Treatment of Basal Cell Carcinoma with Electrochemotherapy: Insights from the InspECT Registry (2008–2019)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Procedure
2.3.1. Protocol
2.3.2. Anaesthesia
2.3.3. Chemotherapy
2.3.4. Electric Pulse Delivery
2.3.5. Postprocedural Care
2.4. Outcome Assessment
2.5. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Treatment
3.3. Toxicity
3.4. Tumour Response
3.5. Predictors of Response
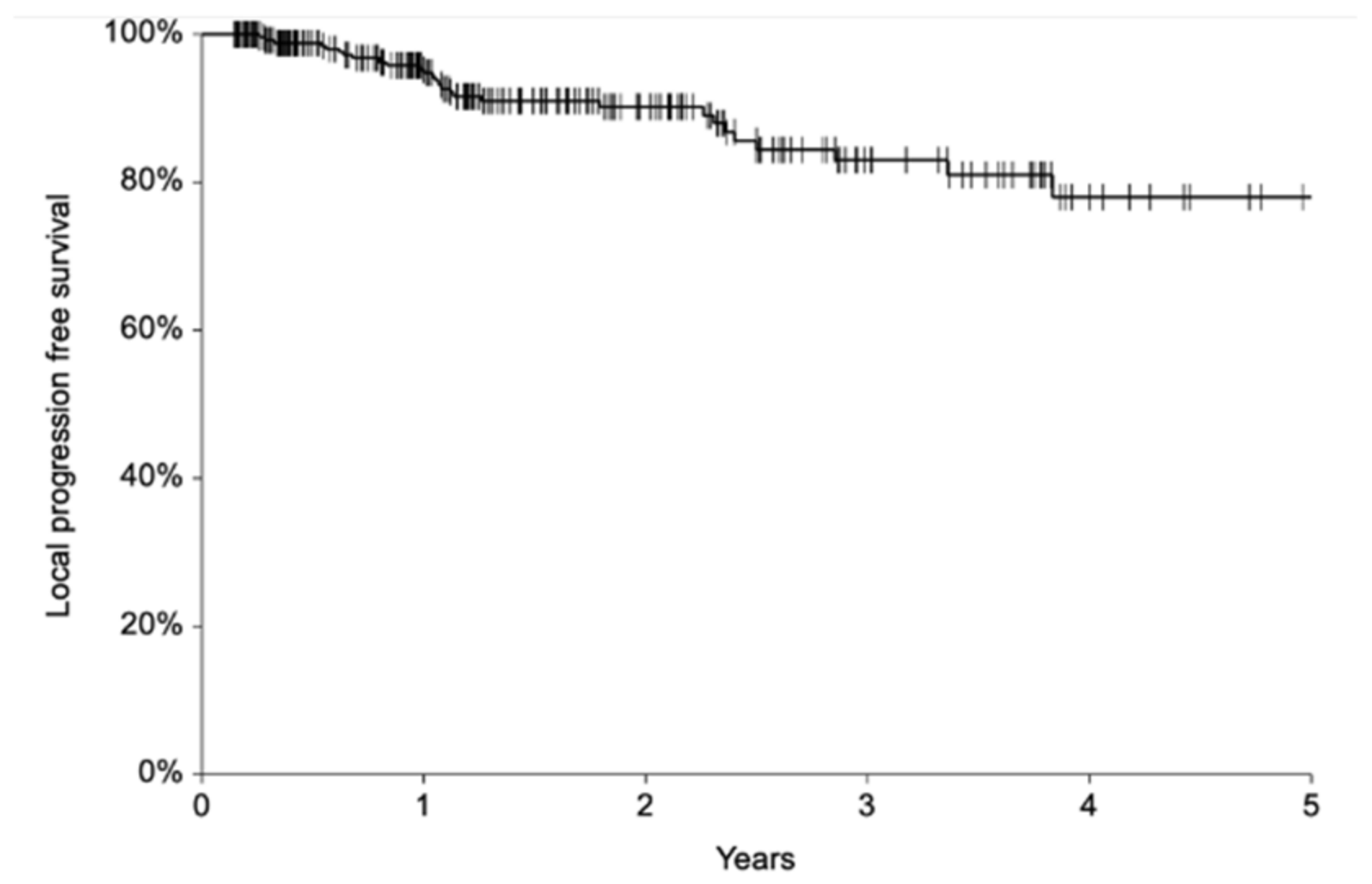
3.6. Local Control and Patient Survival
4. Discussion
4.1. ECT Safety
4.2. Predictors of Response
4.3. Local Control
4.4. Previous Clinical Experiences
4.5. Comparison with Other Treatments
4.6. The Role of ECT in Locally Advanced BCC
4.7. The Role of ECT in an Ageing Population
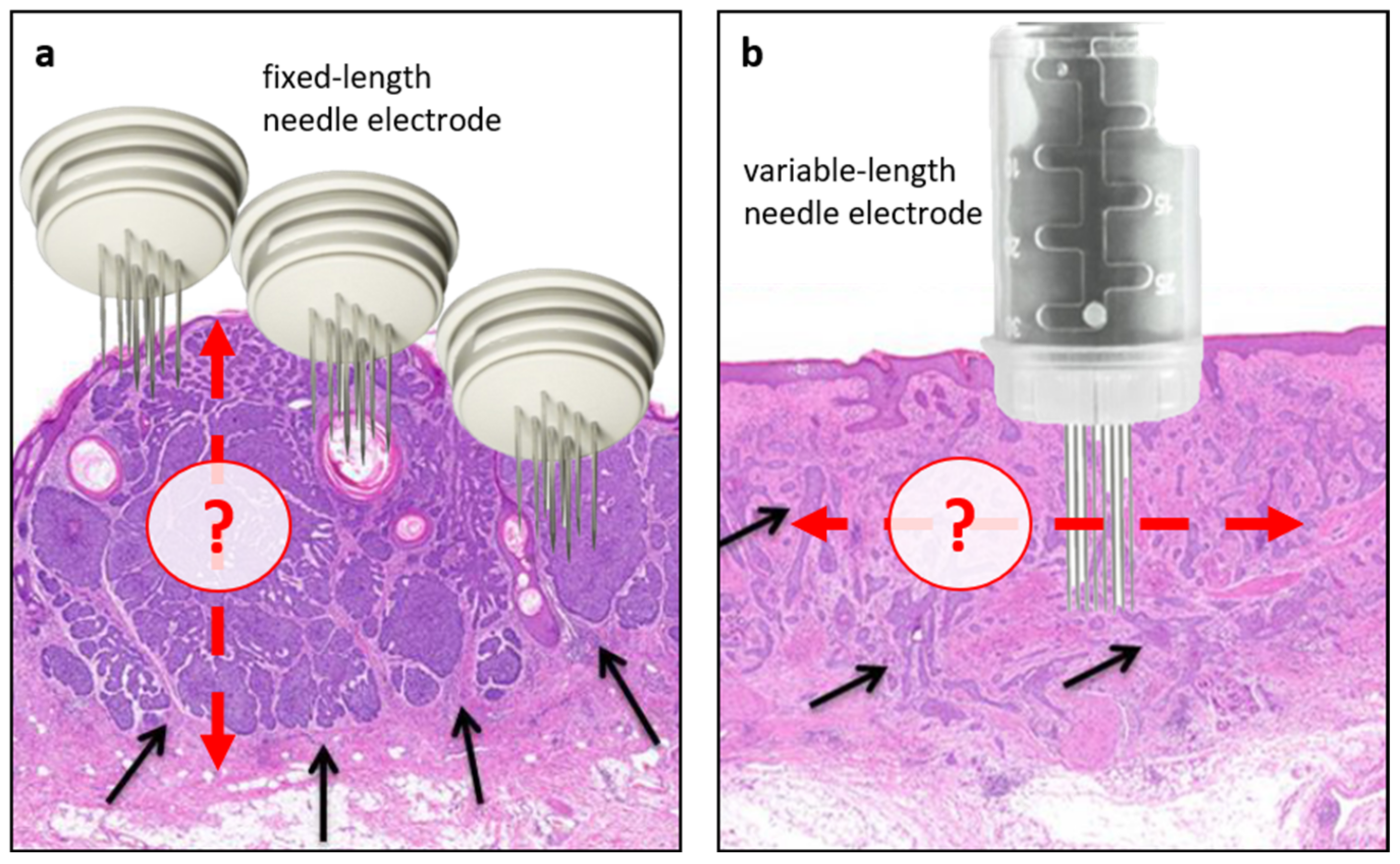
4.8. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Migden, M.R.; Chang, A.L.S.; Dirix, L.; Stratigos, A.J.; Lear, J.T. Emerging trends in the treatment of advanced basal cell carcinoma. Cancer Treat. Rev. 2018, 64, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, C.M. Mohs’ micrographic surgery for basal cell carcinoma: Mohs’ surgery for BCC. Clin. Exp. Dermatol. 1999, 24, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Stratigos, A.J.; Sekulic, A.; Peris, K.; Bechter, O.; Prey, S.; Kaatz, M.; Lewis, K.D.; Basset-Seguin, N.; Chang, A.L.; Dalle, S.; et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2021, 22, 848–857. [Google Scholar] [CrossRef]
- Campana, L.G.; Miklavčič, D.; Bertino, G.; Marconato, R.; Valpione, S.; Imarisio, I.; Dieci, M.V.; Granziera, E.; Cemazar, M.; Alaibac, M.; et al. Electrochemotherapy of superficial tumours—Current status. Semin. Oncol. 2019, 46, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Jarm, T.; Cemazar, M.; Miklavcic, D.; Sersa, G. Antivascular effects of electrochemotherapy: Implications in treatment of metastases. Expert. Rev. Anticance. Ther. 2012, 10, 729–746. [Google Scholar] [CrossRef]
