Long-Term Survival after Linac-Based Stereotactic Radiosurgery and Radiotherapy with a Micro-Multileaf Collimator for Brain Metastasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. SRS and fSRT
2.3. Clinical and Radiological Follow-Up
2.4. Statistics
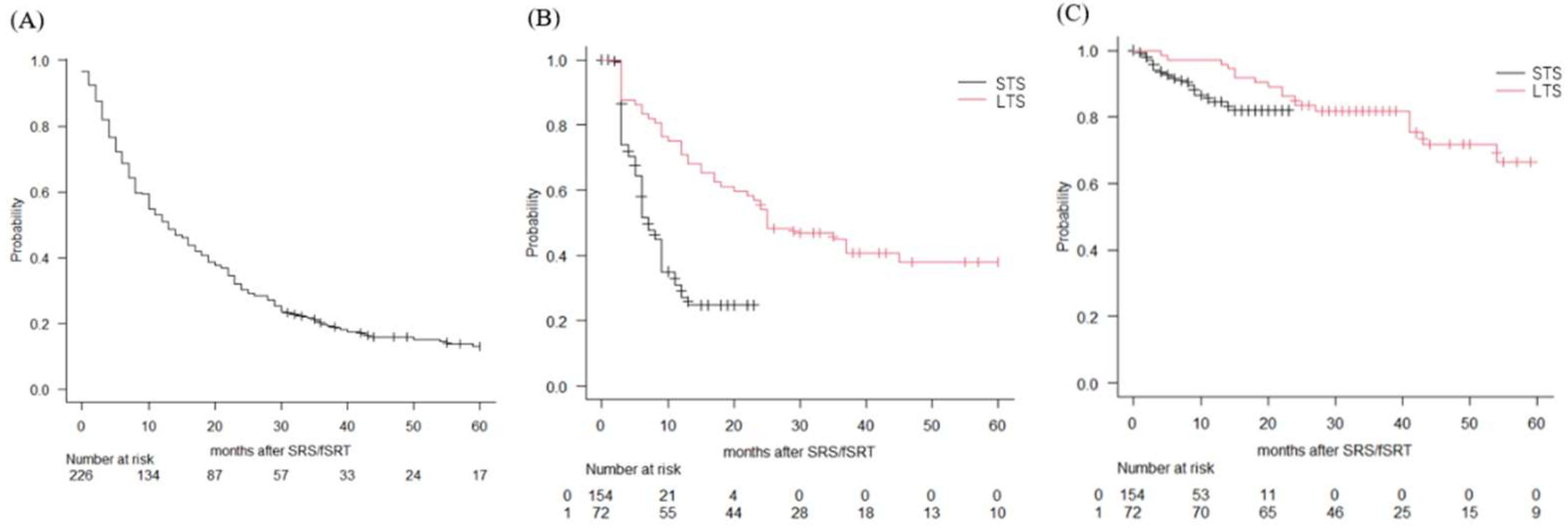
3. Results
3.1. Patient Characteristics in Both Groups
3.2. Treatment Outcomes
3.3. Factors Associated with Survival
4. Discussion
4.1. Long-Term Survival after SRT/fSRT for BM
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferguson, S.D.; Wagner, K.M.; Prabhu, S.S.; McAleer, M.F.; McCutcheon, I.E.; Sawaya, R. Neurosurgical management of brain metastases. Clin. Exp. Metastasis 2017, 34, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Kvint, S.; Chachoua, A.; Pavlick, A.; Wilson, M.; Donahue, B.; Golfinos, J.G.; Silverman, J.; Kondziolka, D. Toward the complete control of brain metastases using surveillance screening and stereotactic radiosurgery. J. Neurosurg. 2018, 128, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Matsuda, R.; Tamamoto, T.; Hontsu, S.; Yamaki, K.; Miura, S.; Park, Y.S.; Nakase, H.; Hasegawa, M. Linac-Based Fractionated Stereotactic Radiotherapy with a Micro-Multileaf Collimator for Brainstem Metastasis. World Neurosurg. 2019, 132, e680–e686. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, R.; Tamamoto, T.; Sugimoto, T.; Hontsu, S.; Yamaki, K.; Miura, S.; Takeshima, Y.; Tamura, K.; Yamada, S.; Nishimura, F.; et al. Linac-based fractionated stereotactic radiotherapy with a micro-multileaf collimator for large brain metastasis unsuitable for surgical resection. J. Radiat. Res. 2020, 61, 546–553. [Google Scholar] [CrossRef]
- Matsuda, R.; Hasegawa, M.; Tamamoto, T.; Ochi, T.; Miyasaka, T.; Inooka, N.; Hontsu, S.; Miura, S.; Takeshima, Y.; Tamura, K.; et al. Linac-based stereotactic radiosurgery and fractionated stereotactic radiotherapy with a micro-multileaf collimator for brain metastasis in the primary motor cortex. J. Radiat. Res. 2022, 63, 63–70. [Google Scholar] [CrossRef]
- Kondziolka, D.; Martin, J.J.; Flickinger, J.C.; Friedland, D.M.; Brufsky, A.M.; Baar, J.; Agarwala, S.; Kirkwood, J.M.; Lunsford, L.D. Long-term survivors after gamma knife radiosurgery for brain metastases. Cancer 2005, 104, 2784–2791. [Google Scholar] [CrossRef]
- Siddiqui, Z.A.; Squires, B.S.; Johnson, M.D.; Baschnagel, A.M.; Chen, P.Y.; Krauss, D.J.; Olson, R.E.; Meyer, K.D.; Grills, I.S. Predictors of radiation necrosis in long-term survivors after Gamma Knife stereotactic radiosurgery for brain metastases. Neurooncol. Pract. 2020, 7, 400–408. [Google Scholar] [CrossRef]
- Gogineni, E.; Vargo, J.A.; Glaser, S.M.; Flickinger, J.C.; Burton, S.A.; Engh, J.A.; Amankulor, N.M.; Beriwal, S.; Quinn, A.E.; Ozhasoglu, C.; et al. Long-Term Survivorship Following Stereotactic Radiosurgery Alone for Brain Metastases: Risk of Intracranial Failure and Implications for Surveillance and Counseling. Neurosurgery 2018, 83, 203–209. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kawabe, T.; Higuchi, Y.; Sato, Y.; Nariai, T.; Barfod, B.E.; Kasuya, H.; Urakawa, Y. Delayed complications in patients surviving at least 3 years after stereotactic radiosurgery for brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 53–60. [Google Scholar] [CrossRef]
