Cost-Effectiveness of Pyrotinib Plus Capecitabine versus Lapatinib Plus Capecitabine for the Treatment of HER2-Positive Metastatic Breast Cancer in China: A Scenario Analysis of Health Insurance Coverage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Treatment
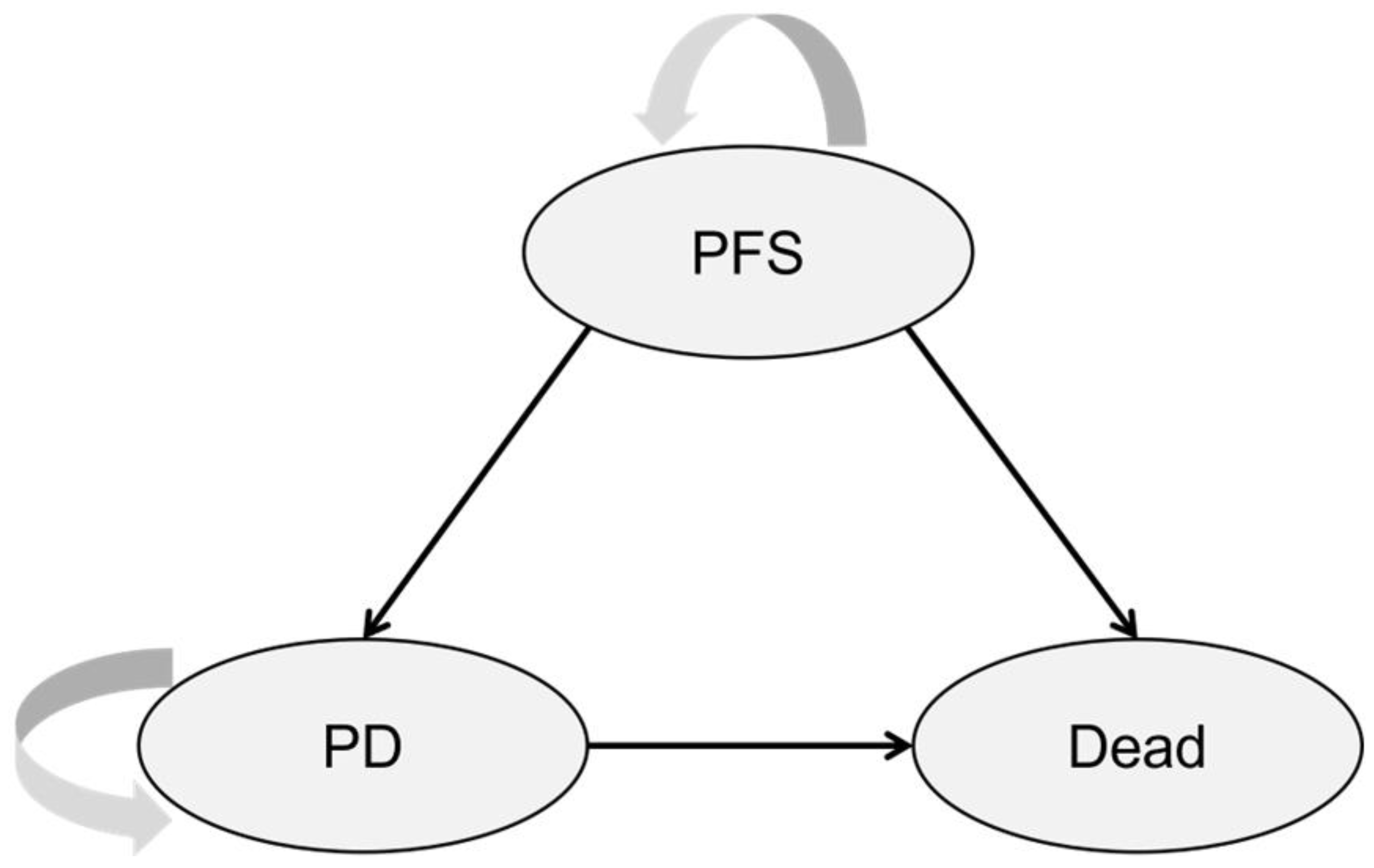
2.2. Model Structure
2.3. Cost Data
2.4. Adverse Events
2.5. Utility Values
2.6. Sensitivity Analysis
3. Results
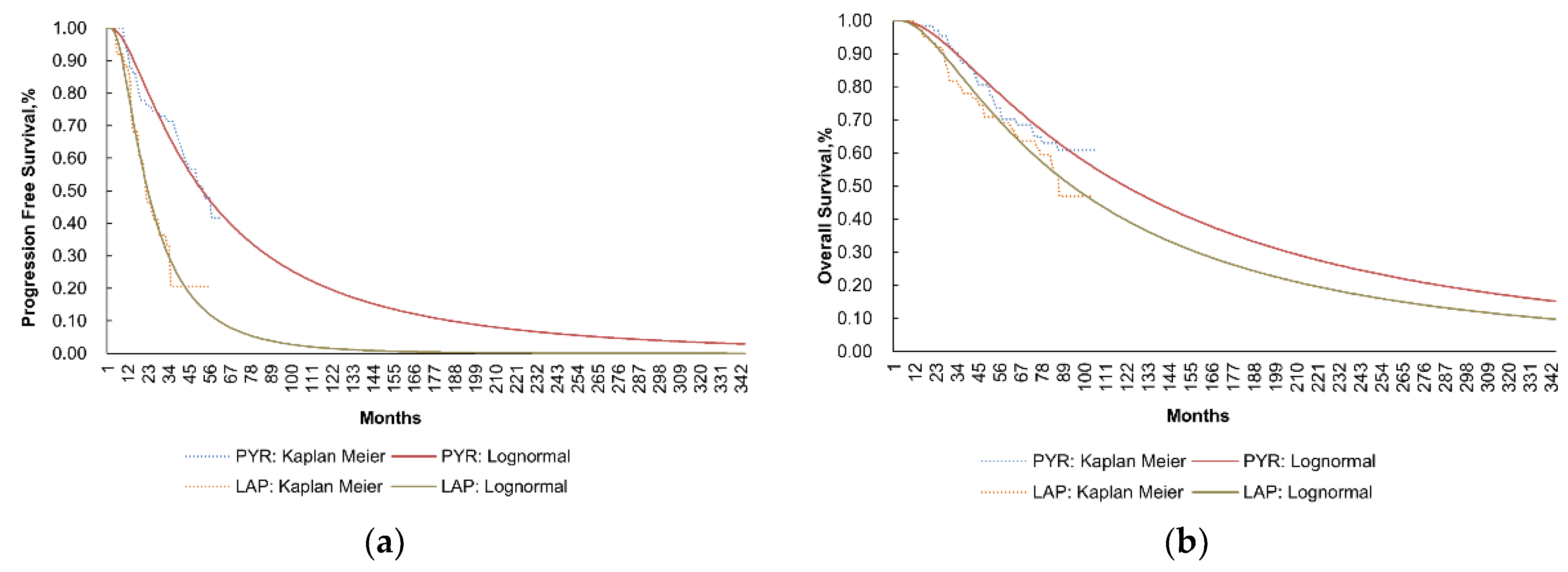
3.1. Base Case Analysis
3.2. Sensitivity Analysis
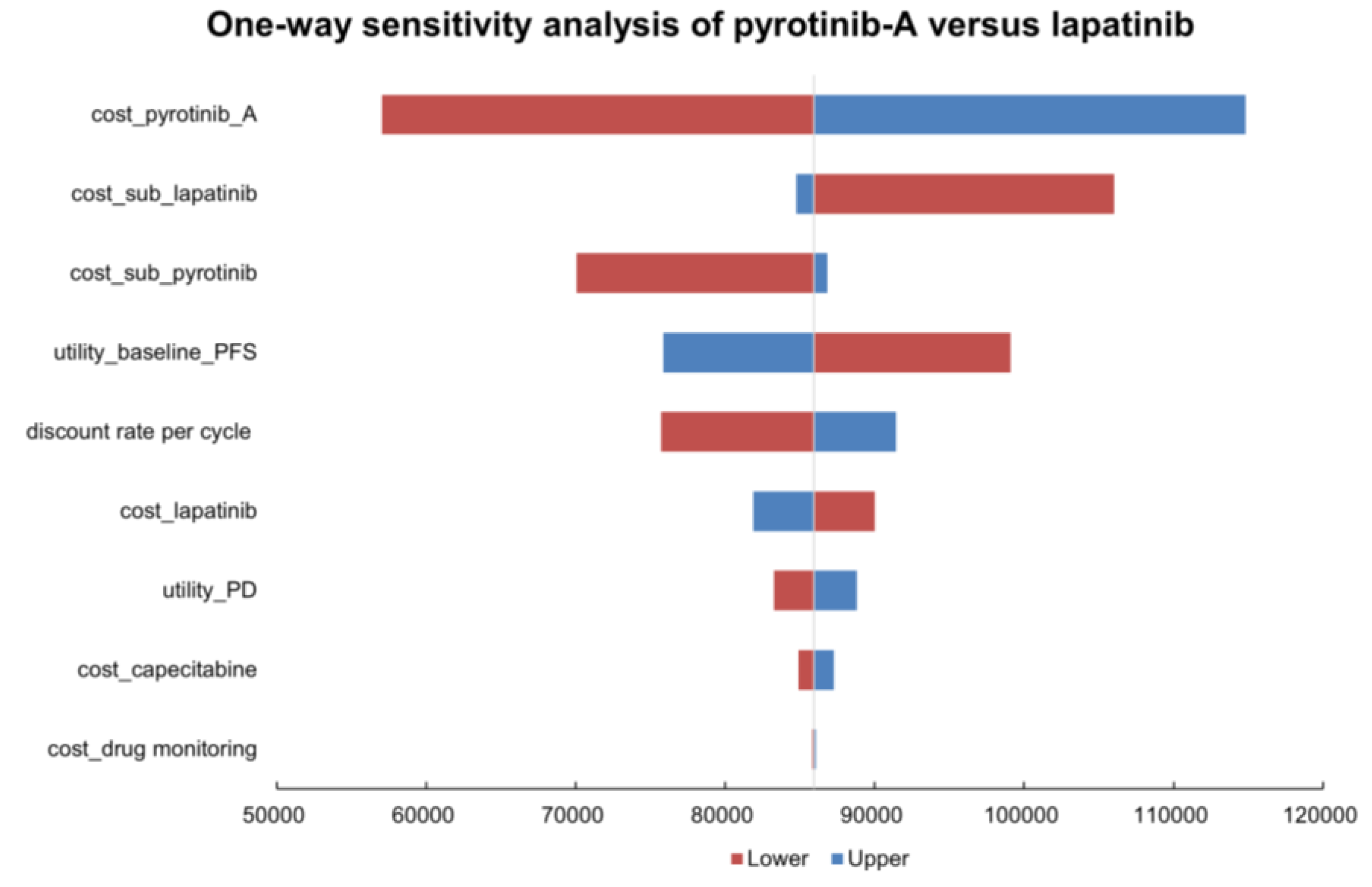
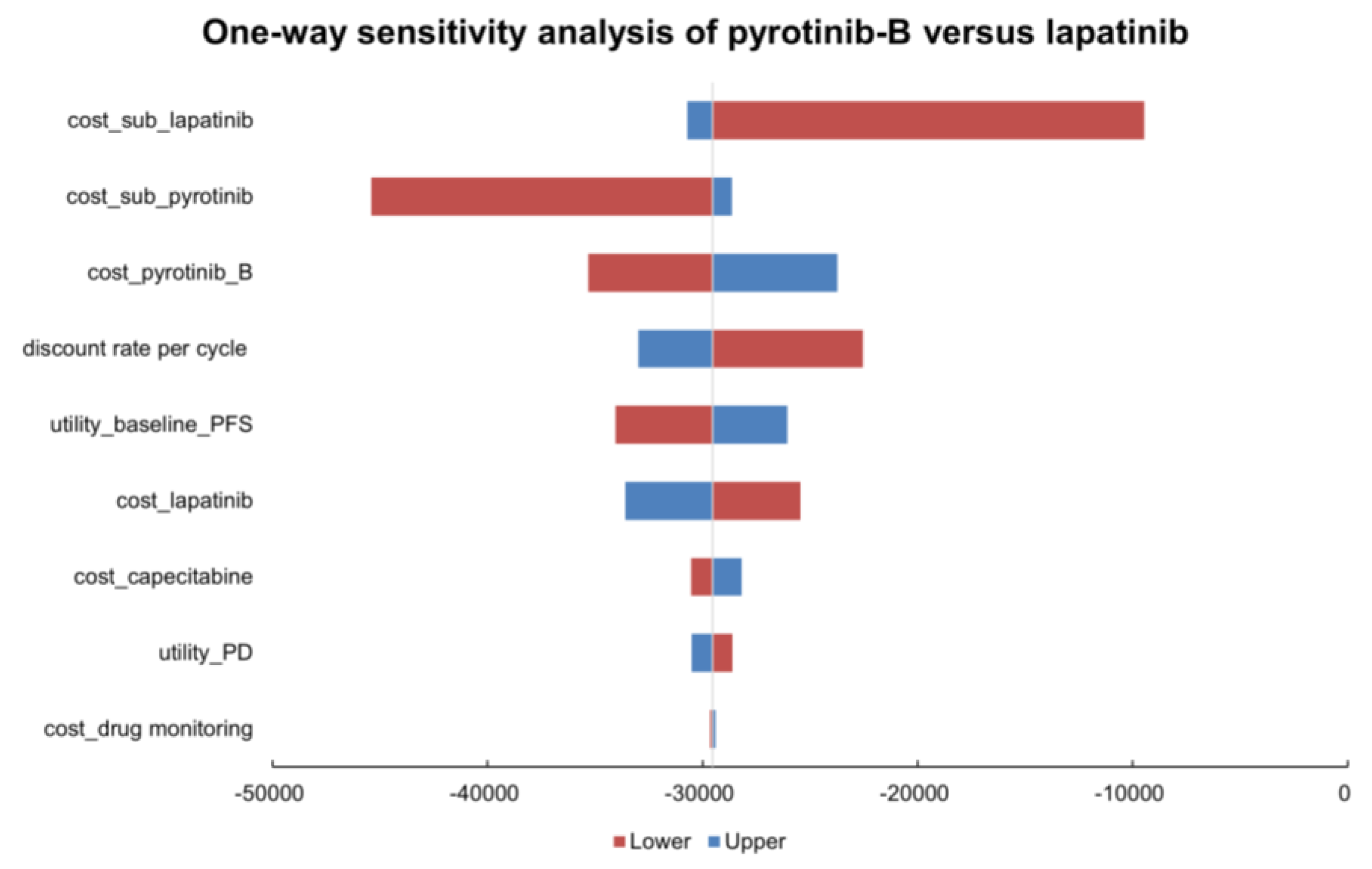
3.2.1. One-Way Sensitivity Analysis
3.2.2. Probability Sensitivity Analysis
3.3. Scenario Analysis
3.3.1. Scenario A: Time Horizon
3.3.2. Scenario B: Price Adjustment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| AEs, (Grade ≥ 3) | Proportion of PYR + CAP, % | Proportion of LAP + CAP, % | Reference |
|---|---|---|---|
| Diarrhea | 30.6 | 8.3 | [26] |
| Hand-foot syndrome | 16.4 | 15.2 | [26] |
| Vomiting | 6.0 | 2.3 | [26] |
| White blood cell count | 7.4 | 0.8 | [26] |
| Neutrophil count decreased | 7.5 | 3.8 | [26] |
| Variables (Range) | Maximum ICER | Minimum ICER |
|---|---|---|
| cost_drug monitoring (26.84 to 40.27) | 86,066.0394 | 85,808.4278 |
| cost_capecitabine (29.65 to 105.48) | 87,282.3157 | 84,918.7577 |
| utility_PD (0.421 to 0.515) | 88,811.8946 | 83,258.0974 |
| cost_lapatinib (868.03 to 1302.04) | 81,873.6848 | 90,000.7823 |
| discount rate per cycle (0.0044 to 0) | 91,416.5327 | 75,715.0284 |
| utility_baseline_PFS (0.666 to 0.814) | 75,870.0543 | 99,106.3971 |
| cost_sub_pyrotinib (767.24 to 1087.61) | 86,833.6547 | 70,047.7306 |
| cost_sub_lapatinib (1194.82 to 1524.87) | 84,761.6212 | 106,008.2993 |
| cost_pyrotinib_A (2316.00 to 3474.00) | 114,827.4397 | 57,047.0274 |
| Variables (Range) | Maximum ICER | Minimum ICER |
|---|---|---|
| cost_drug monitoring (26.84 to 40.27) | −29,398.4940 | −29,656.1056 |
| utility_PD (0.421 to 0.515) | −30,515.0088 | −28,606.7715 |
| cost_capecitabine (29.65 to 105.48) | −28,182.2177 | −30,545.7757 |
| cost_lapatinib (868.03 to 1302.04) | −33,590.8486 | −25,463.7511 |
