Abstract
Breast cancer recurrence is an important outcome for patients and healthcare systems, but it is not routinely reported in cancer registries. We developed an algorithm to identify patients who experienced recurrence or a second case of primary breast cancer (combined as a “second breast cancer event”) using administrative data from the population of Ontario, Canada. A retrospective cohort study design was used including patients diagnosed with stage 0-III breast cancer in the Ontario Cancer Registry between 1 January 2009 and 31 December 2012 and alive six months post-diagnosis. We applied the algorithm to healthcare utilization data from six months post-diagnosis until death or 31 December 2013, whichever came first. We validated the algorithm’s diagnostic accuracy against a manual patient record review (n = 2245 patients). The algorithm had a sensitivity of 85%, a specificity of 94%, a positive predictive value of 67%, a negative predictive value of 98%, an accuracy of 93%, a kappa value of 71%, and a prevalence-adjusted bias-adjusted kappa value of 85%. The second breast cancer event rate was 16.5% according to the algorithm and 13.0% according to manual review. Our algorithm’s performance was comparable to previously published algorithms and is sufficient for healthcare system monitoring. Administrative data from a population can, therefore, be interpreted using new methods to identify new outcome measures.
1. Introduction
Breast cancer recurrence is an important outcome for patients and healthcare systems, but recurrence is not routinely reported in cancer registries or other administrative datasets [1,2,3,4]. Ontario Health (Cancer Care Ontario) is an agency of the government of Ontario, Canada, that measures cancer system performance, among other functions. Measuring breast cancer recurrence in the population of Ontario could inform healthcare system planning and quality improvement since recurrence has been associated with modifiable factors such as margin positivity after surgery [5,6] and treatment selection [5,7,8], and treating recurrence requires significant healthcare resources [9]. Moreover, many breast cancer survivors worry about recurrence [10,11] and both recurrences and second primary breast cancers have been associated with reduced survival [5,12,13], so recurrence rates could inform discussions of risk.
The gold standard for identifying cancer recurrence is a manual review of patient information, which is not feasible at the population level. Researchers have used other methods to identify breast cancer recurrences, such as surveying patients directly [14], or developing algorithms for identifying breast cancer recurrences [3,15,16,17,18] or second breast cancer events (SBCEs) [1,2,19], which combine local and distant recurrences and second primary breast cancers. However, at the population level, patient surveys are impractical, and some algorithms may not be appropriate: some algorithms have been developed from highly selected breast cancer cohorts (potentially with specific treatment patterns), and some did not identify second primary breast cancers as well as local and distant recurrences. Developing an algorithm that could be applied across a population could support system-level decision making, increase algorithm generalizability, and ensure sufficient numbers of SBCEs to provide precise estimates of algorithm accuracy since breast cancer recurrence rates are generally low. Since algorithms developed in other jurisdictions would need to be validated before they could be applied to the Ontario population, and some existing algorithms incorporate data that are inaccessible in Ontario or Canada, we aimed to:
- (1)
- Develop a novel algorithm for measuring SBCE rates (recurrences and second primary breast cancers) in a population using routinely collected administrative data;
- (2)
- Validate the algorithm’s diagnostic accuracy using the results of a manual record review in a large sub-cohort of patients.
For this study, we defined an SBCE as evidence of a local, regional, or distant breast cancer recurrence or a new primary breast cancer observed more than 180 days after the incident breast cancer diagnosis.
2. Materials and Methods
2.1. Patient Selection and Data Sources
This retrospective cohort study included all female patients 18 years old or older diagnosed with stage 0-III breast cancer in the Ontario Cancer Registry [20] between 1 January 2009 and 31 December 2012. Patients with a prior diagnosis of breast or other cancer were included, as prior diagnoses were not expected to change the outcome of interest (detection of recurrence after the incident date). Healthcare utilization data from incident diagnosis until 31 December 2013 or patient death, whichever came first, were retrieved for analysis. Patients were excluded if they were diagnosed with lymphoma in the breast or skin cancer on the breast or died within 180 days (six months) of diagnosis.
Patients’ unique Ontario Health Insurance Plan numbers [21] were used to link data. The Ontario Registrar General provided the cause-of-death data. Stage data, including tumor characteristics, were retrieved from the Ontario Cancer Registry [20]. Inpatient procedure data, including associated diagnosis codes, were retrieved from the Discharge Abstract Database [22]. Emergency department visit data, outpatient procedure data, and associated diagnosis codes were retrieved from the National Ambulatory Care Reporting System [22]. Data about cancer-related consultations, decisions, and treatments, including systemic therapy and radiation therapy, were retrieved from the Activity Level Reporting database [22]. Data about approved funding requests for systemic therapy were retrieved from the New Drug Funding Program database [22]. Additional data about systemic treatment with targeted or endocrine therapy for Ontario residents age 65 and over or on social assistance were retrieved from the Ontario Drug Benefit database [22]. Due to Ontario Health (Cancer Care Ontario)’s designation as a “prescribed entity” for the purposes of Section 45 (1) of the Personal Health Information Protection Act of 2004, an ethics review was not required.
2.2. Index Test: Developing the Algorithm
An expert panel including surgical, medical, and radiation oncologists with expertise in breast cancer management determined algorithm criteria, i.e., types of healthcare events likely to indicate an SBCE. Criteria were based on standard-of-care curative treatments that each breast cancer patient in Ontario should be offered (Figure 1). Time frames for algorithm criteria were based on clinicians’ expertise and their review of study cohort data indicating when healthcare events for each criterion occurred relative to diagnosis. The algorithm was applied to each patient’s data starting at 180 days post-diagnosis through death or the end of the follow-up period in order to distinguish between treatment for the incident breast cancer and treatment for an SBCE. Breast cancer-related healthcare events that occurred within 180 days after the diagnosis date were considered to indicate management of the initial breast cancer, local progression, or distant disease that was occult at diagnosis.
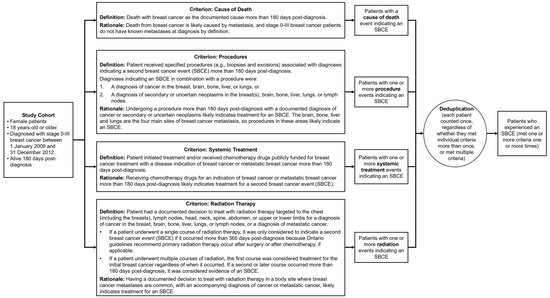
Figure 1.
Algorithm criteria with definitions and rationale. Each criterion was applied to the entire study cohort. Patients could meet a single criterion multiple times or meet multiple criteria. For this study, we considered patients to have experienced a second breast cancer event (SBCE) if they met one criterion one time between 180 days post-diagnosis and their death or the end of follow-up.
All criteria were applied to the entire patient cohort and could be applied in any order. A patient only had to meet one of the criteria one time to be considered as having an SBCE. For the criteria based on procedures and radiotherapy treatments, probable contralateral second primary breast cancers could be identified among SBCEs in the breast based on the laterality of procedures and diagnoses. See Appendix A for code lists for each criterion.
2.3. Manual Record Review
A manual record review, the reference standard test, was conducted for a sub-cohort of patients seen at the Odette Cancer Center in Toronto, Canada, and the Juravinski Cancer Center in Hamilton, Canada. We calculated, a priori, the number of records required for review to accurately validate the algorithm given the prevalence of recurrence in patients with stages I, II, and III breast cancer. Stages I and II breast cancer are diagnosed much more often than stage III breast cancer, but stage III breast cancer patients are more likely to experience an SBCE [23]. To ensure sufficient statistical power (a sufficient number of patients with SBCEs in the validation sub-cohort), we sampled approximately 1000 patients with stages I, II, and III breast cancer, representing each stage at equal proportions rather than picking a random sample that would reflect the natural incidence of each stage in the population. Stage III breast cancer patients, therefore, represented a larger proportion of the validation sub-cohort than their proportion in the entire cohort. Assuming recurrence rates of 2%, 7.7%, and 20% for stage I, II, and III patients, respectively, we aimed to be able to detect an algorithm sensitivity of 75%, 85%, and 90% for stages I, II and III, and specificity of 99%, 95%, and 90% for stages I, II, and III breast cancer patients, respectively. Sampling 1000 patients of each stage (total n = 3000), we expected to observe sensitivity and specificity in the ranges of 52–91% and 98–100% for stage I; 75–92% and 93–96% for stage II; and 85–94% and 88–92% for stage III breast cancer patients. Approximately equal numbers of stage I, II, and III patients were randomly selected from each cancer center for the validation sub-cohort.
Clinical research professionals unaware of the algorithm’s SBCE classifications manually reviewed sub-cohort records. If patients met manual review criteria for experiencing an SBCE, the evidence (clinical, radiological, or tissue-based), anatomical location, and treatment information were documented. When SBCE status was unclear, the study leader at the center (A.E. or J.S.) would adjudicate. If SBCE status remained indeterminate, patients were excluded from the manual record review.
Manual review results were linked to administrative data and algorithm classifications using patients’ medical record numbers. A member of the study team (C.H.) re-reviewed administrative and manually collected data for all false-positive cases (patients classified as experiencing an SBCE by the algorithm but not reviewers). Administrative documents clearly indicative of an SBCE (e.g., a pathology report showing breast cancer or a record of systemic therapy for metastatic breast cancer) were considered more accurate than the results of a manual record review at a single center, as patients may have been diagnosed and/or treated at different centers.
