Recruiting Adolescent and Young Adult Cancer Survivors for Patient-Reported Outcome Research: Experiences and Sample Characteristics of the SURVAYA Study
Abstract
1. Introduction
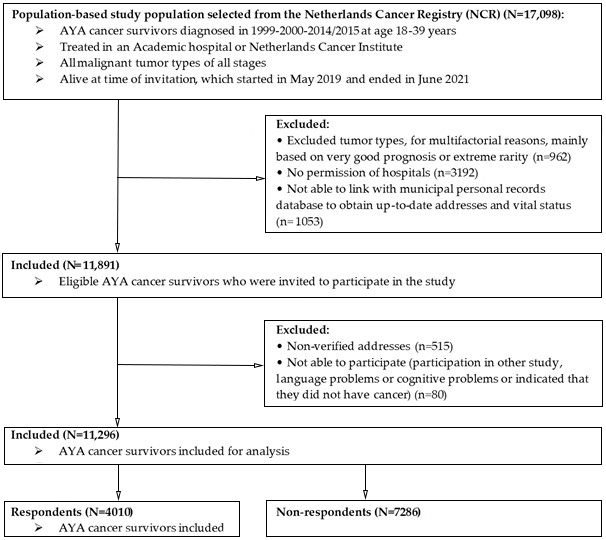
2. Materials and Methods
2.1. Setting and Population
2.2. Data Collection
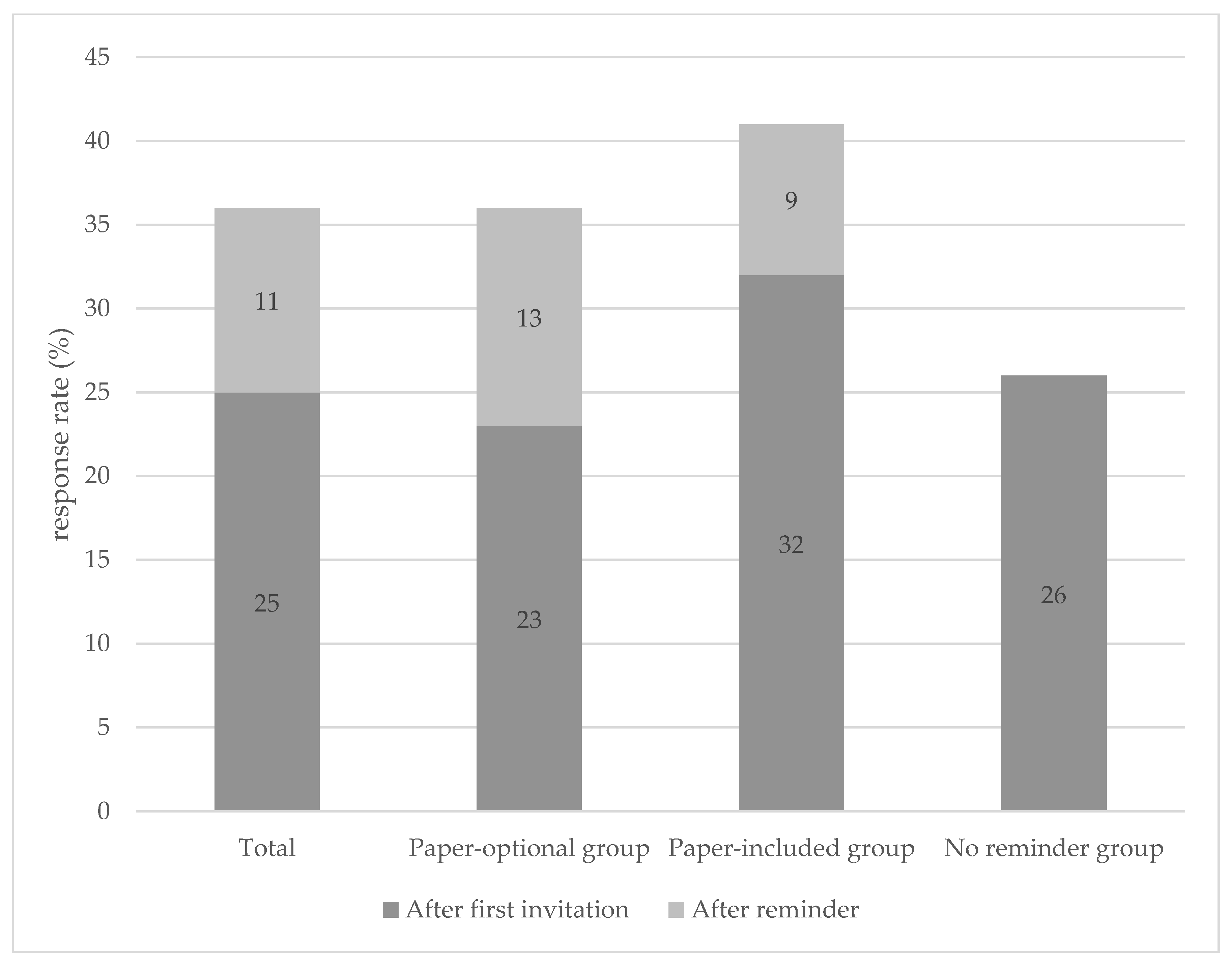
2.2.1. Paper-Optional Group (N = 8291)
2.2.2. No Reminder Group (N = 1671)
2.2.3. Paper Included Group (N = 1334)
2.3. Measures
2.4. Statistical Analyses
3. Results
3.1. Representativeness Study Sample
3.2. Methods of Invitation
3.3. Reasons of Non-Participation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewis, D.R.; Seibel, N.L.; Smith, A.W.; Stedman, M.R. Adolescent and young adult cancer survival. J. Natl. Cancer Inst. Monogr. 2014, 2014, 228–235. [Google Scholar] [CrossRef]
- Galán, S.; de la Vega, R.; Miró, J. Needs of adolescents and young adults after cancer treatment: A systematic review. Eur. J. Cancer Care 2018, 27, e12558. [Google Scholar] [CrossRef]
- Meeneghan, M.R.; Wood, W.A. Challenges for cancer care delivery to adolescents and young adults: Present and future. Acta Haematol. 2014, 132, 414–422. [Google Scholar] [CrossRef]
- Smith, A.W.; Seibel, N.L.; Lewis, D.R.; Albritton, K.H.; Blair, D.F.; Blanke, C.D.; Bleyer, W.A.; Freyer, D.R.; Geiger, A.M.; Hayes-Lattin, B.; et al. Next steps for adolescent and young adult oncology workshop: An update on progress and recommendations for the future. Cancer 2016, 122, 988–999. [Google Scholar] [CrossRef]