- Clover, A.J.P.; de Terlizzi, F.; Bertino, G.; Curatolo, P.; Odili, J.; Campana, L.G.; Kunte, C.; Muir, T.; Brizio, M.; Sersa, G. Electrochemotherapy in the treatment of cutaneous malignancy: Outcomes and subgroup analysis from the cumulative results from the pan-European International Network for Sharing Practice in Electrochemotherapy database for 2482 lesions in 987 patients (2008–2019). Eur. J. Cancer 2020, 138, 30–40. [Google Scholar] [CrossRef]
- Clover, A.J.P.; Salwa, S.P.; Bourke, M.G.; McKiernan, J.; Forde, P.F.; O’Sullivan, S.T.; Kelly, E.J.; Soden, D.M. Electrochemotherapy for the treatment of primary basal cell carcinoma; A randomised control trial comparing electrochemotherapy and surgery with five-year follow-up. Eur. J. Surg. Oncol. 2020, 46, 847–854. [Google Scholar] [CrossRef]
- Campana, L.G.; Marconato, R.; Valpione, S.; Galuppo, S.; Alaibac, M.; Rossi, C.R.; Mocellin, S. Basal cell carcinoma: 10-year experience with electrochemotherapy. J. Transl. Med. 2017, 15, 122. [Google Scholar] [CrossRef]
- Kis, E.G.; Baltás, E.; Ócsai, H.; Vass, A.; Németh, I.B.; Varga, E.; Oláh, J.; Kemény, L.; Tóth-Molnár, E. Electrochemotherapy in the treatment of locally advanced or recurrent eyelid-periocular basal cell carcinomas. Sci. Rep. 2019, 9, 4285. [Google Scholar] [CrossRef]
- Ruggeri, R.; Maurichi, A.; Tinti, M.C.; Cadenelli, P.; Patuzzo, R.; Gallino, G.; Santinami, M. Electrochemotherapy: A good idea in recurrent basal cell carcinoma treatment. Melanoma Manag. 2015, 2, 27–31. [Google Scholar] [CrossRef]
- Salwa, S.P.; Bourke, M.G.; Forde, P.F.; O’Shaughnessy, M.; O’Sullivan, S.T.; Kelly, E.J.; Soden, D.M.; Clover, A.J.P. Electrochemotherapy for the treatment of ocular basal cell carcinoma; a novel adjunct in the disease management. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 403–406. [Google Scholar] [CrossRef]
- Gatti, A.; Stinco, G.; di Meo, N.; Noal, C.; Maione, M.; Trevisan, G. Electrochemotherapy for a locally advanced basal cell carcinoma on the forehead. Indian J. Derm. Venereol. Leprol. 2014, 80, 378–380. [Google Scholar] [CrossRef]