2.4. Statistical Methods
Patient characteristics were summarized as counts with proportions for categorical data and means with standard deviations for continuous data. For continuous variables with skewed distributions, medians and interquartile ranges were used. Patients excluded during the manual record review were compared with patients who remained in the validation sub-cohort using Pearson’s chi-squared tests and a Cochran–Mantel–Haenszel statistic [24] (Appendix B). Algorithm diagnostic accuracy was assessed by calculating agreement statistics: sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, kappa, and prevalence-adjusted bias-adjusted kappa (PABAK), due to criticism of the kappa statistic for its dependence on outcome prevalence [25,26,27,28]. Additional agreement statistics were calculated to verify that including patients with prior cancer diagnoses did not affect algorithm diagnostic accuracy (Appendix C). Analyses were performed using SAS® software version 9.4 for Microsoft Windows. Copyright © 2013 SAS Institute Inc., Cary, NC, USA.
3. Results
3.1. Cohort Characteristics and Algorithm Classifications
The study cohort included 31,782 patients (Figure 2); the median follow-up time was 34 months (approximately 2.8 years; Table 1).
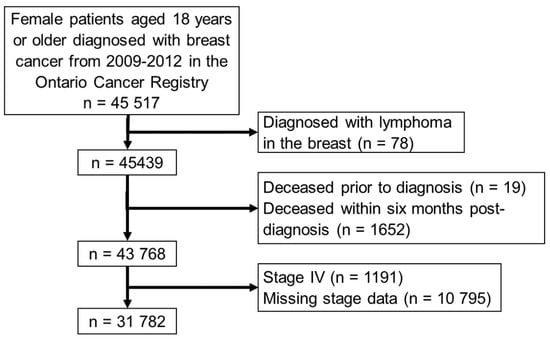
Figure 2.
Patient inclusion/exclusion criteria.

Table 1.
Cohort Description.
The algorithm classified 3796 patients as experiencing an SBCE based on a maximum of 6109 events (true total unavailable due to small cell suppression of cause-of-death data by stage) for an SBCE rate of 11.9% (Table 2). Procedure and diagnosis data classified the most patients as experiencing an SBCE and events as indicating an SBCE of any criterion, followed by radiation data, systemic treatment data, and cause-of-death data (Figure 3). Notably, for all criteria except the cause of death criterion, more healthcare events indicating an SBCE were identified than patients experiencing the events, suggesting that some patients who met the criterion met it based on multiple events.

Table 2.
Algorithm classifications of second breast cancer events (SBCEs) in the entire cohort.
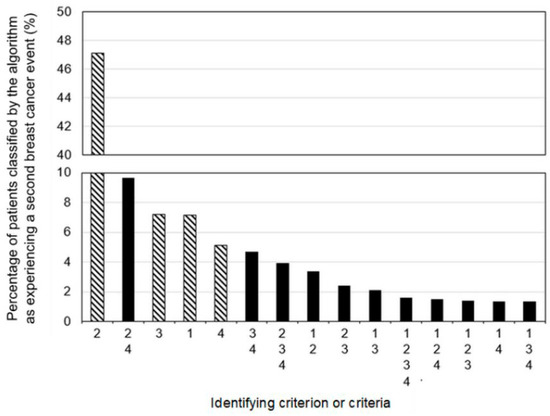
Figure 3.
Proportions of patients classified by the algorithm as experiencing a second breast cancer event based on a single criterion (lined bars) or combinations of criteria (solid bars). Criterion/criteria groups are mutually exclusive and collectively exhaustive. All criteria were applied to the entire cohort and could be applied in any order: 1—death from breast cancer; 2—procedure and associated diagnosis; 3—systemic treatment; 4—radiotherapy.
3.2. Exclusions during Manual Review and Validation Sub-Cohort Characteristics
Of the 3258 patients selected for the manual record review, 1013 patients were excluded because their records could not be retrieved, they did not have sufficient records for review at a study center, or their SBCE status was indeterminate. The remaining validation sub-cohort was 2245 patients (Table 3).

Table 3.
Validation sub-cohort characteristics.
Pearson’s chi-squared tests indicated a potential relationship between stage at diagnosis and likelihood of exclusion during manual review based on a marginally significant p-value of 0.044 (Table A8). The Cochran–Mantel–Haenszel statistic [24] demonstrated that after controlling for the stage at diagnosis, more excluded patients were classified by the algorithm as having an SBCE (Table A9; p-value < 0.0136).
3.3. Algorithm Diagnostic Accuracy
After a case-by-case review of false-positive results (patients classified as experiencing an SBCE by the algorithm but not by manual review), 16 patients’ manual review SBCE statuses were revised due to definitive evidence of SBCEs in administrative data, making them true positive. Algorithm and manual review SBCE classifications after this revision are compared in Table 4A,B. The algorithm had a sensitivity of 85%, a specificity of 94%, a PPV of 67%, an NPV of 98%, a kappa of 71%, and a PABAK of 85% (Table 4C).

Table 4.
(A) Algorithm and manual review classifications of second breast cancer events (SBCEs) in the validation sub-cohort; (B) comparison of algorithm and manual record review classifications of patients as experiencing a second breast cancer event (SBCE); (C) algorithm diagnostic accuracy at classifying patients as experiencing a second breast cancer event (SBCE).
Prior cancer history did not observably affect the algorithm’s diagnostic accuracy, though this may be attributable to the small proportion of patients with prior cancer history (Appendix C).
4. Discussion
Our study demonstrates the feasibility of quantifying SBCE rates in populations by analyzing administrative data using new methods. The sensitivity and specificity of our algorithm were comparable or superior to previously published SBCE [1,2,16,19,29] and recurrence identification [3,15,17] algorithms, though the PPV was slightly lower. Our algorithm may, therefore, be useful in scenarios where the overestimation of the SBCE rate is less important (e.g., system capacity planning). High specificity and NPV make our algorithm useful for identifying patients unlikely to have experienced an SBCE (e.g., for studies about interventions to reduce recurrence rates). The overall accuracy of 92% supports our algorithm’s appropriateness for use in health system monitoring and exceeds the acceptable accuracy threshold chosen by Livaudais-Toman et al. [30].
The sensitivity of the algorithm was limited by the lack of important data in administrative databases. Some patients with SBCEs likely received treatments that were not specific to breast cancer, such as palliative care, or treatments not reported in administrative data, such as endocrine therapy in patients under age 65 and not on social assistance. Since the proportions of such patients are likely to remain constant, it may be possible to apply a correction to, or acknowledge a probable false-negative rate in, estimates of SBCE prevalence.
The relatively low PPV was attributable to false-positive SBCE classifications by the algorithm, i.e., treatments meeting criteria though they were probably not indicated for SBCEs. For example, surgical procedures occurring more than six months following a diagnosis such as a mastectomy with or without reconstruction may have reflected prophylactic treatment, patients’ aesthetic preferences, or potentially primary treatment after neoadjuvant chemotherapy. Other false positives were attributable to the limitations of manual record reviews: Some patients were erroneously determined not to have an SBCE during the manual review because they received care at multiple centers due to treatment availability or personal relocation. This likely also explains the increased rate of SBCEs according to the algorithm among patients whose records were excluded from the manual review.
Each algorithm criterion appears relevant since each criterion identified different patients. Procedure and associated diagnosis data seem especially useful, though further research is required to determine the accuracy of each criterion. Investigating why some patients were only identified posthumously based on the cause-of-death data could elucidate gaps or suggest how many patients do not receive SBCE-specific therapy.
Although we developed our algorithm from a population, a larger and more diverse group than some other authors used to develop algorithms, adjusting individual criteria or the data observation period to align with previously published algorithms could potentially improve performance. Other authors analyzed data starting after a longer time post-diagnosis or after completion of each patient’s primary treatment [1,2,3]; similar changes might reduce our false-positive rate and improve PPV. Other SBCE and breast cancer recurrence identification algorithms have incorporated different types of healthcare events [3,19], numbers [1,3] or rates of occurrence [1,2,19] of events, or intervals between events [1,2]. Promisingly, some SBCE algorithms generated by machine learning used similar criteria to those chosen by clinical experts for our algorithm [1,2].
There are some limitations to our study. Excluding patients from the validation sub-cohort during the manual record review may have led to unmeasured differences between the final sub-cohort and the entire cohort. Reviewing patient records at academic tertiary care centers offering specialized treatments may have increased the inclusion of patients who received care at multiple centers, impeding the review of comprehensive treatment records. Inter-rater reliability was not measured, though chart reviewers and study leaders met regularly to maximize consistency. Finally, we applied our algorithm to data from six months post-breast cancer diagnosis to a maximum of four years post-diagnosis, which does not represent the entire at-risk period for SBCEs. The algorithm’s accuracy may differ depending on the duration of follow-up.
5. Conclusions
Despite these limitations, we calculated an SBCE rate with acceptable accuracy for healthcare system monitoring by applying an algorithm to administrative data. The algorithm may be applicable to other patient populations or other cancer types with similar patterns of treatment since the data types used to identify second cancer events were not specific to breast cancer. Future developments may include adjusting algorithm criteria, incorporating additional administrative datasets, or experimenting with machine learning methods, which could potentially improve algorithm performance and expand algorithm utility.
Author Contributions
Conceptualization, C.M.B.H., K.F., B.G., A.E. and J.S.; methodology, C.M.B.H., O.S., M.E., K.F., P.M., B.G., A.E. and J.S.; validation, C.M.B.H., M.E., A.E. and J.S.; formal analysis, O.S., M.E., P.M. and A.V.E.; investigation, C.M.B.H., A.E. and J.S.; data curation, O.S., M.E., P.M. and A.V.E.; writing—original draft preparation, C.M.B.H. and K.F.; writing—review and editing, C.M.B.H., K.F., M.E., A.E. and J.S.; supervision, C.M.B.H., K.F., A.E. and J.S.; project administration, K.F.; funding acquisition, C.M.B.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Ontario Health (Cancer Care Ontario), specifically the Data and Decision Sciences and Disease Pathway Management groups, through funding provided by the Ontario Ministry of Health. The opinions, results, views, and conclusions reported in this publication are those of the authors and do not necessarily reflect those of Ontario Health (Cancer Care Ontario). No endorsement by Ontario Health (Cancer Care Ontario) is intended or should be inferred. Initial work on this project was supported by a Cancer Care Ontario grant.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the fact that this study exclusively analyzed routinely collected administrative data that Ontario Health (Cancer Care Ontario) is authorized to collect due to its status as a “prescribed entity” for the purposes of Section 45 (1) of the Personal Health Information Protection Act (PHIPA) of 2004. As a prescribed entity, Ontario Health (Cancer Care Ontario) is authorized to collect personal health information from health information custodians without the consent of the patient and to use such personal health information for the purpose of analysis or compiling statistical information with respect to the management, evaluation, or monitoring of the allocation of resources to or planning for all or part of the health system, including the delivery of services.