- Cohen-Inbar, O.; Melmer, P.; Lee, C.C.; Xu, Z.; Schlesinger, D.; Sheehan, J.P. Leukoencephalopathy in long term brain metastases survivors treated with radiosurgery. J. Neurooncol. 2016, 126, 289–298. [Google Scholar] [CrossRef]
- Bilger, A.; Frenzel, F.; Oehlke, O.; Wiehle, R.; Milanovic, D.; Prokic, V.; Nieder, C.; Grosu, A.L. Local control and overall survival after frameless radiosurgery: A single center experience. Clin. Transl. Radiat. Oncol. 2017, 7, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Hashizume, C.; Kobayashi, T.; Shibamoto, Y.; Kosaki, K.; Nagai, A. Stereotactic radiotherapy using Novalis for skull base metastases developing with cranial nerve symptoms. J. Neurooncol. 2010, 98, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, T.; Engels, B.; Garibaldi, C.; Verellen, D.; Deconinck, P.; Duchateau, M.; Reynders, T.; Tournel, K.; De Ridder, M. Implementation of HybridArc treatment technique in preoperative radiotherapy of rectal cancer: Dose patterns in target lesions and organs at risk as compared to helical Tomotherapy and RapidArc. Radiat. Oncol. 2012, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Co, J.; Winter, J.; Millar, B.A.; Laperriere, N.; Tsang, D.S.; van Prooijen, M.; Damyanovich, A.; Heaton, R.; Coolens, C.; et al. Clinicopathologic and Treatment Features of Long-Term Surviving Brain Metastasis Patients. Curr. Oncol. 2021, 28, 549–559. [Google Scholar] [CrossRef]
- Sato, Y.; Yamamoto, M.; Serizawa, T.; Yamada, K.I.; Higuchi, Y.; Kasuya, H. A graded prognostic model for patients surviving 3years or more (GPM ≥ 3Ys) after stereotactic radiosurgery for brain metastasis. Radiother. Oncol. 2021, 156, 29–35. [Google Scholar] [CrossRef]
- McKay, W.H.; McTyre, E.R.; Okoukoni, C.; Alphonse-Sullivan, N.K.; Ruiz, J.; Munley, M.T.; Qasem, S.; Lo, H.W.; Xing, F.; Laxton, A.W.; et al. Repeat stereotactic radiosurgery as salvage therapy for locally recurrent brain metastases previously treated with radiosurgery. J. Neurosurg. 2017, 127, 148–156. [Google Scholar] [CrossRef]
- Kano, H.; Kondziolka, D.; Zorro, O.; Lobato-Polo, J.; Flickinger, J.C.; Lunsford, L.D. The results of resection after stereotactic radiosurgery for brain metastases. J. Neurosurg. 2009, 111, 825–831. [Google Scholar] [CrossRef]
- Rana, N.; Pendyala, P.; Cleary, R.K.; Luo, G.; Zhao, Z.; Chambless, L.B.; Cmelak, A.J.; Attia, A.; Stavas, M.J. Long-term Outcomes after Salvage Stereotactic Radiosurgery (SRS) following In-Field Failure of Initial SRS for Brain Metastases. Front. Oncol. 2017, 7, 279. [Google Scholar] [CrossRef]
- Alattar, A.A.; Carroll, K.; Hirshman, B.R.; Joshi, R.S.; Sanghvi, P.; Chen, C.C. Cystic Formation After Stereotactic Radiosurgery of Brain Metastasis. World Neurosurg. 2018, 114, e719–e728. [Google Scholar] [CrossRef]