- Ferrari, A.; Stark, D.; Peccatori, F.A.; Fern, L.; Laurence, V.; Gaspar, N.; Bozovic-Spasojevic, I.; Smith, O.; De Munter, J.; Derwich, K.; et al. Adolescents and young adults (AYA) with cancer: A position paper from the AYA Working Group of the European Society for Medical Oncology (ESMO) and the European Society for Paediatric Oncology (SIOPE). ESMO Open 2021, 6, 100096. [Google Scholar] [CrossRef]
- van der Meer, D.J.; Karim-Kos, H.E.; van der Mark, M.; Aben, K.K.H.; Bijlsma, R.M.; Rijneveld, A.W.; van der Graaf, W.T.A.; Husson, O. Incidence, Survival, and Mortality Trends of Cancers Diagnosed in Adolescents and Young Adults (15–39 Years): A Population-Based Study in The Netherlands 1990–2016. Cancers 2020, 12, 3421. [Google Scholar] [CrossRef]
- Geue, K.; Mehnert-Theuerkauf, A.; Stroske, I.; Brock, H.; Friedrich, M.; Leuteritz, K. Psychosocial Long-Term Effects of Young Adult Cancer Survivors: Study Protocol of the Longitudinal AYA-LE Long-Term Effects Study. Front. Psychol. 2021, 12, 688142. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network Guidelines for patients. Adolescents and Young adults with Cancer, 2019. Available online: https://www.nccn.org/patients/guidelines/content/PDF/aya-patient.pdf (accessed on 12 July 2022).
- Adolescent and Young Adult Oncology Progress Review Group. Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer, 2006. Available online: https://www.livestrong.org/content/closing-gap-research-and-careimperatives-adolescents-and-young-adults-cancer (accessed on 12 July 2022).
