role in Optimizing Patient Outcome. Nat. Rev. Neurol. 2013, 9, 141–151. [Google Scholar] [CrossRef]
- Wijnenga, M.M.J.; French, P.J.; Dubbink, H.J.; Dinjens, W.N.M.; Atmodimedjo, P.N.; Kros, J.M.; Smits, M.; Gahrmann, R.; Rutten, G.-J.; Verheul, J.B. The Impact of Surgery in Molecularly Defined Low-Grade Glioma: An Integrated Clinical, Radiological, and Molecular Analysis. Neuro-Oncol. 2018, 20, 103–112. [Google Scholar] [CrossRef]
- Winther, R.R.; Hjermstad, M.J.; Skovlund, E.; Aass, N.; Helseth, E.; Kaasa, S.; Yri, O.E.; Vik-Mo, E.O. Surgery for Brain Metastases—Impact of the Extent of Resection. Acta Neurochir. 2022, 1–8. [Google Scholar] [CrossRef]
- Nimsky, C.; Ganslandt, O.; Cerny, S.; Hastreiter, P.; Greiner, G.; Fahlbusch, R. Quantification of, Visualization of, and Compensation for Brain Shift Using Intraoperative Magnetic Resonance Imaging. Neurosurgery 2000, 47, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, A.; Black, P.M.; Gering, D.T.; Westin, C.-F.; Mehta, V.; Pergolizzi Jr, R.S.; Ferrant, M.; Warfield, S.K.; Hata, N.; Schwartz, R.B. Serial Intraoperative Magnetic Resonance Imaging of Brain Shift. Neurosurgery 2001, 48, 787–798. [Google Scholar] [PubMed]
- Nimsky, C. Intraoperative MRI in Glioma Surgery: Proof of Benefit? Lancet Oncol. 2011, 11, 982–983. [Google Scholar] [CrossRef]
- Coburger, J.; Nabavi, A.; König, R.; Wirtz, C.R.; Pala, A. Contemporary Use of Intraoperative Imaging in Glioma Surgery: A Survey among EANS Members. Clin. Neurol. Neurosurg. 2017, 163, 133–141. [Google Scholar] [CrossRef]