- Gatterbauer, B.; Hirschmann, D.; Eberherr, N.; Untersteiner, H.; Cho, A.; Shaltout, A.; Gobl, P.; Fitschek, F.; Dorfer, C.; Wolfsberger, S.; et al. Toxicity and efficacy of Gamma Knife radiosurgery for brain metastases in melanoma patients treated with immunotherapy or targeted therapy-A retrospective cohort study. Cancer Med. 2020, 9, 4026–4036. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, R.; Morimoto, T.; Tamamoto, T.; Inooka, N.; Ochi, T.; Miyasaka, T.; Hontsu, S.; Yamaki, K.; Miura, S.; Takeshima, Y.; et al. Salvage Surgical Resection after Linac-Based Stereotactic Radiosurgery for Newly Diagnosed Brain Metastasis. Curr. Oncol. 2021, 28, 5255–5265. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Number | Median Survival Time (Months) | Log-Rank Test |
|---|---|---|---|
| Sex | |||
| Male 140 | 10 | 0.045 | |
| Female 86 | 17.5 | ||
| Age(years) | |||
| Median (range) 68.6 ± 10.3 | age ≥ 70 12.0 | 0.126 | |
| age < 70 13.5 | |||
| Pretreatment KPS | |||
| Median (range) 90 (40–100) | KPS ≥ 80 17.0 | <0.0001 | |
| <80 5.5 | |||
| Tumor origin | |||
| lung/breast/colon/stomach/kidney/esophagus/others | 189/11/9/4/3/3/7 | lung 15.0 | <0.0001 |
| non- long 5.0 | |||
| Tumor number | |||
| single 131 | single 16.0 | 0.173 | |
| multiple 95 | multiple 10.0 | ||
| Control of primary tumor | |||
| yes 93 | yes 21.0 | <0.0001 | |
| no 133 | no 8.0 | ||
| Extracranial metastasis | |||
| yes 99 | yes 7.0 | <0.0001 | |
| no 127 | no 19.0 | ||
| RPA class | |||
| I 13 | I 29.0 | <0.0001 | |
| II 160 | II 16.0 | ||
| III 53 | III 5.0 |
| Characteristic | Survival <24 Months | Survival ≥24 Months | p-Value |
|---|---|---|---|
| n = 154 | n = 72 | ||
| Sex | |||
| Female | 51 | 35 | 0.028 |
| Male | 103 | 37 | |
| Age(years) | 0.338 | ||
| 69.0 ± 10.5 | 67.6 ± 9.6 | ||
| Pretreatment KPS | 0.002 | ||
| Median(range) | 80 (50–100) | 100 (50–100) | |
| Tumor origin | 0.002 | ||
| lung | 121 | 68 | (lung vs. non-lung) |
| breast | 10 | 1 | |
| colon | 8 | 1 | |
| stomach | 4 | 0 | |
| kidney | 1 | 2 | |
| esophagus | 3 | 0 | |
| other | 7 | 0 | |
| Mutation status in lung cancer | 0.048 | ||
| EGFR positive | 28 | 21 | |
| ALK positive | 3 | 7 | |
| EGFR/ALK negative | 52 | 26 | |
| NA | 38 | 14 | |
| Treatment | 0.695 | ||
| SRS | 59 | 37 | |
| SRS and SRT | 42 | 13 | |
| SRT | 53 | 22 | |
| Tumor number | 0.248 | ||
| median/average/range | 2.16/1/1–9 | 2.08/1/1–9 | |
| single | 85 | 46 | |
| multiple | 69 | 26 | |
| Tumor volume (cc) | 0.881 | ||
| median/average/range | 0.3/2.13/0.01–26.52 | 0.31/1.76/0.02–33.51 | |
| Control of primary tumor | 0.001 | ||
| yes | 52 | 41 | |
| no | 102 | 31 | |
| Extracranial metastasis | <0.001 | ||
| yes | 79 | 20 | |
| no | 75 | 52 | |
| RPA class | <0.001 | ||
| I | 5 | 8 | |
| II | 105 | 55 | |
| III | 44 | 9 |
| Factor | Odds Ratio | 95%CI | p-Value |
|---|---|---|---|
| Sex (female:vs. male) | 1.94 | 1.050–3.60 | 0.0345 |
| Age | 1.02 | 0.989–1.05 | 0.226 |
| Number of metastasis | 1.1 | 0.575–2.09 | 0.782 |
| Pretreatment KPS | 2.54 | 1.150–5.59 | 0.021 |
| Control of primary cancer | 0.638 | 0.332–1.22 | 0.176 |
| Extracranial metastasis | 1.56 | 0.67–3.62 | 0.019 |