- Kis, E.; Baltás, E.; Kinyó, Á.; Varga, E.; Nagy, N.; Gyulai, R.; Kemény, L.; Oláh, J. Successful Treatment of Multiple Basaliomas with Bleomycin-based Electrochemotherapy: A Case Series of Three Patients with Gorlin-Goltz Syndrome. Acta Derm. Venereol. 2012, 92, 648–651. [Google Scholar] [CrossRef]
- Fantini, F.; Gualdi, G.; Cimitan, A.; Giannetti, A. Metastatic Basal Cell Carcinoma with Squamous Differentiation: Report of a Case With Response of Cutaneous Metastases to Electrochemotherapy. Arch. Dermatol. 2008, 144, 1186–1188. [Google Scholar] [CrossRef][Green Version]
- Glass, L.F.; Jaroszeski, M.; Gilbert, R.; Reintgen, D.S.; Heller, R. Intralesional bleomycin-mediated electrochemotherapy in 20 patients with basal cell carcinoma. J. Am. Acad. Dermatol. 1997, 37, 596–599. [Google Scholar] [CrossRef]
- Glass, L.F.; Fenske, N.A.; Jaroszeski, M.; Perrott, R.; Harvey, D.T.; Reintgen, D.S.; Heller, R. Bleomycin-mediated electrochemotherapy of basal cell carcinoma. J. Am. Acad. Dermatol. 1996, 34, 82–86. [Google Scholar] [CrossRef]
- Rotunno, R.; Campana, L.G.; Quaglino, P.; de Terlizzi, F.; Kunte, C.; Odili, J.; Gehl, J.; Ribero, S.; Liew, S.H.; Marconato, R.; et al. Electrochemotherapy of unresectable cutaneous tumours with reduced dosages of intravenous bleomycin: Analysis of 57 patients from the International Network for Sharing Practices of Electrochemotherapy registry. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1147–1154. [Google Scholar] [CrossRef]
- Bertino, G.; Sersa, G.; de Terlizzi, F.; Occhini, A.; Plaschke, C.C.; Groselj, A.; Langdon, C.; Grau, J.J.; McCaul, J.A.; Heuveling, D.; et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: Results of the treatment of skin cancer. Eur. J. Cancer 2016, 63, 41–52. [Google Scholar] [CrossRef]
- Campana, L.G.; Testori, A.; Curatolo, P.; Quaglino, P.; Mocellin, S.; Framarini, M.; Borgognoni, L.; Ascierto, P.A.; Mozzillo, N.; Guida, M. Treatment efficacy with electrochemotherapy: A multi-institutional prospective observational study on 376 patients with superficial tumours. Eur. J. Surg. Oncol. 2016, 42, 1914–1923. [Google Scholar] [CrossRef]