- Rubin, J.M.; Mirfakhraee, M.; Duda, E.E.; Dohrmann, G.J.; Brown, F. Intraoperative Ultrasound Examination of the Brain. Radiology 1980, 137, 831–832. [Google Scholar] [CrossRef]
- Chandler, W.F.; Rubin, J.M. The Application of Ultrasound during Brain Surgery. World J. Surg. 1987, 11, 558–569. [Google Scholar] [CrossRef]
- Prada, F.; Solbiati, L.E.; Martegani, A.E.; DiMeco, F.E. Intraoperative Ultrasound (IOUS) in Neurosurgery. In From Standard B-mode to Elastosonography; Springer International Publishing: Berlin, Germany, 2016. [Google Scholar]
- Šteňo, A.; Buvala, J.; Babkova, V.; Kiss, A.; Toma, D.; Lysak, A. Current Limitations of Intraoperative Ultrasound in Brain Tumor Surgery. Front. Oncol. 2021, 11, 659048. [Google Scholar] [CrossRef]
- Selbekk, T.; Jakola, A.S.; Solheim, O.; Johansen, T.F.; Lindseth, F.; Reinertsen, I.; Unsgård, G. Ultrasound Imaging in Neurosurgery: Approaches to Minimize Surgically Induced Image Artefacts for Improved Resection Control. Acta Neurochir. 2013, 155, 973–980. [Google Scholar] [CrossRef]
- Mair, R.; Heald, J.; Poeata, I.; Ivanov, M. A Practical Grading System of Ultrasonographic Visibility for Intracerebral Lesions. Acta Neurochir. 2013, 155, 2293–2298. [Google Scholar] [CrossRef]
- Schlaier, J.R.; Warnat, J.; Dorenbeck, U.; Proescholdt, M.; Schebesch, K.-M.; Brawanski, A. Image Fusion of MR Images and Real-Time Ultrasonography: Evaluation of Fusion Accuracy Combining Two Commercial Instruments, a Neuronavigation System and a Ultrasound System. Acta Neurochir. 2004, 146, 271–277. [Google Scholar] [CrossRef]
