Rapid Review of Real-World Cost-Effectiveness Analyses of Cancer Interventions in Canada
Abstract
1. Introduction
Background
2. Methods
2.1. Search Strategy
2.2. Information Sources
2.3. Screening Process
2.4. Conversions and Calculations
2.5. Estimates by Study Type
2.6. Estimates of Extra Cost and Extra Effect in Real World Studies of Drugs
3. Results
3.1. Article Inclusion and Exclusion Results
3.2. Overview of the Studies
| Citation | Intervention(s) | Sample Size (n) | Study Population | Study Design, Study Perspective, Time Horizon | Measures of Effectiveness |
|---|---|---|---|---|---|
| Arciero et al., 2022 [17]. JNCI Cancer Spectr. | Comparing cost-effectiveness of using gemcitabine and nab-paclitaxel (Gem-nab) and FOLFIRINOX for patients with advanced pancreatic cancer. | n = 1988; Gem-nab = 928; FOLFIRINOX = 1060 | Aged ≥ 18 years that were prescribed Gem-nab, irinotecan, or oxaliplatin for advanced pancreatic cancer between 17 April 2015–31 March 2019. Mean age (SD) of groups ranged from 61.9(8.8)–69.2(9.0). | Study Design: Dataset; Study Perspective: Healthcare System; Time Horizon: 5 years | QALYs, LYs |
| Cressman et al., 2021 [18]. CMAJ Open | Comparing digital breast tomosynthesis (DBT) plus digital mammography (DM) in high-risk cancer screening to standard care (DM alone). | n = 112,249 | Participants aged 40–74 years who participated in breast cancer screening from the BC Cancer Breast Screening Program and the BC Cancer Registry for all new screening participants, mean age (SD): 49.3 (40–74) years, with an initial, “index,” screening exam received between 1 January 2012, and 31 December 2017. | Study Design: Model; Study Perspective: Healthcare System; Time Horizon: Lifetime | QALYs |
| Cressman et al., 2017 [19]. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. | Comparing high-risk lung cancer screening to standard care. The base case scenario drew a comparison of LDCT- based screening in the HR-NLST (intervention) with the high-risk CXR (HR-CXR) screened arm (comparator) of the NLST, using the assumption that CXR is similar to standard care for early lung cancer detection. | n = 49,775 | Data from the National Lung Cancer Screening Trial (NLST). Participants were separated into high (>2% at 6 years) and low risk (<2% at 6 years) groups. The outcomes data for NLST participants were grouped according to risk (high or low) and screening intervention (LDCT or chest radiography [CXR]). | Study Design: Model; Study Perspective: Public Payer; Time Horizon: 30 years (lifetime horizon) | |
| Cromwell et al., 2011 [20]. Lung Cancer Amst. Neth. | Comparing third-line erlotinib protocol to the next-best alternative of Best Supportive Care (BSC) in BCCA patients. | n = 147; erlotinib = 78, supportive care = 69 | BCCA cases with a diagnosis of stage IIIB/IV advanced NSCLC (including adenocarcinoma, NSC carcinoma, squamous cell and large cell carcinomas, bronchio- alveolar carcinoma, and lung carcinomas not otherwise specified). Median age (SD) erlotinib: 65(39–88); best supportive care: 64(45–77). | Study Design: Dataset; Study Perspective: Healthcare System; Time Horizon: N/A | |
| Cromwell et al., 2011 [21]. J. Thorac. Oncol. Ogg. Publ. Int. Assoc. Study Lung Cancer. | Second-line erlotinib treatment and treatment with docetaxel among patients with non-small cell lung cancer. | n = 201; erlotinib = 133, docetaxel = 68 | Eligible patients were patients treated at the BCCA with a diagnosis of stage IIIb/IV advanced NSCLC who received second-line treatment (including adenocarcinoma, NSC carcinoma, squamous cell and large cell carcinomas, bronchioloalveolar carcinoma, and lung carcinomas not otherwise specified). Median age receiving Erlotinib: 65(39–88). Median age (SD) receiving Docetaxel: 64(45–77). | Study Design: Dataset; Study Perspective: Provincial Healthcare System; Time Horizon: N/A | LYs |
| Dai et al., 2022 [22]. JAMA Oncol. | Treatment with pertuzumab, trastuzumab, and chemotherapy after public funding compared with treatment with trastuzumab and chemotherapy before funding in patients with metastatic breast cancer. | n = 1158 | Patients who received first-line treatments for metastatic breast cancer from 1 January 2008, to 31 March 2018, were identified. Mean age (SD) of patients: 58(12.97 years). | Study Design: Dataset; Study Perspective: Public Payer; Time Horizon: N/A | QALYs, LYs |