- Campana, L.G.; Mali, B.; Sersa, G.; Valpione, S.; Giorgi, C.A.; Strojan, P.; Miklavcic, D.; Rossi, C.R. Electrochemotherapy in nonmelanoma head and neck cancers: A retrospective analysis of the treated cases. Br. J. Oral Maxillofac. Surg. 2014, 52, 957–964. [Google Scholar] [CrossRef]
- Mir, L.M.; Gehl, J.; Sersa, G.; Collins, C.G.; Garbay, J.R.; Billard, V.; Geertsend, P.F.; Rudolf, Z.; O’Sullivan, G.C.; Marty, M. Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses by the Cliniporator™ by means of invasive or non-invasive electrodes. Eur. J. Cancer Suppl. 2006, 4, 14–25. [Google Scholar] [CrossRef]
- Gehl, J.; Sersa, G.; Matthiessen, L.W.; Muir, T.; Soden, D.; Occhini, A.; Quaglino, P.; Curatolo, P.; Campana, L.G.; Kunte, C. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018, 57, 874–882. [Google Scholar] [CrossRef]
- Spratt, D.E.; Gordon Spratt, E.A.; Wu, S.; DeRosa, A.; Lee, N.Y.; Lacouture, M.E.; Barker, C.A. Efficacy of Skin-Directed Therapy for Cutaneous Metastases From Advanced Cancer: A Meta-Analysis. J. Clin. Oncol. 2014, 32, 3144–3155. [Google Scholar] [CrossRef]
- Sersa, G.; Ursic, K.; Cemazar, M.; Heller, R.; Bosnjak, M.; Campana, L.G. Biological factors of the tumour response to electrochemotherapy: Review of the evidence and a research roadmap. Eur. J. Surg. Oncol. 2021, 47, 1836–1846. [Google Scholar] [CrossRef]
- Jacobsen, A.A.; Aldahan, A.S.; Hughes, O.B.; Shah, V.V.; Strasswimmer, J. Hedgehog Pathway Inhibitor Therapy for Locally Advanced and Metastatic Basal Cell Carcinoma: A Systematic Review and Pooled Analysis of Interventional Studies. JAMA Dermatol. 2016, 152, 816–824. [Google Scholar] [CrossRef]
- Sersa, G.; Mascherini, M.; Di Prata, C.; Odili, J.; de Terlizzi, F.; McKenzie, G.A.G.; Clover, A.J.P.; Bertino, G.; Spina, R.; Groselj, A. Outcomes of older adults aged 90 and over with cutaneous malignancies after electrochemotherapy with bleomycin: A matched cohort analysis from the InspECT registry. Eur. J. Surg. Oncol. 2021, 47, 902–912. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Electrochemotherapy for Primary Basal Cell Carcinoma and Primary Squamous Cell Carcinoma. Guidance and Guidelines. 2014. Available online: https://www.nice.org.uk/guidance/ipg478/chapter/7-Further-information (accessed on 20 April 2022).