- Lindseth, F.; Kaspersen, J.H.; Ommedal, S.; Langø, T.; Bang, J.; Hokland, J.; Unsgaard, G.; Nagelhus Hemes, T.A. Multimodal Image Fusion in Ultrasound-Based Neuronavigation: Improving Overview and Interpretation by Integrating Preoperative MRI with Intraoperative 3D Ultrasound. Comput. Aided Surg. 2003, 8, 49–69. [Google Scholar] [CrossRef]
- Coburger, J.; Scheuerle, A.; Kapapa, T.; Engelke, J.; Thal, D.R.; Wirtz, C.R.; König, R. Sensitivity and Specificity of Linear Array Intraoperative Ultrasound in Glioblastoma Surgery: A Comparative Study with High Field Intraoperative MRI and Conventional Sector Array Ultrasound. Neurosurg. Rev. 2015, 38, 499–509. [Google Scholar] [CrossRef]
- Coburger, J.; Scheuerle, A.; Thal, D.R.; Engelke, J.; Hlavac, M.; Wirtz, C.R.; König, R. Linear Array Ultrasound in Low-Grade Glioma Surgery: Histology-Based Assessment of Accuracy in Comparison to Conventional Intraoperative Ultrasound and Intraoperative MRI. Acta Neurochir. 2015, 157, 195–206. [Google Scholar] [CrossRef]
- Moiyadi, A. V Linear Intraoperative Ultrasound Probes and Phased-Array Probes: Two Sides of the Same Coin. Acta Neurochir. 2015, 157, 957–958. [Google Scholar] [CrossRef]