| Gilbert et al., 2020 [23]. J. Comp. Eff. Res. | Treating recurrent high-grade serious ovarian cancer with cytotoxic chemotherapy and after relapse. | n = 66 | Mean age (SD) was 60.6 (8.6) years, and 48% of the women were Caucasian. At diagnosis, 68% had stage IIIC, and 25% had stage IV ovarian cancer. | Study Design: Dataset; Study Perspective: Healthcare System; Time Horizon: N/A | LYs |
| Hannouf et al., 2012 [24]. BMC Cancer | Utilizing a 21-gene recurrence score assay to inform treatment decisions for women with early-stage breast cancer. | n = 498; Pre-menopausal: 109, Post-menopausal: 389 | Pre- and post-menopausal women, average age (≈64 years old) living in Manitoba and have been diagnosed with ER+/PR + LN- ESBC (stage I/II) breast cancer between January 2000–December 2002. | Study Design: Model; Study Perspective: Healthcare System; Time Horizon: Lifetime horizon | QALYs, LYs |
| Hedden et al., 2012 [25]. Eur. J. Cancer Oxf. | Comparing (1) mCRC therapy for all patients in the post-bev era compared to usual mCRC therapy in the pre-bev era; and (2) mCRC therapy for patients diagnosed under age 70 and who received doublet chemotherapy (i.e., those eligible for bevacizumab) in the post-bev era compared to usual mCRC therapy for the same sub-group of patients in the pre-bev era. | n = 943 | All patients with newly diagnosed mCRC referred to BCCA were included; pre-bev cohort: 2003–2004, post-bev cohort: 2006. Mean age at diagnosis: 65. | Study Design: Model; Study Perspective: Healthcare Payer; Time Horizon: 12 years | QALYs, LYs |
| Hedden et al., 2012 [26]. The Oncologist | Adjuvant trastuzumab for operable, HER-2/neu-positive early breast cancer with standard of care treatments in the adjuvant and metastatic settings. | n = 1000 | 50-year-old women with early HER- 2/neu-positive breast cancer, who had successfully completed a surgical resection of disease. Patients entered the model in the postsurgical with trastuzumab or postsurgical without trastuzumab states, depending on the presence or absence of pre-existing low left ventricular ejection fraction (LVEF). | Study Design: Model; Study Perspective: Healthcare System; Time Horizon: 28 years | QALYs, LYs |
| Imran et al., 2019 [27]. Eur. Thyroid J. | Primary versus tertiary care among patients with low-risk differentiated thyroid cancer. | n = 317; Tertiary care = 224, Primary care = 93 | Patients diagnosed with low risk differentiated Thyroid Cancer diagnosed between 1 January 2006 and 31 December 2011. Mean age at diagnosis, tertiary: 47.7; mean age at diagnosis, primary care: 46.0. | Study Design: Dataset; Study Perspective: Healthcare System, Patient Perspective; Time Horizon: N/A | Rate of Recurrence |
| Johnston et al. 2010 [28]. ParmacoeconimcsOutcomes Res. | Cyclophosphamide, doxorubicin, vincristine, and predisone (CHOP) chemotherapy versus CHOP-R (CHOP with the addition of rituximab) in the treatment of large B-cell lymphoma. | n = 785 | Patients with diffuse large B-cell lymphoma. Sample included 266 HIV-negative adults (age > 15 years) initiating treatment with CHOP between September 1997 and June 2000 and 519 HIV-negative adults initiating treatment with CHOP-R between March 2003 and June 2007. | Study Design: Model; Study Perspective: Healthcare Payer; Time Horizon: 15 years | QALYs, LYs, Disease-free LYs |
| Khor et al., 2014 [29]. BMC Cancer | Treating diffuse-large-B-cell lymphoma with rituximab plus cyclophpsphamide, doxorubicin, vincristine, and predisone (CHOP) (RCHOP) as opposed to only CHOP. | n = 4021; RCHOP = 2825, CHOP = 1196 | All ages diagnosed with diffuse-large-B-cell-lymphoma were included in data collection from the date of rituximab approval. Data were collected on patients from 1997–2009. Mean group ages ranged from 56.7–65.5; included patient populations ≥80 years. | Study Design: Dataset; Study Perspective: Healthcare System; Time Horizon: 5 years | LYs |
| Mittmann et al., 2018 [30]. J. Clin. Oncol Off. J. Am. Soc. Clin. Oncol. | Use of 21-gene assay Oncotype Dx test or standard of care for chemotherapy prescription. | n = 1000 | Patients with hormone receptor–positive breast cancer who received endocrine therapy. Median age (SD) at registration: 58 (50–65 years). | Study Design: Database; Study Perspective: Healthcare System; Time Horizon: N/A | Chemotherapy Use Rates |