- Hendel, K.; Jemec, G.B.E.; Haedersdal, M.; Wiegell, S.R. Electrochemotherapy with bleomycin for basal cell carcinomas: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2208–2215. [Google Scholar] [CrossRef]
- Nasr, I.; McGrath, E.J.; Harwood, C.A.; Botting, J.; Buckley, P.; Budny, P.G.; Fairbrother, P.; Fife, K.; Gupta, G.; Hashme, H.; et al. British Association of Dermatologists guidelines for the management of adults with basal cell carcinoma 2021. Br. J. Dermatol. 2021, 185, 899–920. [Google Scholar] [CrossRef]
- Campana, L.G.; Clover, A.J.; Valpione, S.; Quaglino, P.; Gehl, J.; Kunte, C.; Snoj, M.; Cemazar, M.; Rossi, C.R.; Miklavcic, D.; et al. Recommendations for improving the quality of reporting clinical electrochemotherapy studies based on qualitative systematic review. Radiol. Oncol. 2016, 50, 1–13. [Google Scholar] [CrossRef]
- Lear, J.T.; Corner, C.; Dziewulski PFife, K.; Ross, G.L.; Varma, S.; Harwood, C.A. Challenges and new horizons in the management of advanced basal cell carcinoma: A UK perspective. Br. J. Cancer 2021, 14, 1476–1481. [Google Scholar] [CrossRef]
- Baxter, J.M.; Patel, A.N.; Varma, S. Facial basal cell carcinoma. BMJ 2012, 21, e5342. [Google Scholar] [CrossRef] [PubMed]
- Ágoston, D.; Baltás, E.; Ócsai, H.; Rátkai, S.; Lázár, P.G.; Korom, I.; Varga, E.; Németh, I.B.; Dósa-Rácz Viharosné, E.; Gehl, J.; et al. Evaluation of Calcium Electroporation for the Treatment of Cutaneous Metastases: A Double Blinded Randomised Controlled Phase II Trial. Cancers 2020, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, V.; Scarfì, F.; Saqer, E.; Gori, A.; Tomassini, G.M.; Covarelli, P. The use of cisplatin electrochemotherapy in nonmelanoma skin cancers: A single-center study. Dermatol. Ther. 2020, 33, e13547. [Google Scholar] [CrossRef] [PubMed]