Informed Consent Statement
Patient consent was waived because Ontario Health (Cancer Care Ontario) is designated a “prescribed entity” for the purposes of Section 45 (1) of the Personal Health Information Protection Act (PHIPA) of 2004. As a prescribed entity, Ontario Health (Cancer Care Ontario) is authorized to collect personal health information from health information custodians without the consent of the patient and to use such personal health information for the purpose of analysis or compiling statistical information with respect to the management, evaluation, or monitoring of the allocation of resources to or planning for all or part of the health system, including the delivery of services.
Data Availability Statement
Data de-identified to a level suitable for public release may be provided upon request to the corresponding author, due to privacy restrictions. Ontario Health is prohibited from making the data used in this research publicly accessible if they include potentially identifiable personal health information and/or personal information as defined in Ontario law, specifically the Personal Health Information Protection Act (PHIPA) and the Freedom of Information and Protection of Privacy Act (FIPPA).
Acknowledgments
Grace Bannerman assisted with the preparation of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A
Appendix A.1. Algorithm Criteria Codes
Please note that criteria were applied to patient data from six months (180 days) after breast cancer diagnosis through the end of follow-up on 31 December 2013 or patient death, whichever came first. For the radiation therapy criterion, if a patient underwent a single course of radiation therapy, it was only considered to indicate a second breast cancer event (SBCE) if it occurred more than 365 days post-diagnosis because Ontario guidelines recommend primary radiation therapy occur after surgery or after chemotherapy, if applicable. If a patient underwent multiple courses of radiation, the first course was considered treatment for the initial breast cancer regardless of when it occurred. If a second or later course occurred more than 180 days post-diagnosis, it was considered evidence of an SBCE.
Appendix A.2. Death from Breast Cancer Criterion
Patients met the cause of death criterion if their cause of death was coded as breast cancer, as listed below.
- Data Source(s): Death records from the Ontario Registrar General.
- Coding system: International Classification of Diseases, version 10 (ICD10).

Table A1.
Death record code indicating death from a second breast cancer event.
Table A1.
Death record code indicating death from a second breast cancer event.
| Code(s) | Code Description |
|---|---|
| C509 | Malignant neoplasm of breast, unspecified |
Appendix A.3. Procedure and Diagnosis Criterion
Patients met the procedure and diagnosis criterion if they underwent one of the procedures listed associated with one of the diagnoses listed.
Appendix A.3.1. Procedures
- Data Source(s): Discharge Abstract Database, National Ambulatory Care Reporting System.
- Coding system: Canadian Classification of Health Interventions, versions 2009, 2012, and 2015.

Table A2.
Procedure codes for the procedure and associated diagnosis criterion.
Table A2.
Procedure codes for the procedure and associated diagnosis criterion.
| Canadian Classification of Health Interventions Code | Canadian Classification of Health Interventions Code Description |
|---|---|
| 1AA80SZXXL | Repair mening brn cranial flap OA xenogr |
| 1AA87SZ | Excision partial, meninges and dura mater of brain using apposition technique [e.g., suture] |
| 1AA87SZXXN | Excis prt mening brn cranial flap OA synth mat |
| 1AC27JX | Radiation, ventricles of brain using focused beam [e.g., gamma knife, cyber knife stereotactic radiosurgery] |
| 1AC52MBSJ | Drainage, ventricles of brain burr hole technique drainage to skin (of head) catheter or shunt (temporarily) left in situ |
| 1AC52SE | Drainage, ventricles of brain burr hole technique drainage without shunt or catheter left in situ |
| 1AF87DAGX | Excision partial, pituitary region endoscopic (via sinus) approach with device NEC |
| 1AJ87SZAZ | Excision partial, cerebellum open [craniotomy flap] approach with ultrasonic aspirator [e.g., CUSA] |
| 1AJ87SZGX | Excision partial, cerebellum open [craniotomy flap] approach with device NEC |
| 1AN27JA | Radiation, brain using external beam [for teletherapy NEC] |
| 1AN27JX | Radiation, brain using focused beam [e.g., gamma knife, cyber knife stereotactic radiosurgery] |
| 1AN53SEFT | Implantation of internal device, brain burr hole technique for access of [semipermeable] catheter [e.g., for chemical palliative infusion] |
| 1AN53SZFT | Implantation of internal device, brain craniotomy [or craniectomy] flap technique for access of [semipermeable] catheter [e.g., for chemical palliative infusion] |
| 1AN87SEAZ | Excision partial, brain burr hole technique for access with ultrasonic aspirator [e.g., CUSA] |
| 1AN87SZAG | Excision partial, brain craniotomy [or craniectomy] flap technique for access with laser |
| 1AN87SZAZ | Excision partial, brain craniotomy [or craniectomy] flap technique for access with ultrasonic aspirator [e.g., CUSA] |
| 1AN87SZGX | Excision partial, brain craniotomy [or craniectomy] flap technique for access with device NEC |
| 1AW27JA | Radiation, spinal cord using external beam [for teletherapy NEC] |
| 1AX35HAM0 | Pharmacotherapy (local), spinal canal and meninges Percutaneous (needle) approach using antineoplastic agent NEC |
| 1AX35HAP1 | Pharmacotherapy (local), spinal canal and meninges percutaneous [needle] approach using anesthetic agent |
| 1AX52MESJ | Drainage, spinal canal and meninges open approach shunt terminating in abdominal cavity [e.g., lumboperitoneal shunt] |
| 1AX87LAGX | Excision partial, spinal canal and meninges using extradural incision technique [e.g., for space occupying lesion of canal] open approach with combined sources of tissue for closure with device NEC |
| 1AX87WKGX | Excision partial, spinal canal and meninges using intradural incision technique [e.g., for meningeal mass] open approach with apposition technique [e.g., suturing] with device NEC |
| 1EA27JA | Radiation, cranium using external beam |
| 1EA87LANW | Excision partial, cranium open approach no tissue used [for closure of wound] using plate, screw device (with or without wire or mesh) |
| 1EA87LANWN | Excise prt cranium OA &plate/scrw synth mater |
| 1EA92LYXXA | Exc rad w reconstruct cranium cranial base oth appr autogr |
| 1EQ27JA | Radiation, soft tissue of head and neck using external beam |
| 1FM87VW | Excision partial, parotid gland using open approach with preservation of facial nerve technique |
| 1GM59BAGX | Destruction, bronchus NEC using endoscopic per orifice approach and device NEC |
| 1GR87DA | Excision partial, lobe of lung using endoscopic approach [VATS] |
| 1GR87QB | Excision partial, lobe of lung using open thoracic approach |
| 1GR89DA | Excision total, lobe of lung using endoscopic approach [VATS] |
| 1GR89QB | Excision total, lobe of lung using open thoracic approach |
| 1GR91QB | Excision radical, lobe of lung open thoracic approach with simple closure |
| 1GR91QBXXN | Excise rad lobe lung thor OA synth mater |
| 1GT27JA | Radiation, lung NEC using external beam |
| 1GT80LA | Repair, lung NEC using open approach |
| 1GT87DA | Excision partial, lung NEC using endoscopic approach [VATS] |
| 1GT87QB | Excision partial, lung NEC using open thoracic approach |
| 1GT89DA | Excise tot lung EA |
| 1GV52DA | Drainage, pleura using endoscopic approach [VATS] |
| 1GV52DATS | Drainage, pleura using endoscopic approach and leaving drainage tube in situ |
| 1GV52HA | Drainage, pleura using percutaneous (needle) approach |
| 1GV52HAHE | Drainage, pleura using percutaneous catheter (intracostal) with underwater seal drainage system |
| 1GV52HATK | Drainage, pleura using percutaneous catheter with suction pump, (under water seal or negative pressure) |
| 1GV52LA | Drainage, pleura using open approach |
| 1GV52LATS | Drainage, pleura using open approach and leaving drainage tube in situ |
| 1GV54JATS | Management of internal device, pleura of drainage tube [e.g., thoracotomy or pleural cavity drain] using external approach |
| 1GV59DAGX | Destruction, pleura using endoscopic approach [VATS] and device NEC |
| 1GV59DAZ9 | Destruction, pleura using endoscopic approach and chemical agent NEC |
| 1GV59HAZ9 | Destruction, pleura using percutaneous instillation of agent NEC (e.g., blood, talc) |
| 1GV87DA | Excision partial, pleura using endoscopic approach [VATS] |
| 1GV89DA | Excision total, pleura using endoscopic approach [VATS] |
| 1GZ31CAND | Ventilation, respiratory system NEC invasive per orifice approach by endotracheal intubation and positive pressure |
| 1GZ31CBND | Ventilation, respiratory system NEC non-invasive approach and positive pressure ventilation (e.g., CPAP, BIPAP) |
| 1GZ32CAMY | Oxygenation, respiratory system NEC using bulk storage manifold system |
| 1HA87LA | Excision partial, pericardium using open approach |
| 1MC87LA | Excision partial, lymph node(s), cervical using open approach with no tissue |
| 1MC87LAXXE | Excise prt lymph nd neck OA loc flp |
| 1MC89LA | Excision total, lymph node(s), cervical using open approach with no tissue |
| 1MC91LA | Excision radical, lymph node(s), cervical without tissue radical neck dissection |
| 1MC91VB | Excision radical, lymph node(s), cervical without tissue modified radical neck dissection |
| 1MD27JA | Radiation, lymph node(s), axillary using external beam |