| Nazha et al., 2018 [31]. Curr Oncol Tor Ont. | First line treatment in a real world setting for patients with metastatic renal cell carcinoma using sunitinib versus pazopanib. | n = 475; sunitinib = 395, pazopanib = 80 | Patients diagnosed with clear cell metastatic renal cell carcinoma after 1 January 2011. Median age (SD): 63 (56–70). | Study Design: Database; Study Perspective: Healthcare Payer; Time Horizon: N/A | LYs |
| Nazha et al., 2018 [32]. Drug Investig. | Suntinib versus pazopanib in patients with metastatic renal cell carcinoma. | n = 475 | mRCC patients treated with targeted therapy (sunitinib or pazopanib) in first-line with confirmed clear cell histology. Mean age: 64. | Study Design: Model; Study Perspective: Healthcare System; Time Horizon: 5 years | QALYs, LYs |
| Parackal et al. 2020 [33]. Can Urol. Assoc. J. J. Assoc. Urol. Can. | Use of robot-assisted radical prostatectomy (RARP) for prostate cancer or open radical prostatectomy (ORP). | n = 14,396; RARP v ORP developing BCR = 8259, Fail point of BCR = 1485, UI after RP = 2510, ED after RP = 2142 | Men with localized prostate cancer, stages I and II. Mean ages 50–69 years old. | Study Design: Model; Study Perspective: Public Payer; Time Horizon: 10 years | QALYs |
| Pataky et al., 2021 [34]. MDM Policy Pract. | Use of bevacizumab-treated patients versus those treated before bevacizumab funding and contemporaneous controls (receiving chemotherapy without bevacizumab) among patients with metastatic colorectal cancer. | n = 16,250; Ontario = 12,112, Saskatchewan = 1161, British Columbia = 2977 | Metastatic Colorectal Cancer patients (greater than or equal to 18 years old at diagnosis) with a registry-confirmed diagnosis of colorectal cancer (ICD-O-3 codes C18-C20), who initiated irinotecan-based chemotherapy between 1 January 2000, and 31 March 2015 (BC and Ontario), or 1 January 2003, and 31 December 2015 (Saskatchewan). Mean age between 63 and 64 years. | Study Design: Dataset; Study Perspective: Public Payer; Time Horizon: 5 years | QALYs, LYs |
| Raymakers et al., 2020 [35]. BMC Cancer | Use of brentuximab vedotin (BREN + AVD) or ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) as frontline therapy in patients with advanced Hodgkin’s lymphoma. | n = 1519 | Patients diagnosed with advanced stage Hodgkins’s Lymphoma between 2000–2016, ≥18 years old, not pregnant, not HIV positive. | Study Design: Model; Study Perspective: Healthcare System; Time Horizon: 15-year | QALYs |
| Tesch et al., 2022 [36]. Cancer. | Chemotherapy prescription and RS-guided treatment costs post-TAILORx. | n = 2066; pre-funding: 644, post-funding = 739, post-TAILORx cohort = 683 | HR-positive, HER2-negative, node-negative patients with breast cancer defined by diagnosis: before RS funding (cohort 1 [C1]: January 2013–December 2013), after introduction of public RS funding (cohort 2 [C2]: July 2015–June 2016), and after TAILORx results (cohort 3 [C3]: July 2018–June 2019). Patients aged 18–80 years with stage I–III breast cancer. Cohort 1 median age (SD): 62(23–80), Cohort 2: 62(21–80), Cohort 3 61(28–80). | Study Design: Dataset; Study Perspective: Healthcare Payer; Time Horizon: N/A | Chemotherapy Use Rates |
| Thein et al., 2017 [37]. Cancer Med. | Use of transarterial chemoembolization (TACE) + radiofrequency ablation (RFA) versus RFA monotherapy versus no treatment. | n = 2222 | All eligible Hepatocellular carcinoma (HCC) cases aged 18 years and older in Ontario diagnosed between 1 January 2002 and 31 December 2010. | Study Design: Dataset; Study Perspective: Healthcare Payer; Time Horizon: N/A | QALYs, LYs |
| Weymann et al., 2021 [38]. J Community Genet. | Using genomic sequencing to tailor cancer care. | n = 460; usual care = 230, POG intervention = 230 | Adults with varying metastatic, uncurable cancer types. Mean age (SD), usual care: 56.5(11.4); POG patients: 56.2(12.8). | Study Design: Dataset; Study Perspective: Healthcare Payer; Time Horizon: 1 year | LYs |
3.2.1. Description of Study Populations and Participants
3.2.2. Diagnoses
3.2.3. Study Designs
3.2.4. Cost Perspectives
3.2.5. Measures of Effectiveness
3.3. Results of the Studies
| Author(s) | Intervention(s) | ΔE | ΔC | ICER/ICUR(s) | Currency | Uncertainty |
|---|---|---|---|---|---|---|
| Arciero et al., 2022 [17]. JNCI Cancer Spectr. | Comparing cost-effectiveness of using gemcitabine and nab-paclitaxel (Gem-nab) and FOLFIRINOX for patients with advanced pancreatic cancer. | Gem-nab v FOLFIRINOX: −0.25 LY, −0.21 QALY | Gem-nab v FOLFIRINOX: CAD 2366 | - | 2019 CAD | SA, CEAC, Scatter plot |