| HR:hazard ratio | |||
| CI:confidence interval |
| Author (Year) | Modality | Number of Pt | LTS | OS (Range) | Factors in LTS | Cause of Death | Complication |
|---|---|---|---|---|---|---|---|
| Kondziolka (2005) [6] | GK | 44 /681 pts (6.5%) | ≥4 years | 68 months (48–156 months) | Higher pre-KPS Fewer brain metastases Less extracranial disease | 1 pt: brain 27 pts: systemic cancer | 4 pts (9%): permanent neurological deficits 6 pts (13.6%): salvage surgery |
| Yamamoto (2013) [9] | GK | 167 pts (8.4%) | ≥3 years | 49.9 (36–142 months) | NA | 16 pts: brain 76 pts: systemic disease | 17 pts (10.1%): all complications 13 pts (7.8%): salvage surgery |
| Cohen-Inbar (2016) [10] | GK | 92 pts | ≥2 years | Survival rate 3 years: 73.7% 4 years: 51.8% 5 years: 41.7% | NA | NA | 7 pts (7.6%): salvage surgery |
| Gogineni (2018) [8] | CK (86%) Others (14%) | 132 pts (11.8%) | ≥2 years | NA | NA | NA | NA |
| Siddiqui (2020) [7] | GK | 198 pts | ≥ 1 years | 25.2 months (22.9–28.4 months) 2years: 52.8% 3 years: 33.4% 4 years: 22.1% 5 years: 16.6% | NA | NA | 10 pts (5.1%): salvage surgery for radiation necrosis |
| This study (2022) | Linac based | 72 pts (31.8%) | ≥2 years | 43 months (36–73) 3 years: 59.1% 4 years: 49.6% 5 years: 40.7% | Female High pre-KPS no extracranial metastasis | 1 pt: brain 43 pts: systemic cancer | 13 pts (18.0%): salvage surgery for 7 recurrences, 5 radiation necrosis, and 1 cyst formation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuda, R.; Hasegawa, M.; Tamamoto, T.; Inooka, N.; Nikimoto, M.; Ochi, T.; Miyasaka, T.; Hontsu, S.; Yamaki, K.; Miura, S.; et al. Long-Term Survival after Linac-Based Stereotactic Radiosurgery and Radiotherapy with a Micro-Multileaf Collimator for Brain Metastasis. Curr. Oncol. 2022, 29, 6068-6076. https://doi.org/10.3390/curroncol29090477
Matsuda R, Hasegawa M, Tamamoto T, Inooka N, Nikimoto M, Ochi T, Miyasaka T, Hontsu S, Yamaki K, Miura S, et al. Long-Term Survival after Linac-Based Stereotactic Radiosurgery and Radiotherapy with a Micro-Multileaf Collimator for Brain Metastasis. Current Oncology. 2022; 29(9):6068-6076. https://doi.org/10.3390/curroncol29090477
Chicago/Turabian StyleMatsuda, Ryosuke, Masatoshi Hasegawa, Tetsuro Tamamoto, Nobuyoshi Inooka, Mei Nikimoto, Tomoko Ochi, Toshiteru Miyasaka, Shigeto Hontsu, Kaori Yamaki, Sachiko Miura, and et al. 2022. "Long-Term Survival after Linac-Based Stereotactic Radiosurgery and Radiotherapy with a Micro-Multileaf Collimator for Brain Metastasis" Current Oncology 29, no. 9: 6068-6076. https://doi.org/10.3390/curroncol29090477
APA StyleMatsuda, R., Hasegawa, M., Tamamoto, T., Inooka, N., Nikimoto, M., Ochi, T., Miyasaka, T., Hontsu, S., Yamaki, K., Miura, S., Morimoto, T., Mitsui, T., Furuta, T., Yokoyama, S., Kotsugi, M., Yamada, S., Nakagawa, I., Park, Y.-S., & Nakase, H. (2022). Long-Term Survival after Linac-Based Stereotactic Radiosurgery and Radiotherapy with a Micro-Multileaf Collimator for Brain Metastasis. Current Oncology, 29(9), 6068-6076. https://doi.org/10.3390/curroncol29090477