| utility_baseline_PFS (0.666 to 0.814) | −26,068.3030 | −34,052.1120 |
| discount rate per cycle (0.0044 to 0) | −32,983.6944 | −22,545.7296 |
| cost_pyrotinib_B (464.74 to 697.12) | −23,730.0004 | −35,324.5993 |
| cost_sub_pyrotinib (767.24 to 1087.61) | −28,630.8787 | −45,416.8028 |
| cost_sub_lapatinib (1194.82 to 1524.87) | −30,702.9120 | −9456.2341 |
Appendix B

References
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Zheng, R.; Zhang, S.; Wang, S.; Chen, R.; Sun, K.; Zeng, H.; Zhou, J.; Wei, W. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. 2021, 41, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Mello-Thoms, C.; Brennan, P.C. Descriptive epidemiology of breast cancer in China: Incidence, mortality, survival and prevalence. Breast Cancer Res. Treat. 2016, 159, 395–406. [Google Scholar] [CrossRef]
- Cao, M.; Li, H.; Sun, D.; Chen, W. Cancer burden of major cancers in China: A need for sustainable actions. Cancer Commun. 2020, 40, 205–210. [Google Scholar] [CrossRef]
- Xia, C.; Dong, X.; Li, H.; Cao, M.; Sun, D.; He, S.; Yang, F.; Yan, X.; Zhang, S.; Li, N.; et al. Cancer statistics in China and United States, 2022: Profiles, trends, and determinants. Chin. Med. J. 2022, 135, 584–590. [Google Scholar] [CrossRef]
- Fidler, M.M.; Bray, F.; Soerjomataram, I. The global cancer burden and human development: A review. Scand. J. Public Health 2017, 46, 27–36. [Google Scholar] [CrossRef]
- Cao, W.; Chen, H.-D.; Yu, Y.-W.; Li, N.; Chen, W.-Q. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin. Med. J. 2021, 134, 783–791. [Google Scholar] [CrossRef]
- Garrison, L.P., Jr.; Lubeck, D.; Lalla, D.; Paton, V.; Dueck, A.; Perez, E.A. Cost-effectiveness analysis of trastuzumab in the adjuvant setting for treatment of HER2-positive breast cancer. Cancer 2007, 110, 489–498. [Google Scholar] [CrossRef]