- Janssen, S.H.M.; van der Graaf, W.T.A.; van der Meer, D.J.; Manten-Horst, E.; Husson, O. Adolescent and Young Adult (AYA) Cancer Survivorship Practices: An Overview. Cancers 2021, 13, 4847. [Google Scholar] [CrossRef]
- Smith, A.W.; Keegan, T.; Hamilton, A.; Lynch, C.; Wu, X.C.; Schwartz, S.M.; Kato, I.; Cress, R.; Harlan, L. Understanding care and outcomes in adolescents and young adult with Cancer: A review of the AYA HOPE study. Pediatr. Blood Cancer 2019, 66, e27486. [Google Scholar] [CrossRef] [PubMed]
- Patterson, P.; McDonald, F.E.J.; Zebrack, B.; Medlow, S. Emerging Issues Among Adolescent and Young Adult Cancer Survivors. Semin. Oncol. Nurs. 2015, 31, 53–59. [Google Scholar] [CrossRef]
- Chao, C.; Bhatia, S.; Xu, L.; Cannavale, K.L.; Wong, F.L.; Huang, P.S.; Cooper, R.; Armenian, S.H. Chronic Comorbidities Among Survivors of Adolescent and Young Adult Cancer. J. Clin. Oncol. 2020, 38, 3161–3174. [Google Scholar] [CrossRef]
- Schulte, F.S.M.; Chalifour, K.; Eaton, G.; Garland, S.N. Quality of life among survivors of adolescent and young adult cancer in Canada: A Young Adults With Cancer in Their Prime (YACPRIME) study. Cancer 2021, 127, 1325–1333. [Google Scholar] [CrossRef]
- Unger, J.M.; Cook, E.; Tai, E.; Bleyer, A. The Role of Clinical Trial Participation in Cancer Research: Barriers, Evidence, and Strategies. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, 185–198. [Google Scholar] [CrossRef]
- Tai, E.; Buchanan, N.; Eliman, D.; Westervelt, L.; Beaupin, L.; Lawvere, S.; Bleyer, A. Understanding and addressing the lack of clinical trial enrollment among adolescents with cancer. Pediatrics 2014, 133 (Suppl. S3), S98–S103. [Google Scholar] [CrossRef]
- Shnorhavorian, M.; Doody, D.R.; Chen, V.W.; Hamilton, A.S.; Kato, I.; Cress, R.D.; West, M.; Wu, X.C.; Keegan, T.H.M.; Harlan, L.C.; et al. Knowledge of Clinical Trial Availability and Reasons for Nonparticipation Among Adolescent and Young Adult Cancer Patients: A Population-based Study. Am. J. Clin. Oncol. 2018, 41, 581–587. [Google Scholar] [CrossRef]
- Bleyer, W.A.; Tejeda, H.; Murphy, S.B.; Robison, L.L.; Ross, J.A.; Pollock, B.H.; Severson, R.K.; Brawley, O.W.; Smith, M.A.; Ungerleider, R.S. National cancer clinical trials: Children have equal access; adolescents do not. J. Adolesc. Health 1997, 21, 366–373. [Google Scholar] [CrossRef]
- Harlan, L.C.; Lynch, C.F.; Keegan, T.H.; Hamilton, A.S.; Wu, X.C.; Kato, I.; West, M.M.; Cress, R.D.; Schwartz, S.M.; Smith, A.W.; et al. Recruitment and follow-up of adolescent and young adult cancer survivors: The AYA HOPE Study. J. Cancer Surviv. 2011, 5, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Kaal, S.E.J.; Lidington, E.K.; Prins, J.B.; Jansen, R.; Manten-Horst, E.; Servaes, P.; van der Graaf, W.T.A.; Husson, O. Health-Related Quality of Life Issues in Adolescents and Young Adults with Cancer: Discrepancies with the Perceptions of Health Care Professionals. J. Clin. Med. 2021, 10, 1833. [Google Scholar] [CrossRef] [PubMed]
- Murnane, A.; Gough, K.; Thompson, K.; Holland, L.; Conyers, R. Adolescents and young adult cancer survivors: Exercise habits, quality of life and physical activity preferences. Support. Care Cancer 2015, 23, 501–510. [Google Scholar] [CrossRef]