| Cressman et al., 2021 [18]. CMAJ Open | Comparing digital breast tomosynthesis (DBT) plus digital mammography (DM) in high-risk cancer screening to standard care (DM alone). | DBT + DM v DM: 0.027 QALY | DBT + DM v DM: CAD 470 | DBT + DM v DM: CAD 17,149/QALY | 2019 CAD | SA |
| Cressman et al., 2017 [19]. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. | Comparing high-risk lung cancer screening to standard care. The base case scenario drew a comparison of LDCT- based screening in the HR-NLST (intervention) with the high-risk CXR (HR-CXR) screened arm (comparator) of the NLST, using the assumption that CXR is similar to standard care for early lung cancer detection. | High-risk screening v Standard care: 0.032 QALY *Authors’ Assumptions* | High-risk screening v Standard care: CAD 668 *Authors’ Assumptions* | High-risk screening v Standard care: CAD 20,724/QALY *Authors’ Assumptions* | 2015 CAD | SA |
| Cromwell et al., 2011 [20]. Lung Cancer Amst. Neth. | Comparing third-line erlotinib protocol to the next-best alternative of Best Supportive Care (BSC) in BCCA patients. | Third-line erlotinib v best practice: 0.25 LY | Third-line erlotinib v best practice: CAD 11,102 | Third-line erlotinib v best practice: CAD 36,838/LY | 2009 CAD | SA, Scatter plot |
| Cromwell et al., 2011 [21]. J. Thorac. Oncol. Ogg. Publ. Int. Assoc. Study Lung Cancer. | Second-line erlotinib treatment and treatment with docetaxel among patients with non-small cell lung cancer. | Second-line erlotinib v Docetaxel: 0.0027 LY | Second-line erlotinib v Docetaxel: CAD 2891 | Second-line erlotinib v Docetaxel: CAD 1,055,215/LY | 2009 CAD | SA, CEAC, Scatter plot |
| Dai et al., 2022 [22]. JAMA Oncol. | Treatment with pertuzumab, trastuzumab, and chemotherapy after public funding compared with treatment with trastuzumab and chemotherapy before funding in patients with metastatic breast cancer. | Pertuzumab addition v Previous care: 0.61 LY, 0.44 QALY | Pertuzumab addition v Previous care: CAD 192,139 | Pertuzumab addition v Previous care: CAD 316,203/LY, CAD 436,679/QALY | 2018 CAD | CEAC, Scatter plot |
| Gilbert et al., 2020 [23]. J. Comp. Eff. Res. | Treating recurrent high-grade serious ovarian cancer with cytotoxic chemotherapy and after relapse. | One line v Two lines: 1.17 LY Two lines v Three lines: −7.9 months | One line v Two lines: CAD 72,374; Two lines v Three lines: CAD 97,243 | One line v Two lines: CAD 62,040/LY | 2016 CAD | Not stated |
| Hannouf et al., 2012 [24]. BMC Cancer | Utilizing a 21-gene recurrence score assay to inform treatment decisions for women with early-stage breast cancer. | 21-gene assay v Standard clinical practice. Pre-menopausal: 0.05 QALY; Post-menopausal: 0.062 QALY | 21-gene assay v Standard clinical practice. Pre-menopausal: -CAD 50; Post-menopausal: CAD 3700 | 21-gene assay v Standard clinical practice. CAD 60,000/QALY for post-menopausal women | 2010 CAD | SA, CEAC, Scatter plot |
| Hedden et al., 2012 [25]. Eur. J. Cancer Oxf. | Comparing (1) mCRC therapy for all patients in the post-bev era compared to usual mCRC therapy in the pre-bev era; and (2) mCRC therapy for patients diagnosed under age 70 and who received doublet chemotherapy (i.e., those eligible for bevacizumab) in the post-bev era compared to usual mCRC therapy for the same sub-group of patients in the pre-bev era. | Pre- v post- bevacizumab: 0.06 QALY, 0.325 LY | Pre- v post- bevacizumab: CAD 3791 | Pre- v post- bevacizumab: CAD 43,058/QALY, CAD 10,764/LY | 2009 CAD | SA, CEAC, Scatter plot |
| Hedden et al., 2012 [26]. The Oncologist | Adjuvant trastuzumab for operable, HER-2/neu-positive early breast cancer compared with standard of care treatments in the adjuvant and metastatic settings. | Adjuvant trastuzumab v Standard care: 1.38 QALY, 1.17 LY | Adjuvant trastuzumab v Standard care: CAD 18,133 | Adjuvant trastuzumab v Standard care: CAD 13,095/QALY, CAD 15,492/LY | 2009 CAD | SA, CEAC, Scatter plot |
| Imran et al., 2019 [27]. Eur. Thyroid J. | Primary versus tertiary care among patients with low-risk differentiated thyroid cancer. | Tertiary care v Primary care: 0.4% less recurrence *Authors’ Assumptions* | Tertiary care v Primary care: CAD 46.11 | Tertiary care v Primary care: CAD 11,528/recurrence avoided | 2017 CAD | Not stated |