- Durkee, B.Y.; Qian, Y.; Pollom, E.L.; King, M.T.; Dudley, S.A.; Shaffer, J.L.; Chang, D.T.; Gibbs, I.C.; Goldhaber-Fiebert, J.D.; Horst, K.C. Cost-Effectiveness of Pertuzumab in Human Epidermal Growth Factor Receptor 2–Positive Metastatic Breast Cancer. J. Clin. Oncol. 2016, 34, 902–909. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, M.; Zhang, J.; Wang, B.; Tao, Z.; Du, Y.; Zhang, S.; Cao, J.; Wang, L.; Hu, X. Real-World Data of Pyrotinib-Based Therapy in Metastatic HER2-Positive Breast Cancer: Promising Efficacy in Lapatinib-Treated Patients and in Brain Metastasis. Cancer Res. Treat. 2020, 52, 1059–1066. [Google Scholar] [CrossRef]
- Yang, H.; Wang, W. Comparison of pyrotinib or lapatinib with chemotherapy for patients with HER2 positive breast cancer after first-line treatment failure: A retrospective study. Am. J. Transl. Res. 2021, 13, 10863–10870. [Google Scholar]
- Li, Y.; Qiu, Y.; Li, H.; Luo, T.; Li, W.; Wang, H.; Shao, B.; Wang, B.; Ge, R. Pyrotinib Combined with Vinorelbine in HER2-Positive Metastatic Breast Cancer: A Multicenter Retrospective Study. Front. Oncol. 2021, 11, 664429. [Google Scholar] [CrossRef]
- Diaby, V.; Almutairi, R.D.; Babcock, A.; Moussa, R.K.; Ali, A. Cost-effectiveness of treatments for HER2-positive metastatic breast cancer and associated metastases: An overview of systematic reviews. Expert Rev. Pharm. Outcomes Res. 2020, 21, 353–364. [Google Scholar] [CrossRef]
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2016, 389, 2415–2429. [Google Scholar] [CrossRef]
- Chen, Q.; Ouyang, D.; Anwar, M.; Xie, N.; Wang, S.; Fan, P.; Qian, L.; Chen, G.; Zhou, E.; Guo, L.; et al. Effectiveness and Safety of Pyrotinib, and Association of Biomarker with Progression-Free Survival in Patients with HER2-Positive Metastatic Breast Cancer: A Real-World, Multicentre Analysis. Front. Oncol. 2020, 10, 811. [Google Scholar] [CrossRef]
- Dai, M.S.; Feng, Y.H.; Chen, S.W.; Masuda, N.; Yau, T.; Chen, S.T.; Lu, Y.S.; Yap, Y.S.; Ang, P.C.S.; Chu, S.C.; et al. Analysis of the pan-Asian subgroup of patients in the NALA Trial: A randomized phase III NALA Trial comparing neratinib+capecitabine (N+C) vs lapatinib+capecitabine (L+C) in patients with HER2+metastatic breast cancer (mBC) previously treated with two or more HER2-directed regimens. Breast Cancer Res. Treat. 2021, 189, 665–676. [Google Scholar] [CrossRef]