- Bellizzi, K.M.; Smith, A.; Schmidt, S.; Keegan, T.H.; Zebrack, B.; Lynch, C.F.; Deapen, D.; Shnorhavorian, M.; Tompkins, B.J.; Simon, M. Positive and negative psychosocial impact of being diagnosed with cancer as an adolescent or young adult. Cancer 2012, 118, 5155–5162. [Google Scholar] [CrossRef]
- Michel, G.; François, C.; Harju, E.; Dehler, S.; Roser, K. The long-term impact of cancer: Evaluating psychological distress in adolescent and young adult cancer survivors in Switzerland. Psychooncology 2019, 28, 577–585. [Google Scholar] [CrossRef]
- McCarthy, M.C.; McNeil, R.; Drew, S.; Dunt, D.; Kosola, S.; Orme, L.; Sawyer, S.M. Psychological Distress and Posttraumatic Stress Symptoms in Adolescents and Young Adults with Cancer and Their Parents. J. Adolesc. Young Adult Oncol. 2016, 5, 322–329. [Google Scholar] [CrossRef]
- Leuteritz, K.; Friedrich, M.; Nowe, E.; Sender, A.; Taubenheim, S.; Stoebel-Richter, Y.; Geue, K. Recruiting young adult cancer patients: Experiences and sample characteristics from a 12-month longitudinal study. Eur. J. Oncol. Nurs. 2018, 36, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Nichols, H.B.; Baggett, C.D.; Engel, S.M.; Getahun, D.; Anderson, C.; Cannizzaro, N.T.; Green, L.; Gupta, P.; Laurent, C.A.; Lin, P.C.; et al. The Adolescent and Young Adult (AYA) Horizon Study: An AYA Cancer Survivorship Cohort. Cancer Epidemiol. Biomark. Prev. 2021, 30, 857–866. [Google Scholar] [CrossRef]
- van de Poll-Franse, L.V.; Horevoorts, N.; van Eenbergen, M.; Denollet, J.; Roukema, J.A.; Aaronson, N.K.; Vingerhoets, A.; Coebergh, J.W.; de Vries, J.; Essink-Bot, M.L.; et al. The Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship registry: Scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur. J. Cancer 2011, 47, 2188–2194. [Google Scholar] [CrossRef]
- Fritz, A.; Percy, C.; Jack, A.S.; Shanmugaratnam, K.; Sobin, L.; Parkin, D.M.; Whelan, S. International Classification of Diseases for Oncology, 3rd ed.; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Sobin, L.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours; Wiley: New York, NY, USA, 2011. [Google Scholar]
- Rosenberg, A.R.; Junkins, C.C.; Sherr, N.; Scott, S.; Klein, V.; Barton, K.S.; Yi-Frazier, J.P. Conducting Psychosocial Intervention Research among Adolescents and Young Adults with Cancer: Lessons from the PRISM Randomized Clinical Trial. Children 2019, 6, 117. [Google Scholar] [CrossRef]
- Glass, D.C.; Kelsall, H.L.; Slegers, C.; Forbes, A.B.; Loff, B.; Zion, D.; Fritschi, L. A telephone survey of factors affecting willingness to participate in health research surveys. BMC Public Health 2015, 15, 1017. [Google Scholar] [CrossRef]
- Otufowora, A.; Liu, Y.; Young, H., 2nd; Egan, K.L.; Varma, D.S.; Striley, C.W.; Cottler, L.B. Sex Differences in Willingness to Participate in Research Based on Study Risk Level Among a Community Sample of African Americans in North Central Florida. J. Immigr. Minor. Health 2021, 23, 19–25. [Google Scholar] [CrossRef]
- Ryan, J.; Lopian, L.; Le, B.; Edney, S.; Van Kessel, G.; Plotnikoff, R.; Vandelanotte, C.; Olds, T.; Maher, C. It’s not raining men: A mixed-methods study investigating methods of improving male recruitment to health behaviour research. BMC Public Health 2019, 19, 814. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, B.H.; Ezendam, N.P.M.; Mols, F.; Vissers, P.A.J.; Thong, M.S.Y.; Vlooswijk, C.C.P.; Oerlemans, S.; Husson, O.; Horevoorts, N.J.E.; van de Poll-Franse, L.V. Cancer survivors not participating in observational patient-reported outcome studies have a lower survival compared to participants: The population-based PROFILES registry. Qual. Life Res. 2018, 27, 3313–3324. [Google Scholar] [CrossRef]