| Johnston et al. 2010 [28]. ParmacoeconimcsOutcomes Res. | Cyclophosphamide, doxorubicin, vincristine, and predisone (CHOP) chemotherapy versus CHOP-R (CHOP with the addition of rituximab) in the treatment of large B-cell lymphoma. | CHOP-R v CHOP: Younger individuals: 0.6 LY, 0.5 QALY, 0.8 disease-free LY; Older individuals: 1.7 LY, 1.4 QALY, 1.9 disease-free LY | CHOP-R v CHOP. Younger individuals: CAD 9572; Older individuals: CAD 8194 | CHOP-R v CHOP. Younger individuals: CAD 15,953/LY, CAD 19,144/QALY, CAD 11,965/disease-free LY; Older individuals: CAD 4820/LY, CAD 5853/QALY, CAD 4313/disease-free LY | 2006 CAD | SA, Scatter plot |
| Khor et al., 2014 [29]. BMC Cancer | Treating diffuse-large-B-cell lymphoma with rituximab plus cyclophpsphamide, doxorubicin, vincristine, and predisone (CHOP) (RCHOP) as opposed to only CHOP. | RCHOP v CHOP: 0.27 LY | RCHOP v CHOP: CAD 16,298 | RCHOP v CHOP: CAD 61,984/LY | 2009 CAD | CEAC, Scatter plot |
| Mittmann et al., 2018 [30]. J. Clin. Oncol Off. J. Am. Soc. Clin. Oncol. | Use of 21-gene assay Oncotype Dx test or standard of care for chemotherapy prescription. | Addition of assay: 23% less chemotherapy rX | Addition of assay: CAD 3000 | Addition of assay: CAD 13,043/chemo treatment avoided | 2014 CAD | Not stated |
| Nazha et al., 2018 [31]. Curr Oncol Tor Ont. | First line treatment in a real world setting for patients with metastatic renal cell carcinoma using sunitinib versus pazopanib. | Sunitinib v Pazopanib: 0.43 LY | Sunitinib v Pazopanib: CAD 24,232 | Sunitinib v Pazopanib: CAD 56,353/LY | 2017 CAD | Not stated |
| Nazha et al., 2018 [32]. Drug Investig. | Suntinib versus pazopanib in patients with metastatic renal cell carcinoma. | Sunitinib v Pazopanib: 1.21 LY; 0.54 QALY | Sunitinib v Pazopanib: CAD 36,303 | Sunitinib v Pazopanib: CAD 30,002/LY; CAD 67,227/QALY | 2017 CAD | SA, Scatter plot |
| Parackal et al. 2020 [33]. Can Urol. Assoc. J. J. Assoc. Urol. Can. | Use of robot-assisted radical prostatectomy (RARP) for prostate cancer or open radical prostatectomy (ORP). | RARP v ORP: 0.0662 QALY | RARP v ORP: CAD 1701 | RARP v ORP: CAD 25,704/QALY | 2019 CAD | SA, CEAC |
| Pataky et al., 2021 [34]. MDM Policy Pract. | Use of bevacizumab-treated patients versus those treated before bevacizumab funding and contemporaneous controls (receiving chemotherapy without bevacizumab) among patients with metastatic colorectal cancer. | Bevacizumab v Past standard care: 0.4–0.83 LY | Bevacizumab v Past standard care: CAD 31,200–66,600 | Bevacizumab v Past standard care: CAD 78,000–CAD 84,000/LY | 2019 CAD | SA |
| Raymakers et al., 2020 [35]. BMC Cancer | Use of brentuximab vedotin (BREN + AVD) or ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) as frontline therapy in patients with advanced Hodgkin’s lymphoma. | BREN + AVD v ABVD: 0.46 QALY | BREN + AVD v ABVD: CAD 192,336 | BREN + AVD v ABVD: CAD 418,122/QALY | 2018 CAD | SA, CEAC, Scatter plot |
| Tesch et al., 2022 [36]. Cancer. | Chemotherapy prescription and RS-guided treatment costs post-TAILORx. | Use of RS after funding v before: 19% decrease in chemotherapy use; Use of TAILORx v RS: 23% decrease in chemotherapy use | Use of TAILORx v RS: CAD 145,612 | Use of TAILORx c RS: CAD 633,096/chemo treatment avoided | 2021 CAD | SA |
| Thein et al., 2017 [37]. Cancer Med. | Use of transarterial chemoembolization (TACE) + radiofrequency ablation (RFA) versus RFA monotherapy versus no treatment. | TACE + RFA v No treatment: 0.93 QALY; RFA v No treatment: 0.88 QALY | TACE + RFA v No treatment: CAD 2304; RFA v No treatment: CAD 13,697 | TACE + RFA v No treatment: CAD 2465/QALY; RFA v No treatment CAD 15,553/QALY | 2013 USD | SA, CEAC, Scatter plot |
| Weymann et al., 2021 [38]. J Community Genet. | Using genomic sequencing to tailor cancer care. | Genomic sequencing v Usual care: 0.0025 LY | Genomic sequencing v Usual care: CAD 5203 | Genomic sequencing v Usual care: CAD 2,081,200/LY | 2015 CAD | Not stated |
3.3.1. Difference in Effectiveness
3.3.2. Costs
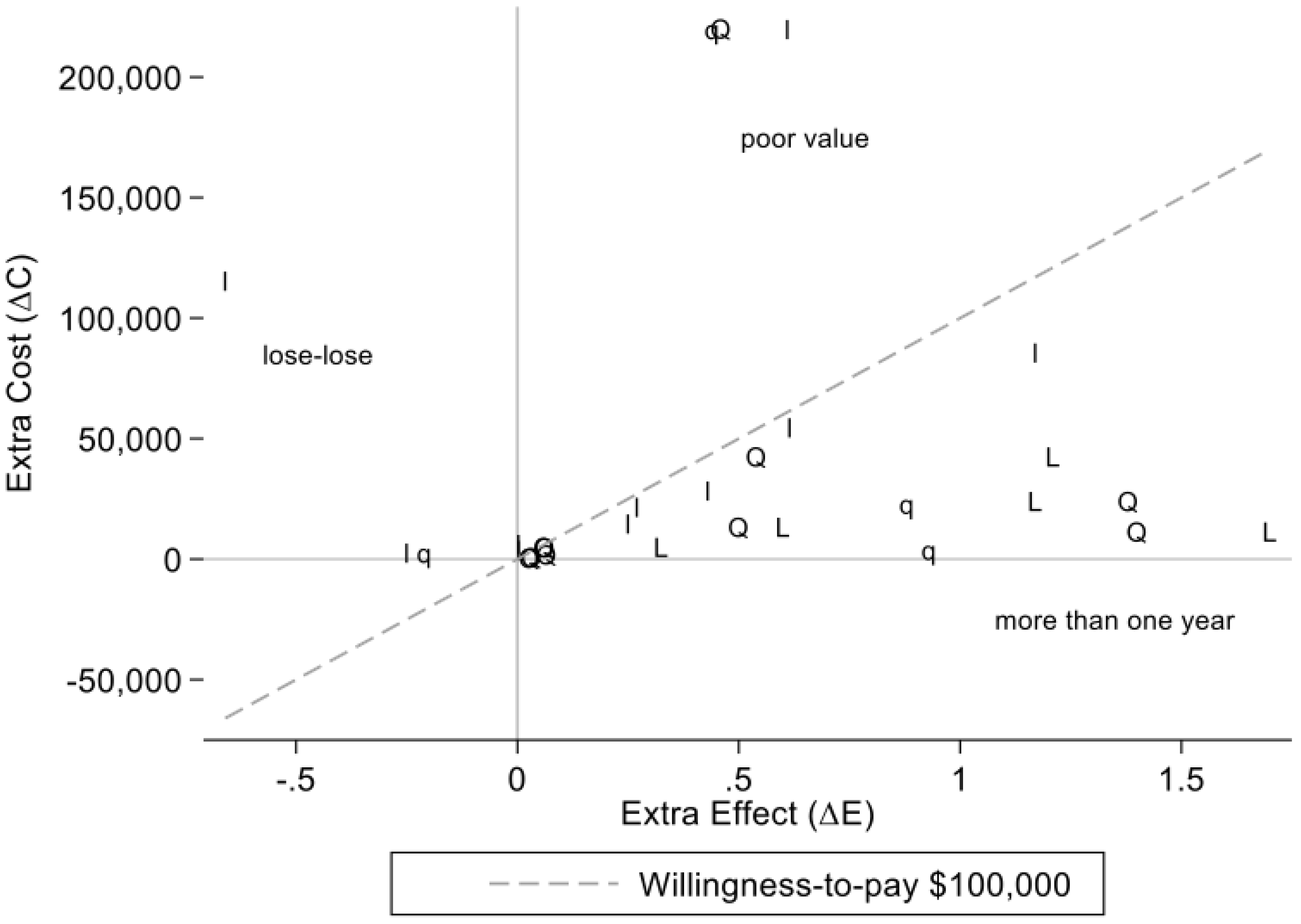
3.3.3. Estimates by Study Type
3.3.4. Estimates of Extra Cost and Extra Effect in Real World Studies of Drugs
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The PCODR Expert Review Committee (PERC)|CADTH. Available online: https://www.cadth.ca/pcodr-expert-review-committee-perc (accessed on 19 August 2022).