- Tronnier, V.M.; Bonsanto, M.M.; Staubert, A.; Knauth, M.; Kunze, S.; Wirtz, C.R. Comparison of Intraoperative MR Imaging and 3D-Navigated Ultrasonography in the Detection and Resection Control of Lesions. Neurosurg. Focus 2001, 10, 1–5. [Google Scholar] [CrossRef]
- Moiyadi, A.V.; Unsgård, G. Navigable Ultrasound, 3D Ultrasound and Fusion Imaging in Neurosurgery. In Intraoperative Ultrasound (IOUS) in Neurosurgery; Springer: Berlin, Germany, 2016; pp. 135–145. [Google Scholar]
- Gronningsaeter, A.; Kleven, A.; Ommedal, S.; Aarseth, T.E.; Lie, T.; Lindseth, F.; Langø, T.; Unsgård, G. SonoWand, an Ultrasound-Based Neuronavigation System. Neurosurgery 2000, 47, 1373–1380. [Google Scholar] [CrossRef]
- Solheim, O.; Selbekk, T.; Jakola, A.S.; Unsgård, G. Ultrasound-Guided Operations in Unselected High-Grade Gliomas—Overall Results, Impact of Image Quality and Patient Selection. Acta Neurochir. 2010, 152, 1873–1886. [Google Scholar] [CrossRef]
- Knauth, M.; Wirtz, C.R.; Tronnier, V.M.; Aras, N.; Kunze, S.; Sartor, K. Intraoperative MR Imaging Increases the Extent of Tumor Resection in Patients with High-Grade Gliomas. Am. J. Neuroradiol. 1999, 20, 1642–1646. [Google Scholar]
- Rainer Wirtz, W.S.; Albert, F.K.; Schwaderer, M.; Heuer, C.; Staubert, A.; Tronnier, V.M.; Knauth, M.; Kunze, S. The Benefit of Neuronavigation for Neurosurgery Analyzed by Its Impact on Glioblastoma Surgery. Neurol. Res. 2000, 22, 354–360. [Google Scholar] [CrossRef]
- Bohinski, R.J.; Kokkino, A.K.; Gaskill-Shipley, M.F.; Kormos, D.W.; Tew, J.M. Glioma Resection in a Shared-Resource Magnetic Resonance Operating Room after Optimal Image-Guided Frameless Stereotactic Resection. Neurosurgery 2001, 48, 731–744. [Google Scholar] [CrossRef]