- Price, K.N.; Lyons, A.B.; Hamzavi, I.H.; Hsiao, J.L.; Shi, V.Y. Facilitating Clinical Trials Participation of Low Socioeconomic Status Patients. Dermatology 2021, 237, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Kripalani, S.; Heerman, W.J.; Patel, N.J.; Jackson, N.; Goggins, K.; Rothman, R.L.; Yeh, V.M.; Wallston, K.A.; Smoot, D.T.; Wilkins, C.H. Association of Health Literacy and Numeracy with Interest in Research Participation. J. Gen. Intern. Med. 2019, 34, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Perneger, T.V.; Chamot, E.; Bovier, P.A. Nonresponse bias in a survey of patient perceptions of hospital care. Med. Care 2005, 43, 374–380. [Google Scholar] [CrossRef]
- Rosenberg, A.R.; Bona, K.; Wharton, C.M.; Bradford, M.; Shaffer, M.L.; Wolfe, J.; Baker, K.S. Adolescent and Young Adult Patient Engagement and Participation in Survey-Based Research: A Report From the “Resilience in Adolescents and Young Adults With Cancer” Study. Pediatr. Blood Cancer 2016, 63, 734–736. [Google Scholar] [CrossRef]
- Richards, J.; Wiese, C.; Katon, W.; Rockhill, C.; McCarty, C.; Grossman, D.; McCauley, E.; Richardson, L.P. Surveying adolescents enrolled in a regional health care delivery organization: Mail and phone follow-up—What works at what cost? J. Am. Board Fam. Med. 2010, 23, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.I.; Ekholm, O.; Kristensen, P.L.; Larsen, F.B.; Vinding, A.L.; Glümer, C.; Juel, K. The effect of multiple reminders on response patterns in a Danish health survey. Eur. J. Public Health 2015, 25, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P.J.; Roberts, I.; Clarke, M.J.; Diguiseppi, C.; Wentz, R.; Kwan, I.; Cooper, R.; Felix, L.M.; Pratap, S. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev. 2009, 2009, Mr000008. [Google Scholar] [CrossRef] [PubMed]
- Akmatov, M.K.; Jentsch, L.; Riese, P.; May, M.; Ahmed, M.W.; Werner, D.; Rösel, A.; Prokein, J.; Bernemann, I.; Klopp, N.; et al. Motivations for (non)participation in population-based health studies among the elderly—Comparison of participants and nonparticipants of a prospective study on influenza vaccination. BMC Med. Res. Methodol. 2017, 17, 18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alkerwi, A.A.; Sauvageot, N.; Couffignal, S.; Albert, A.; Lair, M.-L.; Guillaume, M. Comparison of participants and non-participants to the ORISCAV-LUX population-based study on cardiovascular risk factors in Luxembourg. BMC Med. Res. Methodol. 2010, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Anampa-Guzmán, A.; Freeman-Daily, J.; Fisch, M.; Lou, E.; Pennell, N.A.; Painter, C.A.; Sparacio, D.; Lewis, M.A.; Karmo, M.; Anderson, P.F.; et al. The Rise of the Expert Patient in Cancer: From Backseat Passenger to Co-navigator. JCO Oncol. Pract. 2022, OP2100763. [Google Scholar] [CrossRef]