- Duma, N.; Kothadia, S.M.; Azam, T.U.; Yadav, S.; Paludo, J.; Vera Aguilera, J.; Gonzalez Velez, M.; Halfdanarson, T.R.; Molina, J.R.; Hubbard, J.M.; et al. Characterization of comorbidities limiting the recruitment of patients in early phase clinical trials. Oncol. 2019, 24, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Haslam, A.; Olivier, T.; Thawani, R.; Prasad, V. Duration of treatment in oncology clinical trials: Does the duration change when the same drug moves from the experimental arm to the control arm? ESMO Open 2022, 7, 100480. [Google Scholar] [CrossRef] [PubMed]
- Del Paggio, J.C.; Berry, J.S.; Hopman, W.M.; Eisenhauer, E.A.; Prasad, V.; Gyawali, B.; Booth, C.M. Evolution of the randomized clinical trial in the era of precision oncology. JAMA Oncol. 2021, 7, 379. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.M.; Eisenhauer, E.A. Progression-free survival: Meaningful or simply measurable? J. Clin. Oncol. 2012, 30, 1030–1033. [Google Scholar] [CrossRef]
- Prasad, V.; Kim, C.; Burotto, M.; Vandross, A. The strength of association between surrogate end points and survival in oncology: A systematic review of trial-level meta-analyses. JAMA Intern. Med. 2015, 175, 1389–1398. [Google Scholar] [CrossRef]
- Kovic, B.; Jin, X.; Kennedy, S.A.; Hylands, M.; Pędziwiatr, M.; Kuriyama, A.; Gomaa, H.; Lee, Y.; Katsura, M.; Tada, M.; et al. Evaluating progression-free survival as a surrogate outcome for health-related quality of life in oncology: A systematic review and quantitative analysis. JAMA Intern. Med. 2018, 178, 1586–1596. [Google Scholar] [CrossRef]
- Hwang, T.J.; Gyawali, B. Association between progression-free survival and patients’ quality of life in cancer clinical trials. Int. J. Cancer 2019, 144, 1746–1751. [Google Scholar] [CrossRef]
- Introducing CADTH’s RWE guidance working group|CADTH. Available online: https://www.cadth.ca/news/introducing-cadths-rwe-guidance-working-group (accessed on 19 August 2022).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Covidence—Better Systematic Review Management. Available online: https://www.covidence.org/ (accessed on 19 August 2022).
- Inflation Calculator. Available online: https://www.bankofcanada.ca/rates/related/inflation-calculator/ (accessed on 19 August 2022).
- CPI. Inflation Calculator. Available online: https://www.bls.gov/data/inflation_calculator.htm (accessed on 19 August 2022).
- Historical Currency Converter with Official Exchange Rates from 1953. Available online: https://fxtop.com/en/historical-currency-converter.php?A=12345&C1=USD&C2=CAD&DD=15&MM=05&YYYY=2022&B=1&P=&I=1&btnOK=Go%21 (accessed on 19 August 2022).
- Statistical Software for Data Science|Stata. Available online: https://www.stata.com/ (accessed on 19 August 2022).
- Stinnett, A.A.; Mullahy, J. Net health benefits: A new framework for the analysis of uncertainty in cost-effectiveness analysis. Med. Decis. Mak. Int. J. Soc. Med. Decis. Mak. 1998, 18, S68–S80. [Google Scholar] [CrossRef]
- Arciero, V.; Luo, J.; Parmar, A.; Dai, W.F.; Beca, J.M.; Raphael, M.J.; Isaranuwatchai, W.; Habbous, S.; Tadrous, M.; Earle, C.C.; et al. Real-world cost-effectiveness of first-line gemcitabine + nab-paclitaxel versus folfirinox in patients with advanced pancreatic cancer. JNCI Cancer Spectr. 2022, 6, pkac047. [Google Scholar] [CrossRef]
- Cressman, S.; Mar, C.; Sam, J.; Kan, L.; Lohrisch, C.; Spinelli, J.J. The cost-effectiveness of adding tomosynthesis to mammography-based breast cancer screening: An economic analysis. CMAJ Open 2021, 9, E443–E450. [Google Scholar] [CrossRef] [PubMed]
- Cressman, S.; Peacock, S.J.; Tammemägi, M.C.; Evans, W.K.; Leighl, N.B.; Goffin, J.R.; Tremblay, A.; Liu, G.; Manos, D.; MacEachern, P.; et al. The cost-effectiveness of high-risk lung cancer screening and drivers of program efficiency. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2017, 12, 1210–1222. [Google Scholar] [CrossRef] [PubMed]
- Cromwell, I.; van der Hoek, K.; Malfair Taylor, S.C.; Melosky, B.; Peacock, S. Erlotinib or best supportive care for third-line treatment of advanced non-small-cell lung cancer: A real-world cost-effectiveness analysis. Lung Cancer Amst. Neth. 2012, 76, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Cromwell, I.; van der Hoek, K.; Melosky, B.; Peacock, S. Erlotinib or docetaxel for second-line treatment of non-small cell lung cancer: A real-world cost-effectiveness analysis. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2011, 6, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.F.; Beca, J.M.; Nagamuthu, C.; Liu, N.; de Oliveira, C.; Earle, C.C.; Trudeau, M.; Chan, K.K.W. Cost-effectiveness analysis of pertuzumab with trastuzumab in patients with metastatic breast cancer. JAMA Oncol. 