| 1MD87LA | Excision partial, lymph node(s), axillary using open approach |
| 1MD89LA | Excision total, lymph node(s), axillary using open approach |
| 1MD89LAXXE | Excise tot axil lymph nd OA loc flp |
| 1MD89LAXXG | Excise tot axil lymph nd OA ped flp |
| 1ME87DA | Excision partial, lymph node(s), mediastinal using endoscopic approach |
| 1ME89DA | Excision total, lymph node(s), mediastinal using endoscopic approach |
| 1MF27JA | Radiation, lymph node(s), intrathoracic NEC using external beam |
| 1MF87LA | Excision partial, lymph node(s), intrathoracic NEC using open approach |
| 1MH27JA | Radiation, lymph node(s), pelvic using external beam |
| 1MZ27JA | Radiation, lymphatic system NEC using external beam |
| 1NF90LAXXG | Exc tot w reconstr stom OA w jejnm |
| 1NK87RF | Excision partial, small intestine open approach enteroenterostomy anastomosis technique |
| 1NQ57CJ | Extraction, rectum using per orifice approach and manual technique |
| 1NQ87TF | Excision partial, rectum open abdominal [e.g., anterior] approach colostomy (or ileostomy) with closure of rectal stump [e.g., Hartmann technique] or submucous fistula |
| 1OA27JA | Radiation liver using external beam |
| 1OA59HAAW | Destruction, liver percutaneous approach using radiofrequency |
| 1OA87DA | Excision partial, liver using endoscopic (laparoscopic)approach |
| 1OA87LA | Excision partial, liver using open approach |
| 1OA87LAAZ | Excision partial, liver using ultrasonic aspirator device (for dissection) and open approach |
| 1OE50BANR | Dilate bile dct EPO retro &stent |
| 1OE52GPTS | Drainage, bile ducts using percutaneous transluminal approach [e.g., transhepatic] leaving catheter (tube) in situ |
| 1OE89UF | Excision total, bile ducts using open approach and hepaticojejunostomy technique [for anastomosis] |
| 1OT52HATS | Drain abd cav perc app &tube NOS |
| 1PE52HH | Drainage, renal pelvis using percutaneous approach with insertion of tube (e.g., nephrostomy, pyelostomy) |
| 1PE59BAAG | Destruction, renal pelvis endoscopic per orifice approach Using laser (tissue ablation) |
| 1PM52BATS | Drain bladder EPO &tube NOS |
| 1PM87BA | Excision partial, bladder using endoscopic per orifice approach |
| 1PV52HA | Drainage, surgically created urinary tract using percutaneous needle aspiration |
| 1RD89DA | Excision total, ovary with fallopian tube using endoscopic [laparoscopic] approach |
| 1RD89LA | Excise tot ovary w fallop OA |
| 1RM89AA | Excision total, uterus and surrounding structures using combined laparoscopic and vaginal approach |
| 1SC27JA | Radiation, spinal vertebrae using external beam |
| 1SC74PFNW | Fixation, spinal vertebrae open posterior approach [Includes: posterolateral approach] using screw, screw with plate or rod |
| 1SC75LLKDN | Fuse sp vert ant OA &wire/staple synth mater |
| 1SC75PFGXN | Fuse sp vert post OA &dev NEC synth mater |
| 1SC75PFNWA | Fuse sp vert post OA &plate/scrw autogr |
| 1SC75PFNWN | Fuse sp vert post OA &plate/scrw synth mater |
| 1SC75PFNWQ | Fuse sp vert post OA &plate/scrw combo tis |
| 1SC80HABDN | Repair sp vert perc app w balloon & synth mat |
| 1SC80HAXXN | Repair sp vert perc injct synth mater |
| 1SC80PF | Repair, spinal vertebrae using posterior approach |
| 1SC89LLNWA | Excise tot sp vert ant OA &plate/scrw autogr |
| 1SC89LLNWK | Excise tot sp vert ant OA &plate/scrw homogr |
| 1SC89LLNWN | Excise tot sp vert ant OA &plate/scrw synth mat |
| 1SC89LLNWQ | Excise tot sp vert ant OA &plate/scrw combo tis |
| 1SC89LNNWN | Excis tot sp vert ant w post &plate/scrw syn mat |
| 1SC89PFGX | Excision total, spinal vertebrae posterior approach [posterolateral approach] no tissue used (device only) using device NEC |
| 1SC89PFNWN | Excise tot sp vert post OA &plate/scrw synth mater |
| 1SF74HANW | Fixation, sacrum and coccyx using percutaneous approach and screw, screw with plate |
| 1SH87LAXXE | Excise prt s t back OA loc flp |
| 1SQ27JA | Radiation, pelvis using external beam |
| 1SQ87LAPMN | Excise prt pelvis OA &hip endoprosth synth mat |
| 1SY80LA | Repair m chest & abd OA apposition |
| 1SY87LA | Excision partial, muscles of the chest and abdomen using simple apposition technique [e.g., suture, staple] (for closure of surgical defect) |
| 1SY87LAXXE | Excise prt m chest & abd OA loc flp |
| 1SY87LAXXF | Excise prt m chest & abd non viable free flp |
| 1SZ27JA | Radiation, soft tissue of the chest and abdomen using external beam |
| 1SZ87LA | Excision partial, soft tissue of the chest and abdomen using open approach and apposition [suture, staple] (to close surgical defect) |
| 1SZ87LAXXA | Excise prt s t chest & abd OA autogr |
| 1SZ87LAXXE | Excise prt s t chest & abd OA loc flp |
| 1SZ87LAXXG | Excise prt s t chest & abd OA ped flp |
| 1TK74HALQ | Fixation, humerus percutaneous approach [e.g., with closed or no reduction] fixation device alone using intramedullary nail |
| 1TK74LALQ | Fixation, humerus open approach fixation device alone using intramedullary nail |
| 1TK74LANW | Fixation, humerus open approach fixation device alone using plate, screw |
| 1TK80LAXXN | Repair humerus OA synth mater |
| 1TK87LANWN | Excise prt humerus OA &plate/scrw synth mater |
| 1TV87LA | Excision partial, radius and ulna no tissue used (for closure of defect) using no fixative device |
| 1TZ27JA | Radiation, arm NEC using external beam |
| 1VA74HANV | Fixation, hip joint percutaneous approach [e.g., with closed reduction or no reduction] fixation device alone using pin, nail |
| 1VA74LALQ | Fixation, hip joint open approach fixation device alone using intramedullary nail |
| 1VA74LALQN | Fix hip OA & intramed nail synth mater |
| 1VA74LANV | Fixation, hip joint open approach fixation device alone using pin, nail |
| 1VA74LANW | Fixation, hip joint open approach fixation device alone using plate, screw |
| 1VC74HALQ | Fixation, femur percutaneous approach [e.g., with closed reduction or no reduction] fixation device alone using intramedullary nail |
| 1VC74LALQ | Fixation, femur open approach fixation device alone using intramedullary nail |
| 1VC74LALQN | Fix femur OA &intramed nail synth mater |
| 1VC74LANWQ | Fix femur OA &plate/scrw combo tis |
| 1VC80LAKDQ | Repair femur OA &fix dev NEC combo tis |
| 1VC87LALQ | Excision partial, femur no tissue used (for closure of defect) using intramedullary nail |
| 1VC87LANVN | Excise prt femur OA &pin/nail synth mater |
| 1VC87LANW | Excision partial, femur with synthetic tissue [bone cement, paste] using screw, plate and screw |
| 1VC87LAPMN | Excise prt femur OA &endoprosth synth mat |
| 1VC91LAPNN | Excise rad femur OA &dual comp prosth synth mater |
| 1VD87LAXXA | Excise prt m hip & thigh OA autogr |
| 1VQ74LALQ | Fixation, tibia and fibula open approach fixation device alone using intramedullary nail |
| 1VQ87LANWN | Excise prt tib & fib OA &plate/scrw synth mater |
| 1VZ27JA | Radiation, leg NEC using external beam |
| 1YA87LA | Excision partial, scalp open [excisional] approach Without tissue repair |
| 1YK84LAXXE | Re/construct nipple OA loc flp |
| 1YK84LAXXQ | Re/construct nipple OA combo tis |
| 1YK87LA | Excision partial, nipple using open excisional approach |
| 1YK87LAXXE | Excise prt nipple OA loc flp |
| 1YK89LA | Excision total, nipple using open approach |
| 1YK90LAXXE | Exc tot w reconstr nipple OA loc flp |
| 1YK90LAXXQ | Exc tot w reconstr nipple OA combo tis |
| 1YL87LA | Excision partial, lactiferous duct using open approach |
| 1YL89LA | Excision total, lactiferous duct using open approach |
| 1YM27JA | Radiation, breast using external beam |
| 1YM52HA | Drainage, breast using needle aspiration |
| 1YM52HAAV | Drainage, breast using percutaneous approach with probe |
| 1YM52LA | Drainage, breast using incisional approach |
| 1YM53HAEM | Implantation of internal device, breast of brachytherapy applicator using percutaneous approach |
| 1YM53LAEM | Implantation of internal device, breast of brachytherapy applicator using open approach |
| 1YM54HAG2 | Management of internal device, breast using percutaneous (needle) approach with synthetic agent [e.g., silicone] |
| 1YM54HAW1 | Management of internal device, breast using percutaneous (needle) approach with augmentation agent [e.g., saline, soya] |
| 1YM55LATP | Removal of device, breast without capsulectomy of tissue expander |
| 1YM55WJPM | Removal of device, breast with capsulectomy (with or without inframammary fold repair) of breast implant [prosthesis] |
| 1YM72LA | Release breast OA |
| 1YM74LA | Fixation, breast using open approach |
| 1YM78LAXXE | Repair decr sz breast loc flp |
| 1YM78VQ | Repair by decreasing size, breast using peri areolar round block excisional technique |
| 1YM79LAPM | Repair by increasing size, breast open approach without tissue with implantation of prosthesis |
| 1YM79LATP | Repair by increasing size, breast open approach without tissue with implantation of tissue expander |
| 1YM79LATPG | Augment breast OA w tiss expandr &ped flp |
| 1YM80LA | Repair, breast open approach without tissue with no implantation of device |
| 1YM80LAPM | Repair, breast open approach without tissue with implantation of breast prosthesis |
| 1YM80LAPMA | Repair breast w prosth autogr |
| 1YM80LAPMF | Repair breast OA w prosth free flp |
| 1YM80LAPMG | 2009: Repair, breast using distant pedicled flap (1) with implantation of breast prosthesis 2012: Repair, breast open approach using distant pedicled flap with implantation of breast prosthesis |
| 1YM80LATP | Repair, breast open approach without tissue with implantation of tissue expander |
| 1YM80LATPE | Repair breast w tiss expandr loc flp |