- Barr, R.D.; Ferrari, A.; Ries, L.; Whelan, J.; Bleyer, W.A. Cancer in Adolescents and Young Adults: A Narrative Review of the Current Status and a View of the Future. JAMA Pediatr. 2016, 170, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Barbera, L.; Kerrigan, C.L.; Velikova, G. Implementation of Patient-Reported Outcomes in Routine Medical Care. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Husson, O.; Reeve, B.B.; Darlington, A.S.; Cheung, C.K.; Sodergren, S.; van der Graaf, W.T.A.; Salsman, J.M. Next Step for Global Adolescent and Young Adult Oncology: A Core Patient-Centered Outcome Set. J. Natl. Cancer Inst. 2021, 114, 496–502. [Google Scholar] [CrossRef]
- Salsman, J.M.; Danhauer, S.C.; Moore, J.B.; Canzona, M.R.; Victorson, D.E.; Zebrack, B.J.; Reeve, B.B. Optimizing the measurement of health-related quality of life in adolescents and young adults with cancer. Cancer 2020, 126, 4818–4824. [Google Scholar] [CrossRef] [PubMed]
| Total Population-Based Population | Respondents | Non- Respondents | Excluded | P-Value (Non- Respondents vs. Respondents) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 17,098 | N = 4010 | N = 7286 | N = 5802 | |||||||
| n | % | n | % | n | % | n | % | |||
| Gender | Male | 6997 | 41 | 1549 | 39 | 3082 | 42 | 2366 | 41 | 0.0001 |
| Female | 10,101 | 59 | 2461 | 61 | 4204 | 58 | 3436 | 59 | ||
| Age (at diagnosis), (mean (sd)) | 31.5 (5.9) | 31.6 (5.9) | 31.4 (5.9) | 31.5 (5.8) | ||||||
| 18–24 years | 2672 | 16 | 613 | 15 | 1168 | 16 | 891 | 15 | 0.1354 | |
| 25–34 years | 7782 | 46 | 1786 | 45 | 3328 | 46 | 2668 | 46 | ||
| 35–39 years | 6644 | 39 | 1611 | 40 | 2790 | 38 | 2243 | 39 | ||
| Time since diagnosis, (mean (sd)) | 12.2 (4.6) | 12.4 (4.5) | 11.6 (4.4) | 12.8 (4.8) | ||||||
| 5–10 years | 6362 | 37 | 1386 | 35 | 2990 | 41 | 1986 | 34 | 0.0001 | |
| ≥11–15 years | 558 | 33 | 1397 | 35 | 2496 | 34 | 1687 | 29 | ||
| ≥16–20 years | 5156 | 30 | 1231 | 31 | 1806 | 25 | 2119 | 37 | ||
| Social economic status | Low | 3161 | 19 | 544 | 14 | 1510 | 21 | 1107 | 19 | 0.0001 |
| Intermediate | 5427 | 32 | 1236 | 31 | 2354 | 32 | 1837 | 32 | ||
| High | 8456 | 50 | 2220 | 56 | 3417 | 47 | 2819 | 49 | ||
| Type of cancer | Head and neck | 570 | 3 | 124 | 3 | 305 | 4 | 141 | 2 | 0.0001 |
| Colon and rectal | 368 | 2 | 82 | 2 | 151 | 2 | 135 | 2 | ||
| Digestive tract, other | 262 | 2 | 31 | 1 | 63 | 1 | 168 | 3 | ||
| Respiratory tract | 166 | 1 | 30 | 1 | 71 | 1 | 65 | 1 | ||
| Melanoma | 1221 | 7 | 290 | 7 | 617 | 8 | 314 | 5 | ||
| Skin, other | 105 | 1 | 0 | 0 | 0 | 0 | 105 | 2 | ||
| Breast | 3346 | 20 | 944 | 24 | 1553 | 21 | 849 | 15 | ||
| Female genitalia | 2073 | 12 | 445 | 11 | 878 | 12 | 750 | 13 | ||
| Male genitalia | 24 | 0 | 6 | 0 | 12 | 0 | 6 | 0 | ||
| Urinary tract | 190 | 1 | 46 | 1 | 105 | 1 | 39 | 1 | ||
| Thyroid gland | 1054 | 6 | 248 | 6 | 468 | 6 | 338 | 6 | ||
| Central nervous system | 545 | 3 | 150 | 4 | 231 | 3 | 164 | 3 | ||
| Bone or soft tissue sarcoma | 1256 | 7 | 172 | 4 | 291 | 4 | 793 | 14 | ||
| Germ cell tumors | 2743 | 16 | 692 | 17 | 1394 | 19 | 657 | 11 | ||
| Lymphoid hematological malignancies | 2339 | 14 | 591 | 15 | 903 | 12 | 845 | 15 | ||
| Myeloid hematological malignancies | 671 | 4 | 148 | 4 | 226 | 3 | 297 | 5 | ||
| Other | 165 | 1 | 11 | 0 | 18 | 0 | 136 | 2 | ||
| Tumor stage | I | 7758 | 45 | 1726 | 43 | 3480 | 48 | 2552 | 44 | 0.0001 |
| II | 388 | 23 | 1063 | 27 | 1850 | 25 | 967 | 17 | ||