- Candon, D.; Healy, J.; Crown, J. Modelling the cost-effectiveness of adjuvant lapatinib for early-stage breast cancer. Acta Oncol. 2013, 53, 201–208. [Google Scholar] [CrossRef]
- Le, Q.A.; Hay, J.W. Cost-effectiveness analysis of lapatinib in HER-2-positive advanced breast cancer. Cancer 2009, 115, 489–498. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Gong, R.; Geng, Y.; Li, L. Cost-effectiveness of pertuzumab and trastuzumab as a first-line treatment of HER2-positive metastatic breast cancer in China. Ann. Palliat. Med. 2021, 10, 11382–11393. [Google Scholar] [CrossRef]
- Schlam, I.; Swain, S.M. HER2-positive breast cancer and tyrosine kinase inhibitors: The time is now. NPJ Breast Cancer 2021, 7, 56. [Google Scholar] [CrossRef]
- Wang, C.; Lin, Y.; Zhou, Y.; Mao, F.; Zhu, H.; Guan, J.; Zhang, X.; Shen, S.; Huang, X.; Chen, C.; et al. Pyrotinib with trastuzumab and aromatase inhibitors as first-line treatment for HER2 positive and hormone receptor positive metastatic or locally advanced breast cancer: Study protocol of a randomized controlled trial. BMC Cancer 2020, 20, 653. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, J.; Wang, Y.; Wu, J.; Sun, T. Case Report: Effective Treatment with Pyrotinib and Capecitabine in a Heavily Pretreated Locally Advanced Breast Cancer Harboring Both HER2 Overexpression and Mutant. Front. Oncol. 2021, 11, 715554. [Google Scholar] [CrossRef]
- Ma, F.; Li, Q.; Chen, S.; Zhu, W.; Fan, Y.; Wang, J.; Luo, Y.; Xing, P.; Lan, B.; Li, M.; et al. Phase I Study and Biomarker Analysis of Pyrotinib, a Novel Irreversible Pan-ErbB Receptor Tyrosine Kinase Inhibitor, in Patients with Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer. J. Clin. Oncol. 2017, 35, 3105–3112. [Google Scholar] [CrossRef]
- Yan, M.; Bian, L.; Hu, X.; Zhang, Q.; Ouyang, Q.; Feng, J.; Yin, Y.; Sun, T.; Tong, Z.; Wang, X.; et al. Pyrotinib plus capecitabine for human epidermal growth factor receptor 2-positive metastatic breast cancer after trastuzumab and taxanes (PHENIX): A randomized, double-blind, placebo-controlled phase 3 study. Transl. Breast Cancer Res. 2020, 1, 13. [Google Scholar] [CrossRef]