| 1YM80LATPG | 2009: Repair, breast using distant pedicled flap (1) with implantation of tissue expander 2012: Repair, breast open approach using distant pedicled flap with implantation of tissue expander |
| 1YM80LATPK | Repair breast OA w tiss expandr homogr |
| 1YM80LAXXA | 2009: Repair, breast using autograft with no implantation of device 2012: Repair, breast open approach using autograft with no implantation of device |
| 1YM80LAXXE | Repair breast w loc flp |
| 1YM80LAXXF | 2009: Repair, breast using free flap with no implantation of device 2012: Repair, breast open approach using free flap with no implantation of device |
| 1YM80LAXXG | 2009: Repair, breast using distant pedicled flap with no implantation of device 2012: Repair, breast open approach using distant pedicled flap with no implantation of device |
| 1YM87DA | Excision partial, breast using endoscopic approach with simple apposition |
| 1YM87GB | Excision partial, breast using endoscopic guide wire (or needle hook) excision technique with simple apposition of tissue |
| 1YM87LA | Excision partial, breast using open approach with simple apposition of tissue (e.g., suturing) |
| 1YM87LAXXA | Excise prt breast OA autogr |
| 1YM87LAXXE | Excise prt breast OA loc flp |
| 1YM87UT | Excision partial, breast using open guide wire (or needle hook) excision technique and simple apposition of tissue |
| 1YM88LAPM | Excision partial with reconstruction, breast without tissue with implantation of prosthesis |
| 1YM88LAPME | Exc prt breast w prosth loc flp reconst |
| 1YM88LAPMF | Exc prt breast w prosth free flp reconstr |
| 1YM88LAPMG | Exc prt breast w prosth ped flp reconstr |
| 1YM88LAQF | Exc prt breast w prosth/tis expand reconstr |
| 1YM88LAQFE | Exc prt breast w prosth/tis expand loc flp reconst |
| 1YM88LATP | Excision partial with reconstruction, breast without tissue with implantation of tissue expander |
| 1YM88LATPE | Exc prt breast w tiss expandr &loc flp reconst |
| 1YM88LATPF | Exc prt breast w tiss expand free flp reconstr |
| 1YM88LATPG | Exc prt breast w tiss expand ped flp reconstr |
| 1YM88LATPK | Exc prt breast w tiss expand homogr reconstr |
| 1YM88LAXXE | Exc prt breast w loc flp reconstr |
| 1YM88LAXXF | Exc prt breast w free flp reconstr |
| 1YM88LAXXG | Exc prt breast w ped flp reconstr |
| 1YM89LA | Excision total, breast using open approach |
| 1YM89LAXXA | Excise tot breast w autogr |
| 1YM89LAXXE | Excise tot breast OA loc flp |
| 1YM90LAPM | Excision total with reconstruction, breast simple mastectomy with no node dissection without tissue with implantation of breast prosthesis |
| 1YM90LAPME | Exc tot breast prosth loc flp reconstr |
| 1YM90LAPMF | Exc tot breast prosthesis free flp reconstr |
| 1YM90LAPMG | Exc tot breast prosth ped flp reconstr |
| 1YM90LAQF | Exc tot breast prosth w tiss expand reconstr |
| 1YM90LAQFE | Exc tot breast prosth tis expand loc flp reconst |
| 1YM90LAQFG | Exc tot breast prosth tis expand ped flp reconst |
| 1YM90LATP | Excision total with reconstruction, breast simple mastectomy with no node dissection without tissue with implantation of tissue expander |
| 1YM90LATPF | Exc tot breast tiss expand free flp reconstr |
| 1YM90LATPG | Exc tot breast tiss expand ped flp reconstr |
| 1YM90LAXXF | Exc tot breast free flp reconstr |
| 1YM90LAXXG | Exc tot breast ped flp reconstr |
| 1YM90LAXXQ | Exc tot w reconstr breast OA combo tis |
| 1YM91LA | Excision radical, breast without tissue modified or NOS |
| 1YM91LATP | Excision radical, breast with implantation of tissue expander modified or NOS |
| 1YM91LAXXA | 2009: Excision radical (modified), breast using autograft 2012: Excision radical, breast using autograft modified or NOS |
| 1YM91LAXXE | 2009: Excision (modified) radical, breast using local flap 2012: Excision radical, breast using local flap modified or NOS |
| 1YM91TR | Excision radical, breast without tissue extended [Urban] |
| 1YM91TRXXE | 2009: Excision extended radical, breast using local flap 2012: Excision radical, breast using local flap extended [Urban] |
| 1YM92LAPME | Mod rad mastectmy w prosth loc flp reconst |
| 1YM92LAPMF | Mod rad mastectmy w prosth free flp reconst |
| 1YM92LAPMG | Mod rad mastectmy w prosth ped flp reconst |
| 1YM92LAQFE | Mod rad mastectmy w prosth tiss expand loc flp |
| 1YM92LAQFG | Mod rad mastectmy w prosth tiss expand ped flp |
| 1YM92LATPE | Mod rad mastectmy w tiss expandr loc flp reconst |
| 1YM92LATPF | Mod rad mastectmy w tiss expand free flp reconst |
| 1YM92LATPG | Mod rad mastectmy w tiss expand ped flp reconst |
| 1YM92LAXXF | Mod rad mastectmy w free flp reconst |
| 1YM92LAXXG | Mod rad mastectmy w ped flp reconst |
| 1YM92LAXXQ | 2009: Excision radical with reconstruction, breast modified or NOS with no implanted device using combined sources of tissue (e.g., free and pedicled TRAM flap) 2012: Excision radical with reconstruction, breast modified or NOS using combined sources of tissue (e.g., free and pedicled TRAM flap) with no implanted device |
| 1YM92TRPME | Ext rad mastectmy w prosth loc flp reconst |
| 1YM92TRTPE | Ext rad mastectmy wtiss expand loc flp reconst |
| 1YM92TRXXQ | Exc rad w reconstr breast OA w ext rad excisn combo tis |
| 1YR87LA | Excision partial, skin of axillary region open [excisional] approach with apposition technique (e.g., suture, glue) for closure |
| 1YR87LAXXB | Excise prt sk axilla &splt gr |
| 1YS87LA | Excision partial, skin of abdomen and trunk open [excisional] approach with apposition technique (suture, glue) for closure |
| 1YS87LAXXE | Excise prt sk abd & trunk &loc flp |
| 1ZZ35CAM0 | Pharmacotherapy, total body antineoplastic and immunomodulating agents per orifice (oral) approach antineoplastic agent NOS |
| 1ZZ35CAM2 | Pharmacotherapy, total body antineoplastic and immunomodulating agents per orifice (oral) approach antimetabolite |
| 1ZZ35CAM4 | Pharmacotherapy, total body antineoplastic and immunomodulating agents per orifice (oral) approach cytotoxic antibiotic and related substance |
| 1ZZ35CAM5 | Pharmacotherapy, total body antineoplastic and immunomodulating agents per orifice (oral) approach other antineoplastic |
| 1ZZ35HAK7 | Pharm tx NEC perc app ¯olide/lincosamide |
| 1ZZ35HAM0 | Pharmacotherapy, total body antineoplastic and immunomodulating agents percutaneous needle approach [intramuscular, intravenous, subcutaneous, intradermal] antineoplastic agent NOS |
| 1ZZ35HAM3 | Pharmacotherapy, total body antineoplastic and immunomodulating agents percutaneous approach [intramuscular, intravenous, subcutaneous, intradermal] plant alkaloid and other natural product |
| 1ZZ35HAM4 | Pharmacotherapy, total body antineoplastic and immunomodulating agents percutaneous approach [intramuscular, intravenous, subcutaneous, intradermal] cytotoxic antibiotic and related substance |
| 1ZZ35HAM5 | Pharmacotherapy, total body antineoplastic and immunomodulating agents percutaneous approach [intramuscular, intravenous, subcutaneous, intradermal] other antineoplastic |
| 1ZZ35HAM9 | Pharmacotherapy, total body antineoplastic and immunomodulating agents percutaneous approach [intramuscular, intravenous, subcutaneous, intradermal] Combination [multiple] antineoplastic agents |
| 1ZZ35HAN5 | Pharmacotherapy, total body musculoskeletal system agents percutaneous approach [intramuscular, intravenous, subcutaneous, intradermal] drug for treatment of bone disease |
| 2AX13HA | Specimen collection (diagnostic), spinal canal and meninges using percutaneous (needle) approach |
| 2EQ71HA | Biopsy s t head & neck perc ndle app |
| 2FU71HA | Biopsy thyr gl perc ndle app |
| 2GM71BA | Biopsy, bronchus using endoscopic per orifice approach |
| 2GM71BP | Biopsy, bronchus using endoscopic per orifice approach with needle aspiration |
| 2GM71BR | Biopsy, bronchus using endoscopic per orifice approach with brushing/washing |
| 2GT71BA | Biopsy, lung using endoscopic per orifice approach |
| 2GT71BP | Biopsy, lung using endoscopic per orifice approach and needle aspiration |
| 2GT71HA | Biopsy, lung using percutaneous (needle) approach |
| 2GW71DA | Biopsy mediast endo app |
| 2HZ24JAXJ | ECG NOS (ext applic record electrode) |
| 2ME71BP | Biopsy, mediastinal lymph nodes endoscopic per orifice, with needle aspiration |
| 2ME71DA | Biopsy, mediastinal lymph nodes using endoscopic approach |
| 2ME71LA | Biopsy, mediastinal lymph nodes using open approach |
| 2MZ71HA | Biopsy lymph sys perc ndle app |
| 2NF71BA | Biopsy stomach EPO app |
| 2NK70BABL | Inspect sm intest EPO app & gastroscope |
| 2OT71DA | Biopsy, abdominal cavity using endoscopic [laparoscopic] approach |
| 2SZ71HA | Biopsy s t chest & abd perc ndle app |
| 2WY71HA | Biopsy bone marrow perc ndle app |
| 2YK71HA | Biopsy, nipple using percutaneous approach (needle, punch) |
| 2YK71LA | Biopsy, nipple using open [incisional] approach |
| 2YM70LA | Inspection, breast NOS using open approach |
| 2YM71HA | Biopsy, breast NOS using percutaneous (needle) aspiration |
| 2YM71HAGX | Biopsy, breast NOS percutaneous approach using device NEC |
| 2YM71LA | Biopsy, breast NOS incisional biopsy |
| 2ZZ02ZX | Assessment (examination), total body for determining candidacy for treatment |
| 2ZZ13RA | Specimen collect NEC vn puncture |
| 3AN40WE | MRI brain with & without enhancement |
| 3ER20WC | CT head with enhancement |
| 3OG10WZ | Xray b dct w pancr w endo retrograde injct contr |
| 3OT30DA | U/S abd cav alone |
| 3SC40WE | MRI sp vert with & without enhancement |
| 3WZ70CC | Nuclear study msk sys SPECT tomo |
| 3YM30DA | U/S breast u/s only |
| 7SC08PL | Ministrate NEC personal care chronic pain |
Appendix A.3.2. Diagnoses
- Data Source(s): Discharge Abstract Database, National Ambulatory Care Reporting System.