- Hirschberg, H.; Samset, E.; Hol, P.K.; Tillung, T.; Lote, K. Impact of Intraoperative MRI on the Surgical Results for High-Grade Gliomas. min-Minim. Invasive Neurosurg. 2005, 48, 77–84. [Google Scholar] [CrossRef]
- Schneider, J.P.; Trantakis, C.; Rubach, M.; Schulz, T.; Dietrich, J.; Winkler, D.; Renner, C.; Schober, R.; Geiger, K.; Brosteanu, O. Intraoperative MRI to Guide the Resection of Primary Supratentorial Glioblastoma Multiforme—a Quantitative Radiological Analysis. Neuroradiology 2005, 47, 489–500. [Google Scholar] [CrossRef]
- Hatiboglu, M.A.; Weinberg, J.S.; Suki, D.; Tummala, S.; Rao, G.; Sawaya, R.; Prabhu, S.S. Utilization of Intraoperative Motor Mapping in Glioma Surgery with High-Field Intraoperative Magnetic Resonance Imaging. Stereotact. Funct. Neurosurg. 2010, 88, 345–352. [Google Scholar] [CrossRef]
- Pamir, M.N.; Özduman, K.; Dinçer, A.; Yildiz, E.; Peker, S.; Özek, M.M. First Intraoperative, Shared-Resource, Ultrahigh-Field 3-Tesla Magnetic Resonance Imaging System and Its Application in Low-Grade Glioma Resection. J. Neurosurg. 2010, 112, 57–69. [Google Scholar] [CrossRef]
- Senft, C.; Bink, A.; Franz, K.; Vatter, H.; Gasser, T.; Seifert, V. Intraoperative MRI Guidance and Extent of Resection in Glioma Surgery: A Randomised, Controlled Trial. Lancet Oncol. 2011, 12, 997–1003. [Google Scholar] [CrossRef]