2022, 8, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.; Ramanakumar, A.V.; Festa, M.C.; Jardon, K.; Zeng, X.; Martins, C.; Shbat, L.; Alsoud, M.A.; Borod, M.; Wolfson, M.; et al. Real-world direct healthcare costs of treating recurrent high-grade serous ovarian cancer with cytotoxic chemotherapy. J. Comp. Eff. Res. 2020, 9, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Hannouf, M.B.; Xie, B.; Brackstone, M.; Zaric, G.S. Cost-effectiveness of a 21-gene recurrence score assay versus canadian clinical practice in women with early-stage estrogen- or progesterone-receptor-positive, axillary lymph-node negative breast cancer. BMC Cancer 2012, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Hedden, L.; Kennecke, H.; Villa, D.; Johnston, K.; Speers, C.; Kovacic, L.; Renouf, D.J.; Peacock, S. Incremental cost-effectiveness of the pre- and post-bevacizumab eras of metastatic colorectal cancer therapy in British Columbia, Canada. Eur. J. Cancer Oxf. Engl. 1990 2012, 48, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Hedden, L.; O’Reilly, S.; Lohrisch, C.; Chia, S.; Speers, C.; Kovacic, L.; Taylor, S.; Peacock, S. Assessing the real-world cost-effectiveness of adjuvant trastuzumab in HER-2/Neu positive breast cancer. The Oncologist 2012, 17, 164–171. [Google Scholar] [CrossRef]
- Imran, S.A.; Chu, K.; Rajaraman, M.; Rajaraman, D.; Ghosh, S.; De Brabandere, S.; Kaiser, S.M.; Van Uum, S. Primary versus tertiary care follow-up of low-risk differentiated thyroid cancer: Real-world comparison of outcomes and costs for patients and health care systems. Eur. Thyroid J. 2019, 8, 208–214. [Google Scholar] [CrossRef]
- Johnston, K.M.; Marra, C.A.; Connors, J.M.; Najafzadeh, M.; Sehn, L.; Peacock, S.J. Cost-effectiveness of the addition of rituximab to chop chemotherapy in first-line treatment for diffuse large b-cell lymphoma in a population-based observational cohort in British Columbia, Canada. Value Health J. Int. Soc. Pharmacoecon. Outcomes Res. 2010, 13, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Khor, S.; Beca, J.; Krahn, M.; Hodgson, D.; Lee, L.; Crump, M.; Bremner, K.E.; Luo, J.; Mamdani, M.; Bell, C.M.; et al. Real world costs and cost-effectiveness of rituximab for diffuse large b-cell lymphoma patients: A population-based analysis. BMC Cancer 2014, 14, 586. [Google Scholar] [CrossRef]
- Mittmann, N.; Earle, C.C.; Cheng, S.Y.; Julian, J.A.; Rahman, F.; Seung, S.J.; Levine, M.N. Population-based study to determine the health system costs of using the 21-gene assay. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 238–243. [Google Scholar] [CrossRef]
- Nazha, S.; Tanguay, S.; Kapoor, A.; Jewett, M.; Kollmannsberger, C.; Wood, L.; Bjarnason, G.; Heng, D.; Soulières, D.; Reaume, N.; et al. Use of targeted therapy in patients with metastatic renal cell carcinoma: Clinical and economic impact in a canadian real-life setting. Curr. Oncol. Tor. Ont 2018, 25, e576–e584. [Google Scholar] [CrossRef]
- Nazha, S.; Tanguay, S.; Kapoor, A.; Jewett, M.; Kollmannsberger, C.; Wood, L.; Bjarnason, G.A.G.; Heng, D.; Soulières, D.; Reaume, M.N.; et al. Cost-utility of sunitinib versus pazopanib in metastatic renal cell carcinoma in canada using real-world evidence. Clin. Drug Investig. 2018, 38, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Parackal, A.; Tarride, J.-E.; Xie, F.; Blackhouse, G.; Hoogenes, J.; Hylton, D.; Hanna, W.; Adili, A.; Matsumoto, E.D.; Shayegan, B. Economic evaluation of robot-assisted radical prostatectomy compared to open radical prostatectomy for prostate cancer treatment in Ontario, Canada. Can. Urol. Assoc. J. J. Assoc. Urol. Can. 2020, 14, E350–E357. [Google Scholar] [CrossRef] [PubMed]
- Pataky, R.E.; Beca, J.; Tran, D.; Dai, W.F.; Dvorani, E.; Isaranuwatchai, W.; Peacock, S.; Alvi, R.; Cheung, W.Y.; Earle, C.C.; et al. Real-world cost-effectiveness of bevacizumab with first-line combination chemotherapy in patients with metastatic colorectal cancer: Population-based retrospective cohort studies in three canadian provinces. MDM Policy Pract. 2021, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Raymakers, A.J.N.; Cameron, D.; Tyldesley, S.; Regier, D.A. Cost-effectiveness analysis of stereotactic ablative body radiotherapy for the treatment of oligometastatic tumors versus standard of care. Curr. Oncol. Tor. Ont 2021, 28, 1857–1866. [Google Scholar] [CrossRef]
- Tesch, M.E.; Speers, C.; Diocee, R.M.; Gondara, L.; Peacock, S.J.; Nichol, A.; Lohrisch, C.A. Impact of TAILORx on Chemotherapy prescribing and 21-gene recurrence score-guided treatment costs in a population-based cohort of patients with breast cancer. Cancer 2022, 128, 665–674. [Google Scholar] [CrossRef]
- Thein, H.-H.; Isaranuwatchai, W.; Qiao, Y.; Wong, K.; Sapisochin, G.; Chan, K.K.W.; Yoshida, E.M.; Earle, C.C. Cost-effectiveness analysis of potentially curative and combination treatments for hepatocellular carcinoma with person-level data in a canadian setting. Cancer Med. 2017, 6, 2017–2033. [Google Scholar] [CrossRef]
- Weymann, D.; Laskin, J.; Jones, S.J.M.; Roscoe, R.; Lim, H.J.; Renouf, D.J.; Schrader, K.A.; Sun, S.; Yip, S.; Marra, M.A.; et al. Early-stage economic analysis of research-based comprehensive genomic sequencing for advanced cancer care. J. Community Genet. 2021, 29, 1–16. [Google Scholar] [CrossRef] [PubMed]
- New Drug Funding Program. Available online: https://www.cancercareontario.ca/en/Funding/New_Drug_Funding_Program (accessed on 19 August 2022).