- Coding system: International Classification of Diseases, version 10 (ICD10), 2015.

Table A3.
International Classification of Diseases version 10 diagnosis codes associated with procedures that indicated a second breast cancer event.
Table A3.
International Classification of Diseases version 10 diagnosis codes associated with procedures that indicated a second breast cancer event.
| International Classification of Diseases (Version 10) Codes | International Classification of Diseases (Version 10) Code Descriptions |
|---|---|
| C50 | Malignant neoplasm of breast |
| C22 | Malignant neoplasm of liver and intrahepatic bile ducts (excluding biliary tract NOS, secondary malignant neoplasm of liver) |
| C34 | Malignant neoplasm of bronchus and lung |
| C41 | Malignant neoplasm of bone and articular cartilage of other and unspecified sites |
| D43 | Neoplasm of uncertain or unknown behaviour of brain and central nervous system (excluding peripheral nerves and autonomic nervous system) |
| C71 | Malignant neoplasm of brain (excluding cranial nerves, retrobulbar tissue) |
| C77 | Secondary and unspecified malignant neoplasm of lymph nodes (excluding malignant neoplasm of lymph nodes, specified as primary) |
| C78 | Secondary malignant neoplasm of respiratory and digestive organs |
| C78.0 | Secondary malignant neoplasm of lung |
| C78.3 | Secondary malignant neoplasm of other and unspecified respiratory organs |
| C78.7 | Secondary malignant neoplasm of liver and intrahepatic bile duct |
| D48 | Neoplasm of uncertain or unknown behaviour of other and unspecified sites (excluding neurofibromatosis (nonmalignant)) |
| D48.0 | Bone and articular cartilage (excluding articular cartilage and cartilage of the ear, larynx, and nose; the connective tissue of the eyelid; and synovia). |
| D48.6 | Breast (including connective tissue of breast, cystosarcoma phyllodes; excluding skin of breast) |
| D37 | Neoplasm of uncertain or unknown behaviour of oral cavity and digestive organs |
| D37.6 | Liver, gallbladder and bile ducts |
| D38 | Neoplasm of uncertain or unknown behaviour of middle ear and respiratory and intrathoracic organs (excluding heart) |
| D38.1 | Trachea, bronchus and lung |
| C79 | Secondary malignant neoplasm of other and unspecified sites |
| C79.3 | Secondary malignant neoplasm of brain and cerebral meninges |
| C79.4 | Secondary malignant neoplasm of other and unspecified parts of nervous system |
| C79.5 | Secondary malignant neoplasm of bone and bone marrow |
Appendix A.4. Systemic Therapy Criterion
Patients met the systemic therapy criterion if they received one of the drugs listed, in some cases, for one of the indications listed.
- Data Source(s): Activity Level Reporting database.
- Coding system: Not applicable.

Table A4.
Systemic therapy data types and descriptions that indicated a second breast cancer event.
Table A4.
Systemic therapy data types and descriptions that indicated a second breast cancer event.
| Data Type Analyzed by Algorithm | Description |
|---|---|
| Drug description | PAMIDRONATE |
| CLODRONATE | |
| VINORELBINE | |
| PACLITAXEL | |
| ERIBULIN | |
| PERTUZUMAB | |
| TRASTUZUMAB EMTANSINE |
- Data Source(s): New Drug Funding Program database.
- Coding system: Proprietary to Ontario Health.

Table A5.
Disease indications and funding policy name or name of drug received by patient that indicated a second breast cancer event.
Table A5.
Disease indications and funding policy name or name of drug received by patient that indicated a second breast cancer event.
| Disease Indication | Policy Name/Drug Name |
|---|---|
| Metastatic or Incurable Locally Advanced—Breast Cancer | Eribulin |
| Unresectable Locally Recurrent or Metastatic—Breast Cancer | Pertuzumab with Trastuzumab |
| Trastuzumab Emtansine | |
| Unresectable Locally Advanced or Metastatic Breast Cancer as Third or Subsequent Line of Treatment (Time-Limited) | Trastuzumab Emtansine |
| Metastatic Breast Cancer | Clodronate (IV) |
| Docetaxel | |
| Nab-Paclitaxel | |
| Paclitaxel | |
| Pamidronate | |
| Trastuzumab in combination with Docetaxel | |
| Trastuzumab in combination with Paclitaxel | |
| Trastuzumab in combination with Vinorelbine | |
| Trastuzumab with First Line Docetaxel | |
| Trastuzumab—Single Agent | |
| Vinorelbine | |
| Second Line—Metastatic Breast Cancer | Trastuzumab |
Appendix A.5. Radiation Treatment Criterion
Patients met this criterion if they received radiation therapy in one of the anatomical sites listed to treat one of the associated diagnoses listed in the appropriate time period.
Appendix A.6. Body Regions Where Radiation Was Applied
- Data Source(s): Activity Level Reporting database.
- Coding system: Proprietary to Ontario Health.

Table A6.
Body regions and codes for receiving radiation that indicated a second breast cancer event.
Table A6.
Body regions and codes for receiving radiation that indicated a second breast cancer event.