- Montuori, M.; Santurro, L.; Feliziani, A.; Ricciardi, E.; Gaudio, D.; Campione, E.; Bianchi, L.; Silvi, M.B.; Rossi, P. Electrochemotherapy for basocellular and squamocellular head and neck cancer: Preliminary experience in Day Surgery Unit. G. Ital. Dermatol. Venereol. 2016, 153, 19–25. [Google Scholar] [CrossRef]
- Groselj, A.; Bosnjak, M.; Strojan, P.; Krzan, M.; Cemazar, M.; Sersa, G. Efficiency of electrochemotherapy with reduced bleomycin dose in the treatment of nonmelanoma head and neck skin cancer: Preliminary results. Head Neck 2018, 40, 120–125. [Google Scholar] [CrossRef]
- Rodríguez-Cuevas, S.; Barroso-Bravo, S.; Almanza-Estrada, J.; Cristóbal-Martínez, L.; González-Rodríguez, E. Electrochemotherapy in primary and metastatic skin tumors: Phase II trial using intralesional bleomycin. Arch. Med. Res. 2001, 32, 273–276. [Google Scholar] [CrossRef]
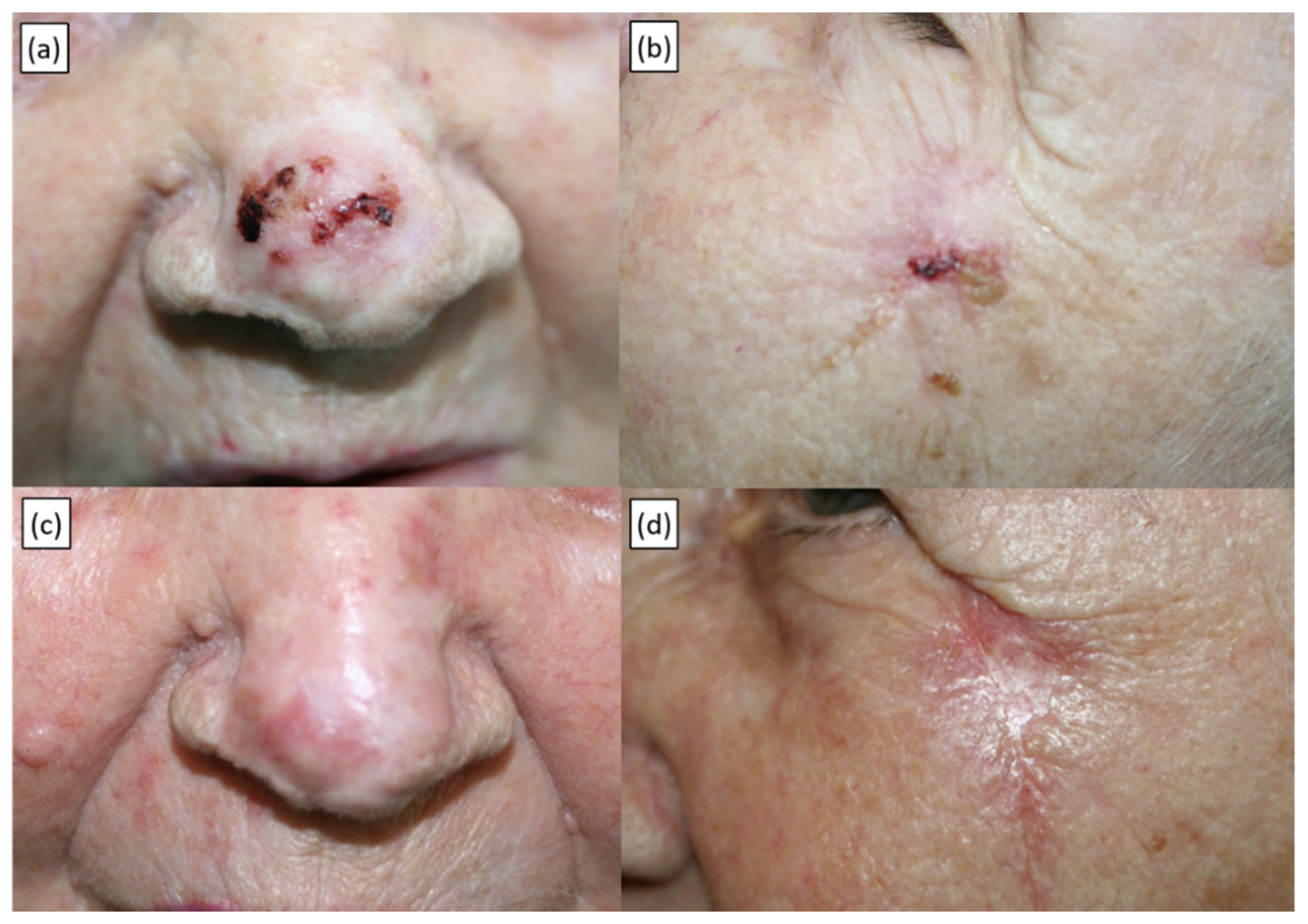
| Characteristic | No. (%) or Median (Range) |
|---|---|
| Sex | |
| Males Females | 205 (62%) 125 (38%) |
| Age (years) | 76 (23–98) |
| No. tumours/patient | 1 (1–7) |
| Tumour presentation | |
| Primary naïve Primary persistent Recurrent | 200 (61%) 79 (24%) 51 (15%) |
| Tumour size (mm) | 13 (5–350) |
| ≤3 cm >3 cm | 560 (90%) 63 (10%) |
| Anatomical location | |
| Head and Neck Trunk Limbs | 496 (80%) 74 (12%) 53 (8%) |
| Previous treatment | |
| None Surgical excision Other a Unknown | 200 (61%) 116 (35%) 12 (3%) 2 (1%) |
| Anaesthesia | |
| Local Local + sedation | 223 (68%) 107 (32%) |
| BLM administration | |
| Intratumoural Intravenous | 146 (44%) 184 (56%) |
| Electrode geometry | |
| Row needle Hexagonal needle Plate Combination | 383 (61.5%) 164 (26.3%) 44 (7.1%) 23 (5.1%) |
| Retreatment (No. of pts) Interval to 2nd ECT (months) | 52 (16%) 6.7 (1.2–47) |
| Score a | Baseline | Post-Procedure | Two Months | Last Follow-Up b |
|---|---|---|---|---|
| Median (range) | 0 (0–7) | 0 (0–8) | 0 (0–10) | 0 (0–9) |
| Mean ± SD | 0.41 ± 1.11 | 0.49 ± 1.14 | 0.32 ± 1.27 | 0.23 ± 1.11 |
| T-test vs. baseline | p = 0.017 | p = 0.347 | p = 0.023 |
| Response | Per-Patient | Per-Tumour | ||