- Xu, B.; Yan, M.; Ma, F.; Hu, X.; Feng, J.; Ouyang, Q.; Tong, Z.; Li, H.; Zhang, Q.; Sun, T.; et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): A multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 351–360. [Google Scholar] [CrossRef]
- Ma, F.; Ouyang, Q.; Li, W.; Jiang, Z.; Tong, Z.; Liu, Y.; Li, H.; Yu, S.; Feng, J.; Wang, S.; et al. Pyrotinib or Lapatinib Combined with Capecitabine in HER2–Positive Metastatic Breast Cancer With Prior Taxanes, Anthracyclines, and/or Trastuzumab: A Randomized, Phase II Study. J. Clin. Oncol. 2019, 37, 2610–2619. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, B.; Li, J.; Peng, L.; Li, S.; Yu, X.; Li, L. Real-World Analysis of the Efficacy and Safety of a Novel Irreversible HER2 Tyrosine Kinase Inhibitor Pyrotinib in Patients with HER2-Positive Metastatic Breast Cancer. Cancer Manag. Res. 2021, ume 13, 7165–7174. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, J.; Chen, J.; Liu, Y.; Wang, X.; Nie, J.; Wang, X.; Hao, C.; Yin, Y.; Wang, S.; et al. Chinese Society of Clinical Oncology (CSCO) Diagnosis and Treatment Guidelines for Breast Cancer 2019, 2019th ed.; People’s Medical Publishing House: Beijing, China, 2019. [Google Scholar]
- Jiang, Z.; Song, E.; Wang, X.; Wang, H.; Wang, X.; Wu, J.; Yin, Y.; Zhang, Q.; Chen, J.; Chen, W.; et al. Chinese Society of Clinical Oncology (CSCO) Diagnosis and Treatment Guidelines for Breast Cancer 2020, 2020th ed.; People’s Medical Publishing House: Beijing, China, 2020. [Google Scholar]
- Zhang, H.; Zhang, Y.; Huang, C.; Wang, J. Cost-effectiveness Analysis of Trastuzumab Emtansine as Second-line Therapy for HER2-Positive Breast Cancer in China. Clin. Drug Investig. 2021, 41, 569–577. [Google Scholar] [CrossRef]
- Ministry of Human Resources and Social Security of the People’s Republic of China. The National Drugs Catalogue of Basic Medical Insurance, Industrial Injury Insurance and Reproductive Insurance (2021 Edition). Available online: http://www.mohrss.gov.cn/xxgk2020/fdzdgknr/shbx_4216/gsbx/202112/t20211203_429397.html (accessed on 31 July 2022).