- Lothes, T.E.; Siekmann, M.; König, R.W.; Wirtz, C.R.; Coburger, J. Surgical Workflow Analysis: Ideal Application of Navigated Linear Array Ultrasound in Low-Grade Glioma Surgery. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2016, 77, 466–473. [Google Scholar]
- Uneri, A.; Otake, Y.; Wang, A.S.; Kleinszig, G.; Vogt, S.; Khanna, A.J.; Siewerdsen, J.H. 3D–2D Registration for Surgical Guidance: Effect of Projection View Angles on Registration Accuracy. Phys. Med. Biol. 2013, 59, 271. [Google Scholar] [CrossRef] [PubMed]
- Jakola, A.S.; Jørgensen, A.; Selbekk, T.; Michler, R.-P.; Solheim, O.; Torp, S.H.; Sagberg, L.M.; Aadahl, P.; Unsgård, G. Animal Study Assessing Safety of an Acoustic Coupling Fluid That Holds the Potential to Avoid Surgically Induced Artifacts in 3D Ultrasound Guided Operations. BMC Med. Imaging 2014, 14, 1–9. [Google Scholar] [CrossRef]
- Unsgård, G.; Sagberg, L.M.; Müller, S.; Selbekk, T. A New Acoustic Coupling Fluid with Ability to Reduce Ultrasound Imaging Artefacts in Brain Tumour Surgery—a Phase I Study. Acta Neurochir. 2019, 161, 1475–1486. [Google Scholar] [CrossRef] [PubMed]
- Bastos, D.C.D.A.; Juvekar, P.; Tie, Y.; Jowkar, N.; Pieper, S.; Wells, W.M.; Bi, W.L.; Golby, A.; Frisken, S.; Kapur, T. Challenges and Opportunities of Intraoperative 3D Ultrasound with Neuronavigation in Relation to Intraoperative MRI. Front. Oncol. 2021, 11, 656519. [Google Scholar] [CrossRef]