- BC. Cancer Registry. Available online: http://www.bccancer.bc.ca/health-professionals/professional-resources/bc-cancer-registry (accessed on 19 August 2022).
- NLST-122: The pan-canadian early detection of lung cancer study:cost-effectiveness of risk-stratification …—Approved Projects—The Cancer Data Access System. Available online: https://cdas.cancer.gov/approved-projects/809/ (accessed on 19 August 2022).
- Ontario Cancer Registry. Available online: https://www.cancercareontario.ca/en/cancer-care-ontario/programs/data-research/ontario-cancer-registry (accessed on 19 August 2022).
- Canadian Kidney Cancer Information System (CKCis). Available online: https://www.kcrnc.ca/ckcis/ (accessed on 19 August 2022).
- Canada, H. Canada Health Act. Available online: https://www.canada.ca/en/health-canada/services/health-care-system/canada-health-care-system-medicare/canada-health-act.html (accessed on 19 August 2022).
- Chan, K.; Nam, S.; Evans, B.; de Oliveira, C.; Chambers, A.; Gavura, S.; Hoch, J.; Mercer, R.E.; Dai, W.F.; Beca, J.; et al. Developing a framework to incorporate real-world evidence in cancer drug funding decisions: The canadian real-world evidence for value of cancer drugs (CanREValue) collaboration. BMJ Open 2020, 10, e032884. [Google Scholar] [CrossRef] [PubMed]

| Study Inclusion Criteria |
|
| Study Exclusion Criteria |
|
| Study Type | Extra Cost (ΔC) In 2022 Canadian Dollars (CAD) | Extra Effect (ΔE), as Either Life Years or QALYs | Incremental Cost-Effectiveness Ratio (ICER) (CAD) | |||
|---|---|---|---|---|---|---|
| Mean | Median | Mean | Median | Mean | Median | |
| Drug | ||||||
| Yes (n = 24) | 50,396.70 | 22,283.62 | 0.58 | 0.52 | 149,963.21 | 47,085.67 |
| No (n = 5) | 2872.24 | 1908.17 | 0.04 | 0.03 | 530,733.24 | 28,824.32 |
| p-value for difference | 0.003 ** | 0.06 | <0.001 *** | 0.06 | 0.49 | 0.93 |
| Model | ||||||
| Yes (n = 15) | 28,081.67 | 11,441.67 | 0.64 | 0.5 | 63,058.95 | 25,092.81 |
| No (n = 14) | 57,332.64 | 22,283.62 | 0.32 | 0.35 | 417,247.72 | 76,724.32 |
| p-value for difference | 0.25 | 0.2 | 0.13 | 0.35 | 0.14 | 0.04 * |
| QALYs as outcome | ||||||
| Yes (n = 15) | 41,002.70 | 8238.84 | 0.47 | 0.45 | 105,812.63 | 26,731.66 |
| No (n = 14) | 43,322.95 | 21,650.43 | 0.5 | 0.43 | 326,949.47 | 62,577.42 |
| p-value for difference | 0.93 | 0.35 | 0.9 | 0.85 | 0.29 | 0.56 |
| After 2017 | ||||||
| Yes (n = 14) | 74,524.06 | 42,566.94 | 0.32 | 0.44 | 338,009.02 | 76,044.77 |
| No (n = 15) | 12,036.35 | 11,441.67 | 0.64 | 0.5 | 126,449.90 | 25,092.81 |
| p-value for difference | 0.02 * | 0.2 | 0.13 | 0.85 | 0.36 | 0.18 |
| Total (n = 29) | 42,202.83 | 13,365.83 | 0.48 | 0.44 | 220,476.18 | 35,179.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guggenbickler, A.M.; Barr, H.K.; Hoch, J.S.; Dewa, C.S. Rapid Review of Real-World Cost-Effectiveness Analyses of Cancer Interventions in Canada. Curr. Oncol. 2022, 29, 7285-7304. https://doi.org/10.3390/curroncol29100574
Guggenbickler AM, Barr HK, Hoch JS, Dewa CS. Rapid Review of Real-World Cost-Effectiveness Analyses of Cancer Interventions in Canada. Current Oncology. 2022; 29(10):7285-7304. https://doi.org/10.3390/curroncol29100574
Chicago/Turabian StyleGuggenbickler, Andrea M., Heather K. Barr, Jeffrey S. Hoch, and Carolyn S. Dewa. 2022. "Rapid Review of Real-World Cost-Effectiveness Analyses of Cancer Interventions in Canada" Current Oncology 29, no. 10: 7285-7304. https://doi.org/10.3390/curroncol29100574
APA StyleGuggenbickler, A. M., Barr, H. K., Hoch, J. S., & Dewa, C. S. (2022). Rapid Review of Real-World Cost-Effectiveness Analyses of Cancer Interventions in Canada. Current Oncology, 29(10), 7285-7304. https://doi.org/10.3390/curroncol29100574