- Liu, G.E. China Guidelines for Pharmacoeconomic Evaluations, 2020th ed.; China Market Press: Beijing, China, 2020. [Google Scholar]
- National Bureau of Statistics of China. Statistical Communiqué of the People’s Republic of China on National Economic and Social Development in 2021; 28 February 2022. Available online: http://www.stats.gov.cn/xxgk/sjfb/zxfb2020/202202/t20220228_1827971.html (accessed on 28 February 2022).
- National Health Commission of the People’s Republic of China. Report on the Nutrition and Chronic Disease Status of Chinese Residents, 2020th ed.; People’s Medical Publishing House: Beijing, China, 2020. [Google Scholar]
- Yang, Q.; Yu, X.; Zhang, W. Health variations among breast-cancer patients from different disease states: Evidence from China. BMC Health Serv. Res. 2020, 20, 1013. [Google Scholar] [CrossRef]
- Lloyd, A.; Nafees, B.; Narewska, J.; Dewilde, S.; Watkins, J. Health state utilities for metastatic breast cancer. Br. J. Cancer 2006, 95, 683–690. [Google Scholar] [CrossRef]
- Annual Drug Review Report in 2018. Available online: https://www.nmpa.gov.cn/directory/web/nmpa/xxgk/fgwj/gzwj/gzwjyp/20190701175801236.html (accessed on 1 July 2019).
- Oh, D.-Y.; Bang, Y.-J. HER2-targeted therapies—A role beyond breast cancer. Nat. Rev. Clin. Oncol. 2019, 17, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, B.; Pearson, S.-A.; Viney, R. Economic evaluations of trastuzumab in HER2-positive metastatic breast cancer: A systematic review and critique. Eur. J. Health Econ. 2013, 15, 93–112. [Google Scholar] [CrossRef] [PubMed]
- Derakhshani, A.; Rezaei, Z.; Safarpour, H.; Sabri, M.; Mir, A.; Sanati, M.A.; Vahidian, F.; Moghadam, A.G.; Aghadoukht, A.; Hajiasgharzadeh, K.; et al. Overcoming trastuzumab resistance in HER2-positive breast cancer using combination therapy. J. Cell. Physiol. 2019, 235, 3142–3156. [Google Scholar] [CrossRef] [PubMed]