- Incekara, F.; Smits, M.; Dirven, L.; Bos, E.M.; Balvers, R.K.; Haitsma, I.K.; Schouten, J.W.; Vincent, A.J.P.E. Intraoperative B-Mode Ultrasound Guided Surgery and the Extent of Glioblastoma Resection: A Randomized Controlled Trial. Front. Oncol. 2021, 11, 649797. [Google Scholar] [CrossRef]
- Giammalva, G.R.; Ferini, G.; Musso, S.; Salvaggio, G.; Pino, M.A.; Gerardi, R.M.; Brunasso, L.; Costanzo, R.; Paolini, F.; Di Bonaventura, R.; et al. Intraoperative Ultrasound: Emerging Technology and Novel Applications in Brain Tumor Surgery. Front. Oncol. 2022, 12, 818446. [Google Scholar] [CrossRef]
- Saß, B.; Zivkovic, D.; Pojskic, M.; Nimsky, C.; Bopp, M.H.A. Navigated Intraoperative 3D Ultrasound in Glioblastoma Surgery: Analysis of Imaging Features and Impact on Extent of Resection. Front. Neurosci. 2022, 16, 883584. [Google Scholar] [CrossRef]
- Della Pepa, G.M.; Ius, T.; La Rocca, G.; Gaudino, S.; Isola, M.; Pignotti, F.; Rapisarda, A.; Mazzucchi, E.; Giordano, C.; Dragonetti, V.; et al. 5-Aminolevulinic Acid and Contrast-Enhanced Ultrasound: The Combination of the Two Techniques to Optimize the Extent of Resection in Glioblastoma Surgery. Neurosurgery 2020, 86, E529–E540. [Google Scholar] [CrossRef]
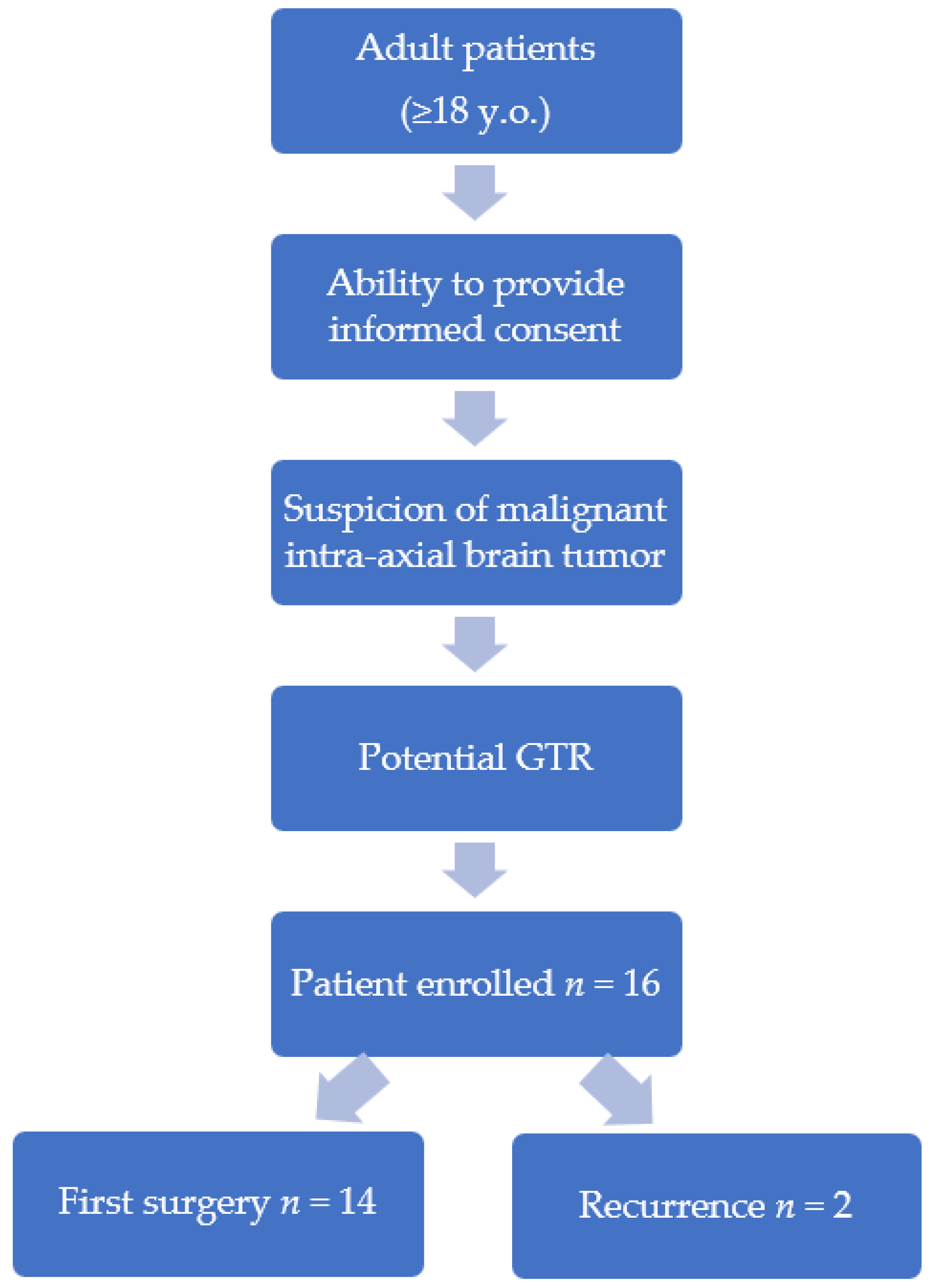
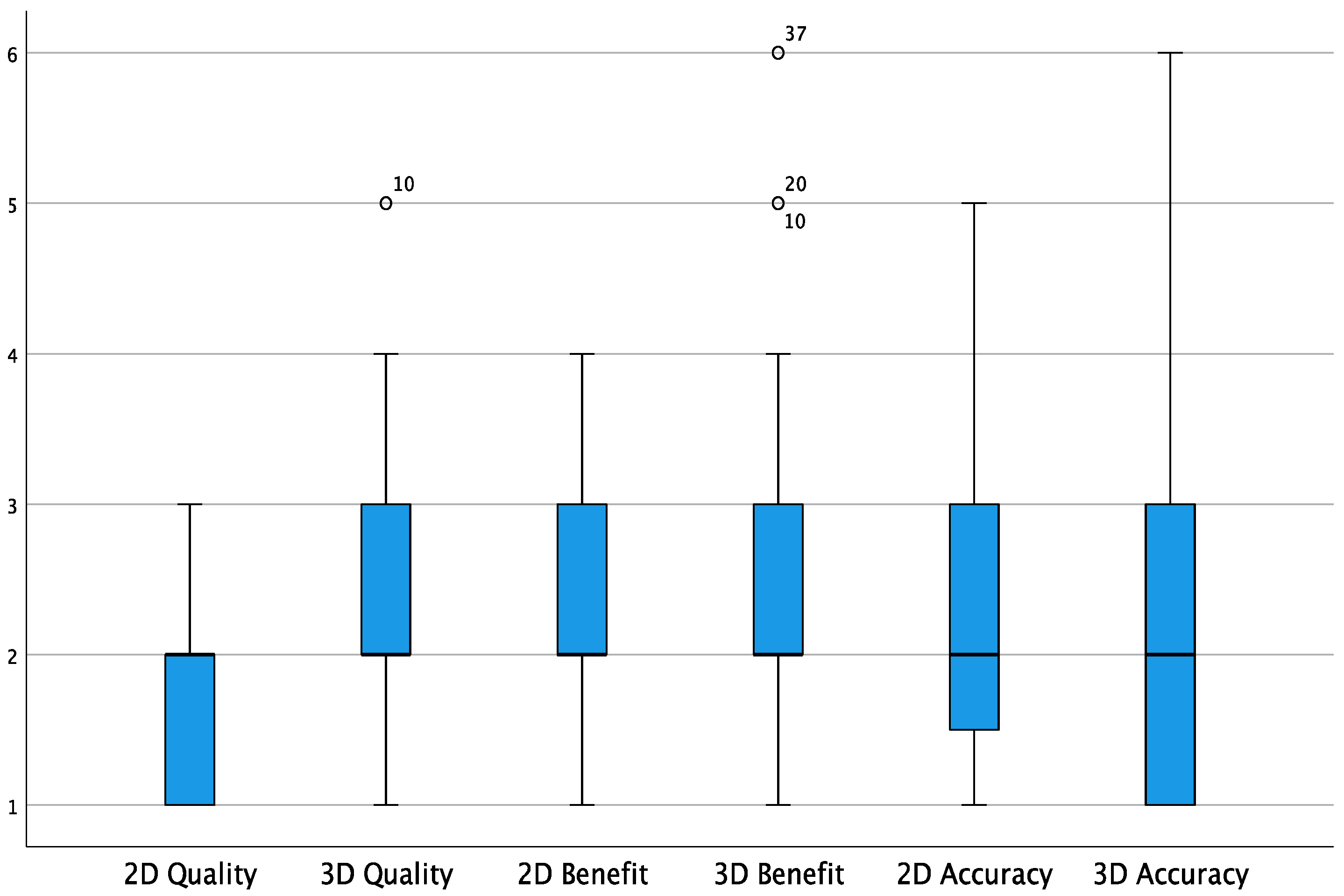
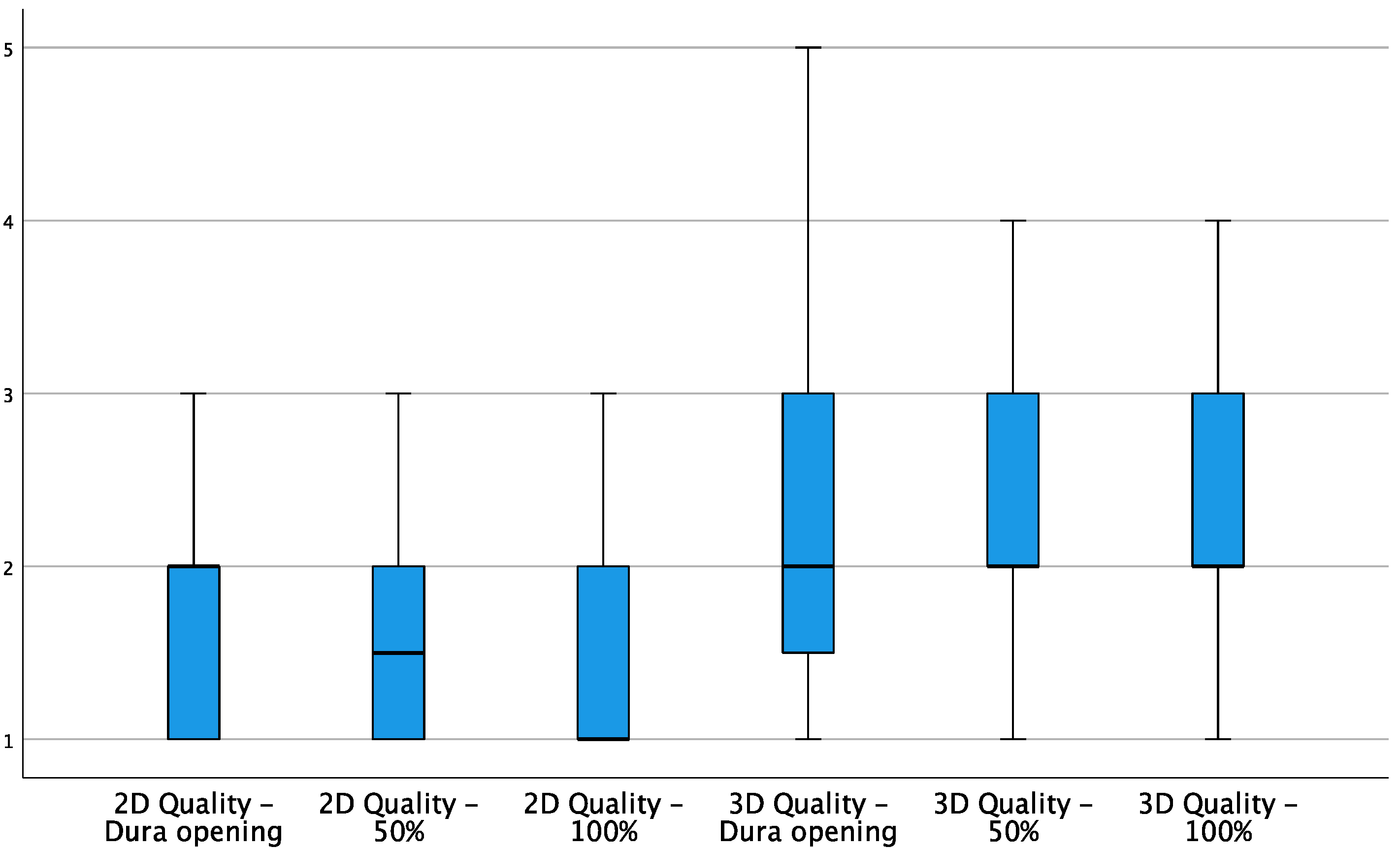
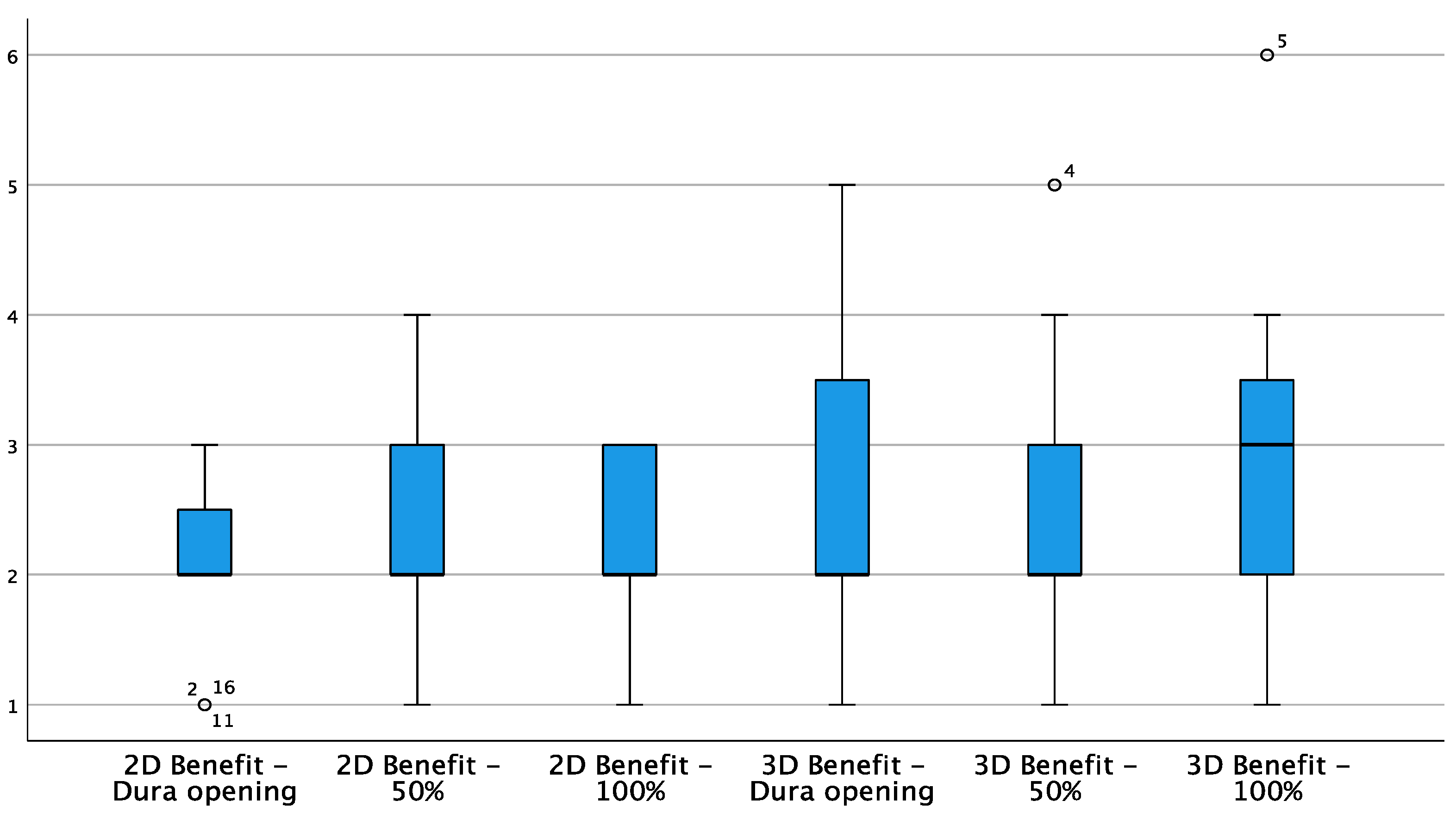



| 2D | 3D | |||||
|---|---|---|---|---|---|---|
| Stage of Surgery | After Dura Opening | 50% Resection | 100% Resection | After Dura Opening | 50% Resection | 100% Resection |