| Body Region Group | Body Region Code | Body Region Code Description |
|---|---|---|
| ABDOMEN | ABDL | Left abdomen |
| ABDOMEN (continued) | ABDO | Whole abdomen |
| ABDR | Right abdomen | |
| ABLB | Lower abdomen | |
| ABLL | Left lower abdomen | |
| ABLR | Right lower abdomen | |
| ABUB | Upper abdomen | |
| ABUL | Left upper abdomen | |
| ABUR | Right upper abdomen | |
| ADRL | Left adrenal | |
| ADRR | Right adrenal | |
| BILE | Bile duct | |
| COLN | Colon | |
| EPIG | Epigastrium | |
| GALL | Gall bladder | |
| INVY | Inverted ‘y’ (dog-leg, hockey-stick) | |
| KIDL | Left kidney | |
| KIDR | Right kidney | |
| LIVR | Liver | |
| PANC | Pancreas | |
| PARA | Para-aortic nodes | |
| SPLE | Spleen | |
| STOM | Stomach | |
| CHEST | AXIL | Left axilla |
| AXIR | Right axilla | |
| BREB | Bilateral breast | |
| BREL | Left breast | |
| BRER | Right breast | |
| CHEB | Bilateral chest lung & area involve | |
| CHEL | Left chest | |
| CHER | Right chest | |
| CHWB | Bilateral chest wall (w/o breast) | |
| CHWL | Left chest wall | |
| CHWR | Right chest wall | |
| CLAB | Bilateral clavicle | |
| CLAL | Left clavicle | |
| CLAR | Right clavicle | |
| ESOI | Lower esophagus | |
| ESOM | Middle esophagus | |
| ESOS | Upper esophagus | |
| ESOW | Entire esophagus | |
| HEML | Left hemimantle | |
| HEMR | Right hemimantle | |
| HERT | Heart | |
| CHEST (continued) | IMCB | Bilateral internal mammary chain |
| LUNB | Bilateral lung | |
| LUNL | Left lung | |
| LUNR | Right lung | |
| MANT | Mantle | |
| MEDI | Mediastinum | |
| PLEL | Left pleura (as in mesothelioma) | |
| PLER | Right pleura | |
| RIBL | Left ribs | |
| RIBR | Right ribs | |
| SCAB | Bilateral scapula | |
| SCAL | Left scapula | |
| SCAR | Right scapula | |
| SCNB | Bilateral supraclavicular nodes | |
| SCNL | Left supraclavicular nodes | |
| SCNR | Right supraclavicular nodes | |
| STER | Sternum | |
| HEAD | ANTB | Bilateral antrum (bull’s eye) |
| ANTL | Left antrum | |
| ANTR | Right antrum | |
| BRAI | Brain | |
| CHKL | Left cheek | |
| CHKR | Right cheek | |
| EARL | Left ear | |
| EARR | Right ear | |
| ETHM | Ethmoid sinus | |
| EYEB | Bilateral eyes | |
| EYEL | Left eye | |
| EYER | Right eye | |
| FACB | Bilateral face | |
| FACL | Left face | |
| FACR | Right face | |
| FLOO | Floor of mouth (boosts) | |
| FOSS | Posterior fossa | |
| GING | Gingiva | |
| HEAD | Head | |
| LACB | Bilateral lacrimal gland | |
| LACL | Left lacrimal gland | |
| LACR | Right lacrimal gland | |
| LIPB | Both lip(s) | |
| HEAD (continued) | LIPI | Lower lip |
| LIPS | Upper lip | |
| MANB | Bilateral mandible | |
| MANL | Left mandible | |
| MANR | Right mandible | |
| MAXB | Bilateral maxilla | |
| MAXL | Left maxilla | |
| MAXR | Right maxilla | |
| NASA | Nasal fossa | |
| NASO | Nasopharynx | |
| ORAL | Oral cavity/buccal mucosa | |
| ORBB | Bilateral orbit | |
| ORBL | Left orbit | |
| ORBR | Right orbit | |
| OROP | Oropharynx | |
| PALH | Hard palate | |
| PALS | Soft palate | |
| PALX | Palate unspecified | |
| PARL | Left parotid | |
| PARR | Right parotid | |
| PITU | Pituitary | |
| SALL | Left salivary gland | |
| SALR | Right salivary gland | |
| SKUL | Skull | |
| SPHE | Sphenoid sinus | |
| SUBM | Submandibular glands | |
| TONG | Tongue | |
| TONS | Tonsil | |
| UVUL | Uvula | |
| LOWER LIMB | ANKB | Bilateral ankle |
| ANKL | Left ankle | |
| ANKR | Right ankle | |
| FEMB | Bilateral femur | |
| FEML | Left femur | |
| FEMR | Right femur | |
| FIBL | Left fibula | |
| FIBR | Right fibula | |
| LOWER LIMB (continued) | FOOB | Bilateral feet |
| FOOL | Left foot | |
| FOOR | Right foot | |
| HEEB | Bilateral heel | |
| HEEL | Left heel | |
| HEER | Right heel | |
| HIPB | Bilateral hip | |
| HIPL | Left hip | |
| HIPR | Right hip | |
| KNEB | Bilateral knee | |
| KNEL | Left knee | |
| KNER | Right knee | |
| LEGB | Bilateral leg | |
| LEGL | Left leg | |
| LEGR | Right leg | |
| LELB | Lower bilateral leg | |
| LELL | Lower left leg | |
| LELR | Lower right leg | |
| LEUB | Upper bilateral leg | |
| LEUL | Upper left leg | |
| LEUR | Upper right leg | |
| TIBL | Left tibia | |
| TIBR | Right tibia | |
| TOEL | Left toes | |
| TOER | Right toes | |
| NECK | HYPO | Hypopharynx |
| LARP | Larygopharynx | |
| LARY | Larynx | |
| NECB | Bilateral neck includes nodes | |
| NECL | Left neck includes nodes | |
| NECR | Right neck includes nodes | |
| PYRI | Pyriform fossa (sinuses) | |
| THYB | Thyroid | |
| TRAC | Trachea | |
| SPINE | COCC | Coccyx |
| SACR | Sacrum | |
| SPCT | Cervical & thoracic spine | |
| SPIC | Cervical spine | |
| SPIL | Lumbar spine | |
| SPIT | Thoracic spine | |
| SPIW | Whole spine | |
| SPLS | Lumbo-sacral spine | |
| SPTL | Thoracic & lumbar spine | |
| UPPER LIMB | ARLL | Lower left arm |
| ARLR | Lower right arm | |
| ARMB | Bilateral arms | |
| ARML | Left arm | |
| ARMR | Right arm | |
| ARUL | Upper left arm | |
| ARUR | Upper right arm | |
| FING | Finger (including thumbs) | |
| HANB | Bilateral hand | |
| HANL | Left hand | |
| HANR | Right hand | |
| HUML | Left humerus | |
| HUMR | Right humerus | |
| RADL | Left radius | |
| RADR | Right radius | |
| SHOB | Bilateral shoulder | |
| SHOL | Left shoulder | |
| SHOR | Right shoulder | |
| ULNL | Left ulna | |
| ULNR | Right ulna |
Appendix A.7. Diagnoses Associated with Radiation
- Data Source(s): Activity Level Reporting database.
- Coding system: International Classification of Diseases, version 10 (ICD10), 2015.

Table A7.
International Classification of Diseases version 10 diagnosis codes that indicated a second breast cancer event.
Table A7.
International Classification of Diseases version 10 diagnosis codes that indicated a second breast cancer event.
| Codes | Code Description (ICD-10 Version 2015) |
|---|---|
| C50 | Malignant neoplasm of breast |
| C34 | Malignant neoplasm of bronchus and lung |
| C40 | Malignant neoplasm of bone and articular cartilage of limbs |
| C71 | Malignant neoplasm of brain |
| C77 | Secondary and unspecified malignant neoplasm of lymph nodes |
| C78 | Secondary malignant neoplasm of respiratory and digestive organs |
| C79 | Secondary malignant neoplasm of other and unspecified sites |
Appendix B
Exclusions during Manual Record Review and Comparison to Final Validation Sub-Cohort
Of the 3258 patients selected for manual record review, 1013 patients were excluded because their records could not be retrieved, they did not have sufficient records for review at a study center, or their SBCE status was indeterminate. The remaining validation sub-cohort was 2245 patients (main text Table 3). We conducted additional statistical analyses to determine whether patients excluded during manual record review differed from patients who remained in the validation sub-cohort. Pearson’s Chi-squared tests were used to determine whether patients excluded during manual record review differed from the patients remaining in the validation sub-cohort based on stage at diagnosis and algorithm classification as having or not having an SBCE. A Cochran-Mantel-Haenszel statistic [24] was used to test for conditional independence between remaining in the validation sub-cohort and algorithm SBCE classification after controlling for stage at diagnosis.
Pearson’s chi-squared tests indicated a potential relationship between stage at diagnosis and likelihood of exclusion during manual review based on a marginally significant p-value of 0.044 (main text Table 4A). The Cochran-Mantel-Haenszel statistic [24] demonstrated that after controlling for stage at diagnosis, the algorithm classified more excluded patients as having an SBCE (main text Table 4B; p-value < 0.0136).

Table A8.
Stage at diagnosis among patients excluded during manual review and patients remaining in the validation sub-cohort.
Table A8.
Stage at diagnosis among patients excluded during manual review and patients remaining in the validation sub-cohort.
| Patient Group | Stage at Diagnosis | |||
|---|---|---|---|---|
| Stage 1 N (%) | Stage 2 N (%) | Stage 3 N (%) | Total N | |
| Remaining validation sub-cohort | 701 (31.2%) | 812 (36.2%) | 732 (32.6%) | 2245 |
| Excluded during manual review | 347 (34.3%) | 322 (31.8%) | 344 (34.0%) | 1013 |
Abbreviations: N, number.

Table A9.
Algorithm classification as experiencing a second breast cancer event (SBCE) stratified by stage at diagnosis and exclusion during manual review.
Table A9.
Algorithm classification as experiencing a second breast cancer event (SBCE) stratified by stage at diagnosis and exclusion during manual review.
| Stage at Diagnosis | Patient Group | Algorithm SBCE Classification | |
|---|---|---|---|
| SBCE N (Row%) | No SBCE N (Row%) | ||
| Stage 1 | Remaining validation sub-cohort | 48 (6.8%) | 653 (93.2%) |
| Excluded during manual review | 27 (7.8%) | 320 (92.2%) | |
| Stage 2 | Remaining validation sub-cohort | 107 (13.2%) | 705 (86.8%) |
| Excluded during manual review | 61 (18.9%) | 261 (81.1%) | |
| Stage 3 | Remaining validation sub-cohort | 216 (29.5%) | 516 (70.5%) |
| Excluded during manual review | 114 (33.1%) | 230 (66.9%) | |
Abbreviations: N, number; SBCE, second breast cancer event.
Appendix C
Algorithm Diagnostic Accuracy by Prior Cancer History
Algorithm diagnostic accuracy was assessed for patients with a history of cancer prior to the breast cancer diagnosis that qualified them for inclusion in this study. Diagnostic accuracy was similar for the entire cohort, patients with no prior cancer, and patients with no prior breast cancer, though sensitivity decreased for patients with any prior cancer (prior breast or non-breast cancer, or both). Patients with prior breast cancers constituted too small a group to analyze separately. The comparable diagnostic accuracy for patients with no prior cancer and no prior breast cancer suggests that inclusion of patients with prior non-breast cancers did not meaningfully affect algorithm performance.

Table A10.
Algorithm diagnostic accuracy at classifying patients as experiencing a second breast cancer event (SBCE), stratified by prior cancer status.
Table A10.