| III | 1994 | 12 | 573 | 14 | 923 | 13 | 498 | 9 | ||
| IV | 618 | 4 | 179 | 4 | 298 | 4 | 141 | 2 | ||
| Missing | 2848 | 17 | 469 | 12 | 735 | 10 | 1644 | 28 | ||
| Primary treatment modality | Surgery | 13,145 | 77 | 3126 | 78 | 5863 | 81 | 4156 | 72 | 0.0017 |
| Chemotherapy | 8199 | 48 | 2239 | 56 | 3632 | 50 | 2328 | 40 | 0.0001 | |
| Radiotherapy | 7681 | 45 | 1902 | 47 | 3293 | 45 | 2486 | 43 | 0.0213 | |
| Hormone therapy | 1734 | 10 | 484 | 12 | 786 | 11 | 464 | 8 | 0.0382 | |
| Targeted therapy | 1119 | 7 | 308 | 8 | 514 | 7 | 297 | 5 | 0.2178 | |
| Stem cell therapy | 523 | 3 | 142 | 4 | 207 | 3 | 174 | 3 | 0.0392 | |
| Marital status (at time of questionnaire) | Partner | NA | NA | 3333 | 83 | NA | NA | NA | NA | |
| Education level | No education or primary education | NA | NA | 28 | 1 | NA | NA | NA | NA | |
| Secondary education | 266 | 7 | ||||||||
| Secondary (vocational) education | 1456 | 36 | ||||||||
| Higher (vocational) education | 1374 | 34 | ||||||||
| University education | 878 | 22 | ||||||||
| Mode of completion | Paper | NA | NA | 647 | 16 | NA | NA | NA | NA | |
| Online | 3363 | 84 | ||||||||
| Respondents | Non-Respondents | Odds of Respondents vs. Non-Respondents | ||||||
|---|---|---|---|---|---|---|---|---|
| N = 4010 | N = 7286 | OR | 95% CI | p- value | ||||
| Gender | Male | 1549 | 39 | 3082 | 42 | 1.00 (ref) | ||
| Females | 2461 | 61 | 4204 | 58 | 1.246 | 1.108–1.400 | 0.0002 | |
| Age (at diagnosis) | 18–24 years | 613 | 15 | 1168 | 16 | 1.00 (ref) | ||
| 25–34 years | 1786 | 45 | 3328 | 46 | 1.074 | 0.953–1.210 | 0.2420 | |
| 35–39 years | 1611 | 40 | 2790 | 38 | 1.123 | 0.989–1.275 | 0.0734 | |
| Years since diagnosis | 5–10 years | 1386 | 35 | 2990 | 41 | 1.00 (ref) | ||
| ≥11–15 years | 1397 | 35 | 2496 | 34 | 1.210 | 1.103–1.328 | 0.0001 | |
| ≥16–20 years | 1231 | 31 | 1806 | 25 | 1.489 | 1.348–1.645 | 0.0001 | |
| Social economic status | Low | 544 | 14 | 1510 | 21 | 1.00 (ref) | ||
| Intermediate | 1236 | 31 | 2354 | 32 | 1.406 | 1.245–1.587 | 0.0001 | |
| High | 2220 | 56 | 3417 | 47 | 1.763 | 1.575–1.974 | 0.0001 | |
| Type of cancer | Head and neck | 124 | 3 | 305 | 4 | 0.933 | 0.711–1.224 | 0.6153 |
| Colon and rectal | 82 | 2 | 151 | 2 | 1.080 | 0.793–1.472 | 0.6246 | |
| Digestive tract, other | 31 | 1 | 63 | 1 | 1.146 | 0.719–1.827 | 0.5673 | |
| Respiratory tract | 30 | 1 | 71 | 1 | 0.991 | 0.626–1.570 | 0.9696 | |
| Melanoma | 290 | 7 | 617 | 8 | 1.069 | 0.856–1.334 | 0.5576 | |
| Breast | 944 | 24 | 1553 | 21 | 1.00 (ref) | |||
| Female genitalia | 445 | 11 | 878 | 12 | 1.042 | 0.864–1.257 | 0.6642 | |
| Male genitalia | 6 | 0 | 12 | 0 | 1.258 | 0.460–3.439 | 0.6546 | |
| Urinary tract | 46 | 1 | 105 | 1 | 1.086 | 0.736–1.604 | 0.6769 | |
| Thyroid gland | 248 | 6 | 468 | 6 | 1.213 | 0.976–1.508 | 0.0816 | |
| Central nervous system | 150 | 4 | 231 | 3 | 1.402 | 0.998–1.969 | 0.0515 | |
| Bone or soft tissue sarcoma | 172 | 4 | 291 | 4 | 1.289 | 1008–1.648 | 0.0430 | |
| Germ cell tumors | 692 | 17 | 1394 | 19 | 1.124 | 0.920–1.374 | 0.2532 | |
| Lymphoid hematological malignancies | 591 | 15 | 903 | 12 | 1.381 | 1.048–1.820 | 0.0220 | |
| Myeloid hematological malignancies | 148 | 4 | 226 | 3 | 1.294 | 0.877–1.908 | 0.1939 | |
| Other | 11 | 0 | 18 | 0 | 1.318 | 0.596–2.912 | 0.4951 | |