|---|---|---|---|---|
| n | % | n | % | |
| CR | 242 | 80.7 | 488 | 83.1 |
| PR | 46 | 15.3 | 76 | 12.9 |
| SD | 9 | 3.0 | 15 | 2.6 |
| PD | 0 | 0 | 0 | 0 |
| NE | 3 | 1.0 | 8 | 1.4 |
| Total | 300 | 100 | 587 | 100 |
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| O.R. | 95% C.I. | p | O.R. | 95% C.I. | p | |
| Anatomical location (head/neck vs. other) | 2.75 | 1.30–5.83 | 0.008 | 1.98 | 0.86–4.52 | 0.107 |
| Route of BLM (i.v. vs. i.t.) | 0.60 | 0.33–1.09 | 0.095 | |||
| Previous RT (yes vs. no) | 0.18 | 0.05–0.62 | 0.007 | 0.25 | 0.06–1.00 | 0.051 |
| Lymphedema (yes vs. no) | 0.24 | 0.01–3.84 | 0.311 | |||
| Tumour size (< vs. ≥30 mm) | 2.48 | 1.15–5.35 | 0.020 | 1.68 | 0.71–3.99 | 0.237 |
| Coverage of deep margins (yes vs. no) | 3.46 | 1.79–7.06 | 0.001 | 5.44 | 1.37–21.69 | 0.016 |
| Coverage of lateral margins (yes vs. no) | 2.23 | 1.02–4.88 | 0.045 | 0.37 | 0.08–1.61 | 0.185 |
| Presentation (recurrent vs. primary) | 0.50 | 0.25–1.00 | 0.051 | |||
| No previous treatments | 3.45 | 1.89–6.25 | <0.001 | 2.86 | 1.49–5.26 | 0.001 |
| Author | Year | Study | No Pts | No of Tumours | BCC Subtype | BCC Presentation | T Size (mm) | Drug | Route | CR (%) | Re-ECT (%) | F-Up (mos) | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clover [7] | 2020 | Randomised (ECT vs. surgery) | 52 | 69 | Nodular/Superficial/ Infiltrative/Morpheaform | Primary | 1.7 cm2 (0.2–10) | BLM | i.t. | 86 a | 12 | 60 | 5-year LDFS, 87% (5 recurrences) |
| Kis [9] | 2019 | Case series | 12 | 17 | n.r. | Primary: 3 Recurrent: 9 | 11 (3–43) | BLM | i.v./i.t. | 100 | 33 | 19 | n.r. |
| Campana [10] | 2017 | Case series | 84 | 185 | Superficial/Nodular/Infiltrative/Morpheaform | Primary/recurrent (L/LA/Mts) | 20 (5–267) | BLM | i.v./i.t. | 50 a | 29 | 49 | 5-year LPFS, 70% |
| Ruggeri [10] | 2015 | Case report | 1 | 3 | n.r | Recurrent, multifocal | 4, 7, 8 | BLM | i.v. | 100 | 0 | 7 | no |
| Salwa [11] | 2014 | Case series | 3 | 3 | n.r. | Primary, periocular | 0.5–1 cm2 | BLM | i.t. | 100 | 0 | 5–8 | no |
| Gatti [12] | 2014 | Case report | 1 | 1 | n.r. | Recurrent | n.r. | BLM | i.v. | 100 | 0 | 12 | no |