Algorithm diagnostic accuracy at classifying patients as experiencing a second breast cancer event (SBCE), stratified by prior cancer status.
| Patients’ Cancer Status Prior to Cohort Entry | N | Agreement Statistic % (95% Confidence Interval) | ||||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Accuracy | Kappa 1 | Prevalence-Adjusted Bias-Adjusted Kappa 1 | ||
| Remaining validation sub-cohort | 2245 | 85.3 (80.7–89.1) | 93.8 (92.6–94.8) | 67.1 (62.1–71.9) | 97.7 (96.9–98.3) | 92.7 (91.5–93.7) | 70.9 (66.7–75.0) | 85.3 (83.0–87.4) |
| No prior breast cancer (no prior cancer and prior non-breast cancer) | 2182 | 85.9 (81.3–89.8) | 93.7 (92.5–94.8) | 66.5 (61.3–71.4) | 97.9 (97.1–98.5) | 92.7 (91.5–93.8) | 70.8 (66.5–75.0) | 85.4 (83.1–87.5) |
| Any prior cancer (prior breast cancer, non-breast cancer, or both) | 167 | 79.3 (60.3–92.0) | 93.5 (88.0–97.0) | 71.9 (53.3–86.3) | 95.6 (90.6–98.4) | 91.0 (85.6–94.9) | 69.9 (55.7–84.1) | 82.0 (71.2–89.8) |
| No prior cancer | 2078 | 85.9 (81.1–89.9) | 93.8 (92.6–94.8) | 66.7 (61.4–71.7) | 97.9 (97.1–98.5) | 92.8 (91.6–93.9) | 70.9 (66.6–75.3) | 85.6 (83.2–87.7) |
Abbreviations: N, number; SBCE, second breast cancer event. 1 The Fleiss method of confidence interval calculation was used to calculate the confidence intervals for the kappa and prevalence-adjusted bias-adjusted kappa statistics [28].
References
- Chubak, J.; Yu, O.; Pocobelli, G.; Lamerato, L.; Webster, J.; Prout, M.N.; Ulcickas Yood, M.; Barlow, W.E.; Buist, D.S.M. Administrative data algorithms to identify second breast cancer events following early-stage invasive breast cancer. JNCI J. Natl. Cancer Inst. 2012, 104, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kong, S.; Cheung, W.Y.; Bouchard-Fortier, A.; Dort, J.C.; Quan, H.; Buie, E.M.; McKinnon, G.; Quan, M.L. Development and validation of case-finding algorithms for recurrence of breast cancer using routinely collected administrative data. BMC Cancer 2019, 19, 210. [Google Scholar] [CrossRef]
- Ritzwoller, D.P.; Hassett, M.J.; Uno, H.; Cronin, A.M.; Carroll, N.M.; Hornbrook, M.C.; Kushi, L.C. Development, validation, and dissemination of a breast cancer recurrence detection and timing informatics algorithm. JNCI J. Natl. Cancer Inst. 2017, 110, 273–281. [Google Scholar] [CrossRef] [PubMed]
- In, H.; Simon, C.A.; Phillips, J.L.; Posner, M.C.; Ko, C.Y.; Winchester, D.P. The quest for population-level cancer recurrence data; current deficiencies and targets for improvement. J. Surg. Oncol. 2015, 111, 657–662. [Google Scholar] [CrossRef]
- Maishman, T.; Cutress, R.I.; Hernandez, A.; Gerty, S.; Copson, E.R.; Durcan, L.; Eccles, D.M. Local recurrence and breast oncological surgery in young women with breast cancer: The POSH observational cohort study. Ann. Surg. 2017, 266, 165–172. [Google Scholar] [PubMed]
- Pilewskie, M.; Morrow, M. Margins in breast cancer: How much is enough? Cancer 2018, 124, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Pivot, X.; Asmar, L.; Hortobagyi, G.N.; Theriault, R.; Pastorini, F.; Buzdar, A. A retrospective study of first indicators of breast cancer recurrence. Oncology 2000, 58, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Gray, R.; Braybrooke, J.; Davies, C.; Taylor, C.; McGale, P.; Peto, R.; Pritchard, K.I.; Bergh, J.; Dowsett, M.; et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N. Engl. J. Med. 2017, 377, 1836–1846. [Google Scholar] [CrossRef]
- Will, B.P.; Berthelot, J.-M.; Le Petit, C.; Tomiak, E.M.; Verma, S.; Evans, W.K. Estimates of the lifetime costs of breast cancer treatment in Canada. Eur. J. Cancer 2000, 36, 724–735. [Google Scholar] [CrossRef]
- Hawley, S.T.; Janz, N.K.; Griffith, K.A.; Jagsi, R.; Friese, C.R.; Kurian, A.W.; Hamilton, A.S.; Ward, K.C.; Morrow, M.; Wallner, L.P.; et al. Recurrence risk perception and quality of life following treatment of breast cancer. Breast Cancer Res. Treat. 2017, 161, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Tewari, A.; Chagpar, A.B. Worry about breast cancer recurrence: A population-based analysis. Am. Surg. 2014, 80, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Geurts, Y.M.; Witteveen, A.; Bretveld, R.; Poortmans, P.M.; Sonke, G.S.; Strobbe, L.J.A.; Siesling, S. Patterns and predictors of first and subsequent recurrence in women with early breast cancer. Breast Cancer Res. Treat. 2017, 165, 709–720. [Google Scholar] [CrossRef]
- Soerjomataram, I.; Louwman, M.W.J.; Ribot, J.G.; Roukema, J.A.; Coebergh, J.W.W. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res. Treat. 2008, 107, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Beatty, J.D.; Sun, Q.; Markowitz, D.; Chubak, J.; Huang, B.; Etzioni, R. Identifying breast cancer recurrence histories via patient-reported outcomes. J. Cancer Surviv. 2022, 16, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Hassett, M.J.; Ritzwoller, D.P.; Taback, N.; Carroll, N.; Cronin, A.M.; Ting, G.V.; Schrag, D.; Warren, J.L.; Hornbrook, M.C.; Weeks, J.C. Validating billing/encounter codes as indicators of lung, colorectal, breast, and prostate cancer recurrence using 2 large contemporary cohorts. Med. Care 2014, 52, e65–e73. [Google Scholar] [CrossRef]
- Whyte, J.L.; Engel-Nitz, N.M.; Teitelbaum, A.; Gomez Rey, G.; Kallich, J.D. An evaluation of algorithms for identifying metastatic breast, lung, or colorectal cancer in administrative claims data. Med. Care 2015, 53, e49–e57. [Google Scholar] [CrossRef] [PubMed]
- Cronin-Fenton, D.; Kjærsgaard, A.; Nørgaard, M.; Amelio, J.; Liede, A.; Hernandez, R.K.; Sørensen, H.T. Breast cancer recurrence, bone metastases, and visceral metastases in women with stage II and III breast cancer in Denmark. Breast Cancer Res. Treat. 2018, 167, 517–528. [Google Scholar] [CrossRef]
- Henriques Abreu, P.; Santos, M.; Henriques Abreu, M.; Aveleira Andrade, B.; Silva, D. Predicting breast cancer recurrence using machine learning techniques: A systematic review. ACM Comput. Surv. 2016, 49, 1–40. [Google Scholar]
- Haque, R.; Shi, J.; Schottinger, J.E.; Ahmed, S.A.; Chung, J.; Avila, C.; Lee, V.S.; Cheetham, T.C.; Habel, L.A.; Fletcher, S.W.; et al. A hybrid approach to identify subsequent breast cancer using pathology and automated health information data. Med. Care 2015, 53, 380–385. [Google Scholar] [PubMed]
- How We Collect Cancer Registry Data. Available online: https://www.cancercareontario.ca/en/data-research/accessing-data/technical-information/cancer-registry-data-collection (accessed on 8 July 2022).
- Apply for OHIP and Get a Health Card. Available online: https://www.ontario.ca/page/apply-ohip-and-get-health-card#section-0 (accessed on 8 July 2022).
- Access Data. Available online: https://www.ccohealth.ca/en/access-data (accessed on 8 July 2022).
- Ontario Cancer Statistics 2016. Available online: https://www.cancercareontario.ca/en/statistical-reports/ontario-cancer-statistics-2016 (accessed on 8 July 2022).
- Agresti, A. An Introduction to Categorical Data Analysis, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Byrt, T.; Bishop, J.; Carlin, J.B. Bias, prevalence and kappa. J. Clin. Epidemiol. 1993, 46, 423–429. [Google Scholar] [CrossRef]
- Sim, J.; Wright, C.C. The Kappa statistic in reliability studies: Use, interpretation, and sample size requirements. Phys. Ther. 2005, 85, 257–268. [Google Scholar] [CrossRef]
- Marrie, R.A.; Fisk, J.D.; Yu, B.N.; Leung, S.; Elliott, L.; Caetano, P.; Warren, S.; Evans, C.; Wolfson, C.; Svenson, L.W.; et al. Mental comorbidity and multiple sclerosis: Validating administrative data to support population-based surveillance. BMC Neurol. 2013, 13, 16. [Google Scholar]
- Fleiss, J.L.; Cohen, J.; Everitt, B.S. Large sample standard errors of kappa and weighted kappa. Psychol. Bull. 1969, 72, 323–327. [Google Scholar]
- Kroenke, C.H.; Chubak, J.; Johnson, L.; Castillo, A.; Weltzien, E.; Caan, B.J. Enhancing breast cancer recurrence algorithms through selective use of medical record data. JNCI J. Natl. Cancer Inst. 2015, 108, djv336. [Google Scholar] [CrossRef] [PubMed]
- Livaudais-Toman, J.; Egorova, N.; Franco, R.; Prasad-Hayes, M.; Howell, E.A.; Wisnivesky, J.; Bickell, N.A. A validation study of administrative claims data to measure ovarian cancer recurrence and secondary debulking surgery. EGEMS 2016, 4, 1208. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).