- Escrivá-De-Romaní, S.; Arumí, M.; Bellet, M.; Saura, C. HER2-positive breast cancer: Current and new therapeutic strategies. Breast 2018, 39, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.F.; Beca, J.M.; Nagamuthu, C.; Liu, N.; de Oliveira, C.; Earle, C.C.; Trudeau, M.; Chan, K.K.W. Cost-effectiveness Analysis of Pertuzumab with Trastuzumab in Patients with Metastatic Breast Cancer. JAMA Oncol. 2022, 8, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Younis, T.; Lee, A.; Coombes, M.E.; Bouganim, N.; Becker, D.; Revil, C.; Jhuti, G.S. Economic evaluation of adjuvant trastuzumab emtansine in patients with HER2-positive early breast cancer and residual invasive disease after neoadjuvant taxane and trastuzumab–based treatment in Canada. Curr. Oncol. 2020, 27, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Delea, T.E.; Tappenden, P.; Sofrygin, O.; Browning, D.; Amonkar, M.M.; Karnon, J.; Walker, M.D.; Cameron, D. Cost-effectiveness of lapatinib plus capecitabine in women with HER2+ metastatic breast cancer who have received prior therapy with trastuzumab. Eur. J. Health Econ. 2011, 13, 589–603. [Google Scholar] [CrossRef]
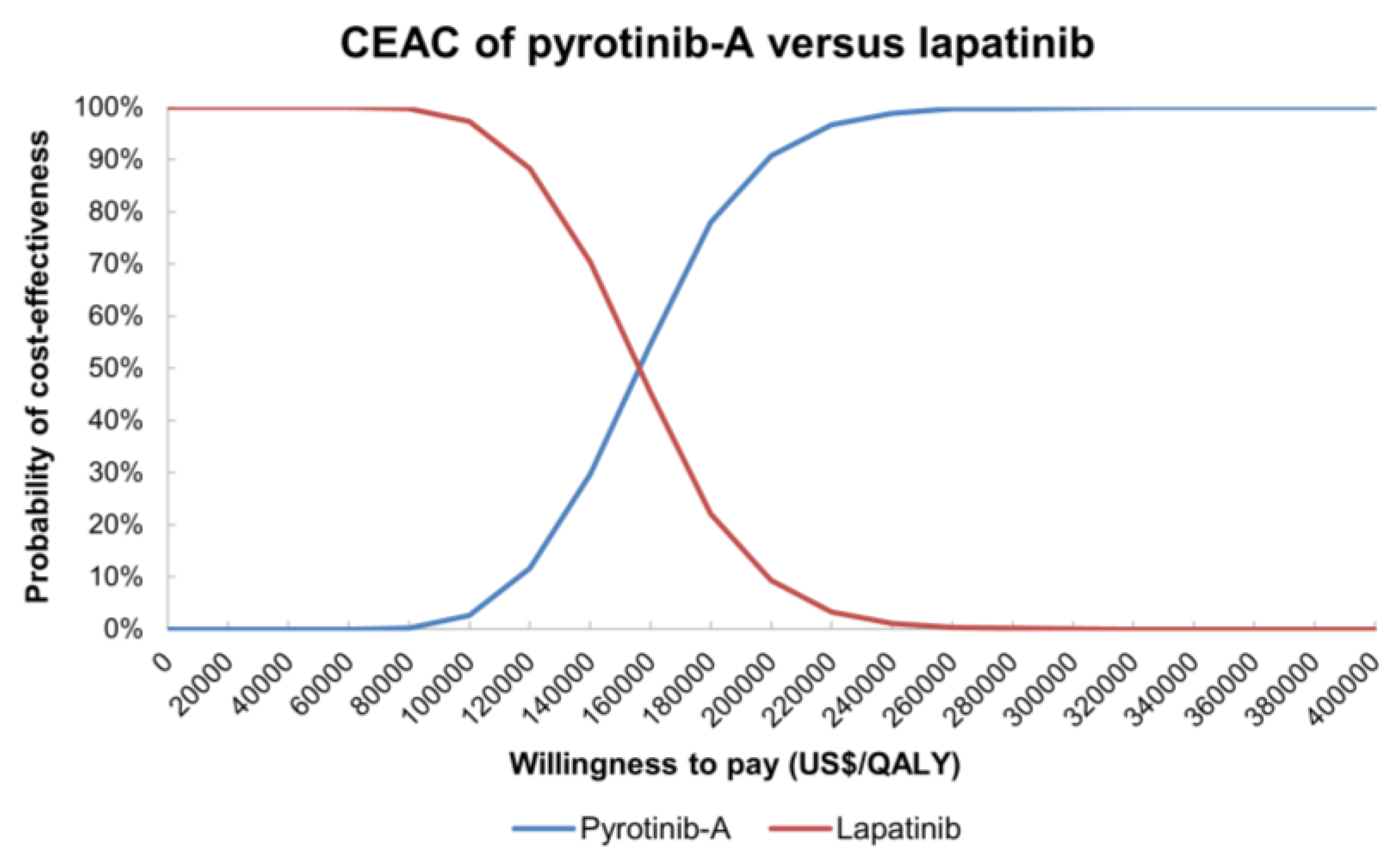
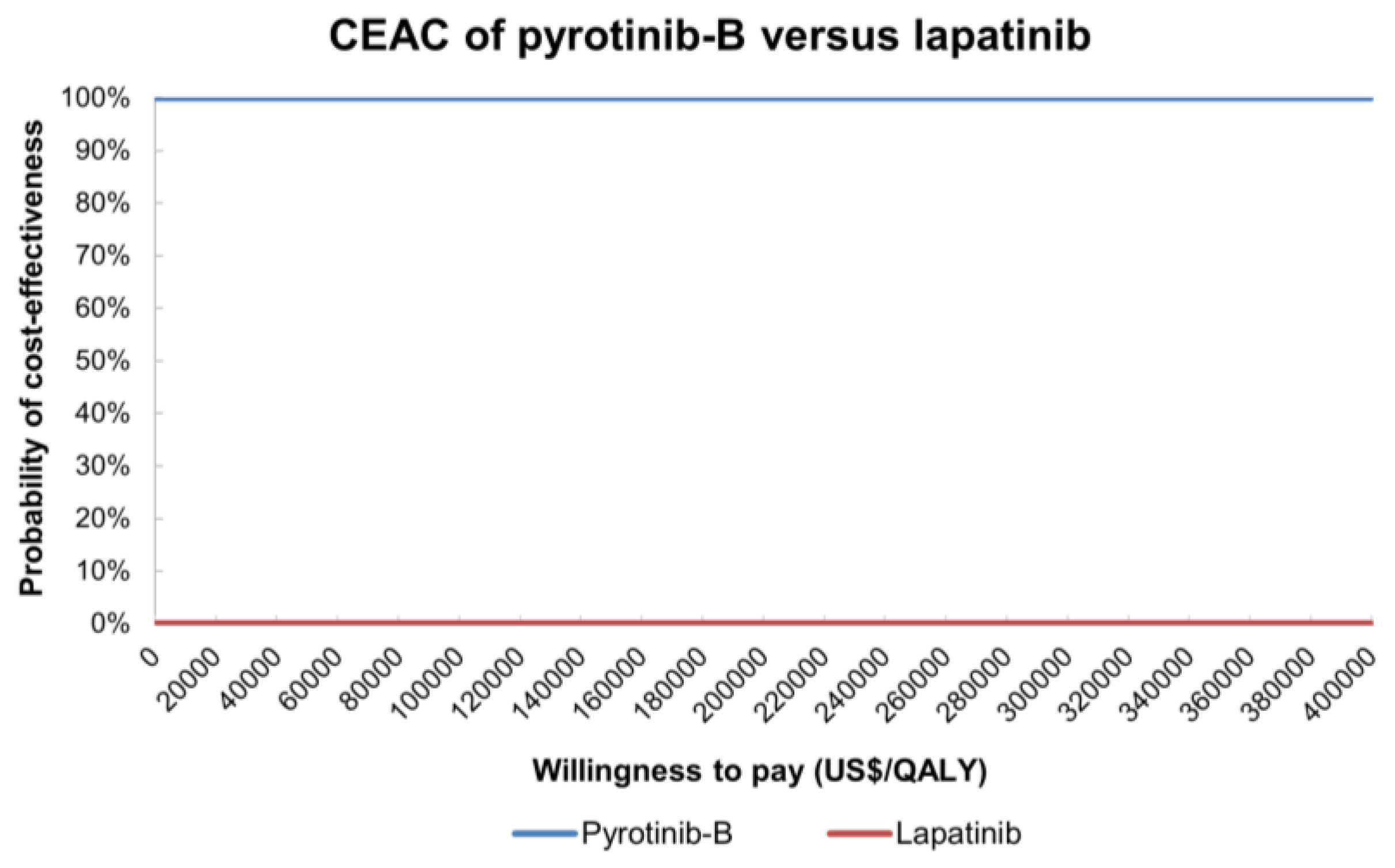
| Variables | Base-Case Values | Upper Limit | Lower Limit | Standard Error | Distribution | Reference |
|---|---|---|---|---|---|---|
| Costs | ||||||
| Pyrotinib-A | 2895.00 | 3474.00 | 2316.00 | 272.94 | Gamma | hospital |
| Pyrotinib-B | 580.93 | 697.12 | 464.74 | 54.77 | Gamma | hospital |
| Lapatinib | 1085.03 | 1302.04 | 868.03 | 102.30 | Gamma | hospital |
| Capecitabine | 62.32 | 105.48 | 29.65 | 17.93 | Gamma | hospital |
| Subsequent_PYR | 1070.50 | 1087.61 | 767.24 | 84.96 | Gamma | expert opinions, [26] |
| Subsequent_LAP | 1506.61 | 1524.87 | 1194.82 | 87.45 | Gamma | expert opinions, [26] |
| AEs_PYR | 62.99 | 75.58 | 50.39 | 5.94 | Gamma | expert opinions, [26] |
| AEs_LAP | 30.4 | 36.48 | 24.32 | 2.87 | Gamma | expert opinions, [26] |
| AEs_Hospitalization | 123.83 | 148.59 | 99.06 | 11.67 | Gamma | hospital, [26] |
| Drug monitoring | 33.56 | 40.27 | 26.84 | 3.16 | Gamma | hospital, [26] |
| Health-state utility values | ||||||
| PFS | 0.740 | 0.814 | 0.666 | 0.0349 | Beta | [36] |
| PD | 0.468 | 0.515 | 0.421 | 0.0221 | Beta | [36,37] |
| AE disutility values | ||||||
| Diarrhea (PYR + LAP) | 0.051 | 0.056 | 0.045 | 0.0024 | Beta | [26,37] |
| Hand-foot (PYR + LAP) | 0.027 | 0.029 | 0.024 | 0.0012 | Beta | [26,37] |