| Image Quality | 1 | 2 | 3 | 2 | 3 | 3 |
| Benefit | 1 | 2 | 3 | 2 | 3 | 2 |
| Accuracy | 2 | 2 | 2 | 1 | 2 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleo, D.; Elshaer, Z.; Pfnür, A.; Schuler, P.J.; Fontanella, M.M.; Wirtz, C.R.; Pala, A.; Coburger, J. Evaluation of a Navigated 3D Ultrasound Integration for Brain Tumor Surgery: First Results of an Ongoing Prospective Study. Curr. Oncol. 2022, 29, 6594-6609. https://doi.org/10.3390/curroncol29090518
Aleo D, Elshaer Z, Pfnür A, Schuler PJ, Fontanella MM, Wirtz CR, Pala A, Coburger J. Evaluation of a Navigated 3D Ultrasound Integration for Brain Tumor Surgery: First Results of an Ongoing Prospective Study. Current Oncology. 2022; 29(9):6594-6609. https://doi.org/10.3390/curroncol29090518
Chicago/Turabian StyleAleo, Danilo, Ziad Elshaer, Andreas Pfnür, Patrick J. Schuler, Marco Maria Fontanella, Christian Rainer Wirtz, Andrej Pala, and Jan Coburger. 2022. "Evaluation of a Navigated 3D Ultrasound Integration for Brain Tumor Surgery: First Results of an Ongoing Prospective Study" Current Oncology 29, no. 9: 6594-6609. https://doi.org/10.3390/curroncol29090518
APA StyleAleo, D., Elshaer, Z., Pfnür, A., Schuler, P. J., Fontanella, M. M., Wirtz, C. R., Pala, A., & Coburger, J. (2022). Evaluation of a Navigated 3D Ultrasound Integration for Brain Tumor Surgery: First Results of an Ongoing Prospective Study. Current Oncology, 29(9), 6594-6609. https://doi.org/10.3390/curroncol29090518