| Tumor stage | I | 1726 | 43 | 3480 | 48 | 1.00 (ref) | ||
| II | 1063 | 27 | 1850 | 25 | 1.026 | 0.916–1.149 | 0.6579 | |
| III | 573 | 14 | 923 | 13 | 1.174 | 1.022–1.348 | 0.0231 | |
| IV | 179 | 4 | 298 | 4 | 1.139 | 0.916–1.417 | 0.2412 | |
| Missing | 469 | 12 | 735 | 10 | 1.121 | 0.892–1.409 | 0.3278 | |
| Primary treatment modality | Surgery | 3126 | 78 | 5863 | 81 | 1.121 | 0.901–1.395 | 0.3059 |
| Chemotherapy | 2239 | 56 | 3632 | 50 | 1.262 | 1.118–1.425 | 0.0002 | |
| Radiotherapy | 1902 | 47 | 3293 | 45 | 1.030 | 0.935–1.135 | 0.5475 | |
| Hormone therapy | 484 | 12 | 786 | 11 | 0.991 | 0.841–1.168 | 0.9156 | |
| Targeted therapy | 308 | 8 | 514 | 7 | 1.013 | 0.862–1.191 | 0.8762 | |
| Stem cell therapy | 142 | 4 | 207 | 3 | 0.996 | 0.772–1.284 | 0.9738 | |
| N | % | |
|---|---|---|
| Not interested in the research | 379 | 49 |
| Don’t want to think about cancer | 243 | 32 |
| Too busy | 148 | 19 |
| Questionnaire is too long/too personal/difficult | 97 | 13 |
| Don’t consider themself a young adult cancer patient | 97 | 13 |
| Have participated in research too many times | 58 | 8 |
| No personal incentive or benefit | 32 | 4 |
| Not capable to participate * | 20 | 3 |
| Don’t see the added value of this research | 21 | 3 |
| Worried about privacy aspects | 16 | 2 |
| Practical problems in participating ** | 16 | 2 |
| Other *** | 16 | 2 |
| Unclear what causes symptoms because of comorbidities | 14 | 2 |
| Prefer an in person invitation | 9 | 1 |
| Multiple answers could be given |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlooswijk, C.; Poll-Franse, L.V.v.d.; Janssen, S.H.M.; Derksen, E.; Reuvers, M.J.P.; Bijlsma, R.; Kaal, S.E.J.; Kerst, J.M.; Tromp, J.M.; Bos, M.E.M.M.; et al. Recruiting Adolescent and Young Adult Cancer Survivors for Patient-Reported Outcome Research: Experiences and Sample Characteristics of the SURVAYA Study. Curr. Oncol. 2022, 29, 5407-5425. https://doi.org/10.3390/curroncol29080428
Vlooswijk C, Poll-Franse LVvd, Janssen SHM, Derksen E, Reuvers MJP, Bijlsma R, Kaal SEJ, Kerst JM, Tromp JM, Bos MEMM, et al. Recruiting Adolescent and Young Adult Cancer Survivors for Patient-Reported Outcome Research: Experiences and Sample Characteristics of the SURVAYA Study. Current Oncology. 2022; 29(8):5407-5425. https://doi.org/10.3390/curroncol29080428
Chicago/Turabian StyleVlooswijk, Carla, Lonneke V. van de Poll-Franse, Silvie H. M. Janssen, Esther Derksen, Milou J. P. Reuvers, Rhodé Bijlsma, Suzanne E. J. Kaal, Jan Martijn Kerst, Jacqueline M. Tromp, Monique E. M. M. Bos, and et al. 2022. "Recruiting Adolescent and Young Adult Cancer Survivors for Patient-Reported Outcome Research: Experiences and Sample Characteristics of the SURVAYA Study" Current Oncology 29, no. 8: 5407-5425. https://doi.org/10.3390/curroncol29080428
APA StyleVlooswijk, C., Poll-Franse, L. V. v. d., Janssen, S. H. M., Derksen, E., Reuvers, M. J. P., Bijlsma, R., Kaal, S. E. J., Kerst, J. M., Tromp, J. M., Bos, M. E. M. M., Hulle, T. v. d., Lalisang, R. I., Nuver, J., Kouwenhoven, M. C. M., van der Graaf, W. T. A., & Husson, O. (2022). Recruiting Adolescent and Young Adult Cancer Survivors for Patient-Reported Outcome Research: Experiences and Sample Characteristics of the SURVAYA Study. Current Oncology, 29(8), 5407-5425. https://doi.org/10.3390/curroncol29080428