| Discount rate, % | ||||||
| Discount rate | 5.00% | 8.00% | 0.00% | 0.0191 | Normal | [33] |
| Table | Costs (USD) | QALYs | Incremental Costs | Incremental QALYs | ICER (US$/QALY) |
|---|---|---|---|---|---|
| PYR-A + CAP 1 | 109726.16 | 1.86 | 45400.64 | 0.53 | 85944.79 |
| PYR-B + CAP 2 | 48726.25 | 1.86 | −15599.27 | 0.53 | −29529.90 |
| LAP + CAP | 64325.52 | 1.33 |
| Price Adjustment | ICERs (PYR-A vs. LAP) | ICERs (PYR-B vs. LAP) |
|---|---|---|
| 10% | 71,498.44 | −27,497.92 |
| 20% | 57,052.07 | −25,465.97 |
| 30% | 42,605.69 | −23,434.02 |
| 40% | 28,159.31 | −21,402.07 |
| 50% | 13,712.94 | −19,370.12 |
| 60% | Dominant | −17,338.17 |
| 70% | Dominant | −15,306.22 |
| 80% | Dominant | −13,274.27 |
| 90% | Dominant | −11,242.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, Y.; Zhang, Z.; He, X.; Cai, L.; Wang, X.; Li, X. Cost-Effectiveness of Pyrotinib Plus Capecitabine versus Lapatinib Plus Capecitabine for the Treatment of HER2-Positive Metastatic Breast Cancer in China: A Scenario Analysis of Health Insurance Coverage. Curr. Oncol. 2022, 29, 6053-6067. https://doi.org/10.3390/curroncol29090476
Bao Y, Zhang Z, He X, Cai L, Wang X, Li X. Cost-Effectiveness of Pyrotinib Plus Capecitabine versus Lapatinib Plus Capecitabine for the Treatment of HER2-Positive Metastatic Breast Cancer in China: A Scenario Analysis of Health Insurance Coverage. Current Oncology. 2022; 29(9):6053-6067. https://doi.org/10.3390/curroncol29090476
Chicago/Turabian StyleBao, Yuwen, Zhuolin Zhang, Xuan He, Lele Cai, Xiao Wang, and Xin Li. 2022. "Cost-Effectiveness of Pyrotinib Plus Capecitabine versus Lapatinib Plus Capecitabine for the Treatment of HER2-Positive Metastatic Breast Cancer in China: A Scenario Analysis of Health Insurance Coverage" Current Oncology 29, no. 9: 6053-6067. https://doi.org/10.3390/curroncol29090476
APA StyleBao, Y., Zhang, Z., He, X., Cai, L., Wang, X., & Li, X. (2022). Cost-Effectiveness of Pyrotinib Plus Capecitabine versus Lapatinib Plus Capecitabine for the Treatment of HER2-Positive Metastatic Breast Cancer in China: A Scenario Analysis of Health Insurance Coverage. Current Oncology, 29(9), 6053-6067. https://doi.org/10.3390/curroncol29090476