| Kis [13] | 2012 | Case series | 3 b | 99 | Superficial/Nodular/Ulcerated/Plaque | primary/recurrent | 9 (3–22) | BLM | i.v. | 87 a | 33 | n.r. | n.r. |
| Fantini[14] | 2008 | Case report | 1 | 3/3/“multiple” | BCC with SCC differentiation | Metastatic | n.r. | BLM | i.t./i.v. | 100 | 0 | 8 | no |
| Glass [15] | 1997 | Case series | 20 | 54 | Nodular | Primary | 9 (4–21) | BLM | i.t. | 98 c | 10 | 18 | no |
| Glass [16] | 1996 | Case report | 2 | 6 | Superficial/ Nodular | n.r. | n.r. | BLM | i.v. | 33 c | 0 | n.r. | n.r. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertino, G.; Muir, T.; Odili, J.; Groselj, A.; Marconato, R.; Curatolo, P.; Kis, E.; Lonkvist, C.K.; Clover, J.; Quaglino, P.; et al. Treatment of Basal Cell Carcinoma with Electrochemotherapy: Insights from the InspECT Registry (2008–2019). Curr. Oncol. 2022, 29, 5324-5337. https://doi.org/10.3390/curroncol29080423
Bertino G, Muir T, Odili J, Groselj A, Marconato R, Curatolo P, Kis E, Lonkvist CK, Clover J, Quaglino P, et al. Treatment of Basal Cell Carcinoma with Electrochemotherapy: Insights from the InspECT Registry (2008–2019). Current Oncology. 2022; 29(8):5324-5337. https://doi.org/10.3390/curroncol29080423
Chicago/Turabian StyleBertino, Giulia, Tobian Muir, Joy Odili, Ales Groselj, Roberto Marconato, Pietro Curatolo, Erika Kis, Camilla Kjaer Lonkvist, James Clover, Pietro Quaglino, and et al. 2022. "Treatment of Basal Cell Carcinoma with Electrochemotherapy: Insights from the InspECT Registry (2008–2019)" Current Oncology 29, no. 8: 5324-5337. https://doi.org/10.3390/curroncol29080423
APA StyleBertino, G., Muir, T., Odili, J., Groselj, A., Marconato, R., Curatolo, P., Kis, E., Lonkvist, C. K., Clover, J., Quaglino, P., Kunte, C., Spina, R., Seccia, V., de Terlizzi, F., Campana, L. G., & the InspECT BCC Working Group. (2022). Treatment of Basal Cell Carcinoma with Electrochemotherapy: Insights from the InspECT Registry (2008–2019). Current Oncology, 29(8), 5324-5337. https://doi.org/10.3390/curroncol29080423








