Abstract
This prospective registry-based study aims to describe electrochemotherapy (ECT) modalities in basal cell carcinoma (BCC) patients and evaluate its efficacy, safety, and predictive factors. The International Network for Sharing Practices of Electrochemotherapy (InspECT) multicentre database was queried for BCC cases treated with bleomycin-ECT between 2008 and 2019 (n = 330 patients from seven countries, with 623 BCCs [median number: 1/patient; range: 1–7; size: 13 mm, range: 5–350; 85% were primary, and 80% located in the head and neck]). The procedure was carried out under local anaesthesia in 68% of cases, with the adjunct of mild sedation in the remaining 32%. Of 300 evaluable patients, 242 (81%) achieved a complete response (CR) after a single ECT course. Treatment naïvety (odds ratio [OR] 0.35, 95% confidence interval [C.I.] 0.19–0.67, p = 0.001) and coverage of deep tumour margin with electric pulses (O.R. 5.55, 95% C.I. 1.37–21.69, p = 0.016) predicted CR, whereas previous radiation was inversely correlated (O.R. 0.25, p = 0.0051). Toxicity included skin ulceration (overall, 16%; G3, 1%) and hyperpigmentation (overall, 8.1%; G3, 2.5%). At a 17-month follow-up, 28 (9.3%) patients experienced local recurrence/progression. Despite no convincing evidence that ECT confers improved outcomes compared with standard surgical excision, it can still be considered an opportunity to avoid major resection in patients unsuitable for more demanding treatment. Treatment naïvety and coverage of the deep margin predict tumour clearance and may inform current patient selection and management.
1. Introduction
In its various forms, surgical resection is the mainstay of treatment for patients with basal cell carcinoma (BCC), the most common form of skin cancer. Generally, standard excision is indicated for small/intermediate-sized tumours in low-risk anatomical areas, whereas Mohs surgery is reserved for large or recurrent lesions, particularly in high-risk areas [1,2].
However, contemporary population ageing poses new therapeutic challenges, and patients with relevant comorbidities are best served by nonsurgical approaches, including radiation, photodynamic therapy, cryotherapy, and topical agents. Additionally, relatively few patients with locally advanced or metastatic BCC can now benefit from systemic treatment with hedgehog (i.e., vismodegib and sonidegib) and, more recently, programmed death-1 (i.e., cemiplimab) inhibitors [1,3].
In pursuing well-tolerated minimally invasive options, electrochemotherapy (ECT) was introduced in the late 1990s in the United States and Europe as a highly effective skin-directed locoregional chemotherapy. ECT harnesses short, high-voltage electric pulses to increase cell permeability to bleomycin or cisplatin, two otherwise poorly permeant drugs [4], and its mechanisms of action include the direct cytotoxic effect, composite antivascular activity, and a local immune response [5]. The procedure is currently widely used, mainly as a palliative measure, to provide local disease control in patients with superficial malignancies from various histotypes.
According to recent studies, ECT is associated with 50–100% clearance rates in BCC [6,7,8]. However, the generalizability of results is blurred by global variation in study designs, treated patients, and the reporting of results [9,10,11,12,13,14,15,16,17,18,19,20]. The present study aimed to describe ECT modalities in the largest available cohort of BCC patients, evaluate treatment efficacy and safety, and identify predictors of response. Its results are clinically relevant because they may inform the current patient selection and help design future clinical trials in more selected populations.
2. Materials and Methods
2.1. Study Design
We analysed the International Network for Sharing Practices of ECT (InspECT) database. The InspECT registry is a prospective database established in 2008 to assess the outcome of patients with skin cancers or cutaneous metastases treated with ECT (https://insp-ect.eu accessed on: 20 May 2022), approved by the National Medical Ethics Committee of the Republic of Slovenia (No. 102/09/14). The study was carried out according to the rules of Good Clinical Practice and tenets of the Declaration of Helsinki. The collected information included patient demographics, tumour characteristics, ECT parameters (anaesthesia regimen, chemotherapy drug, type of pulse applicator, duration of the procedure), tumour response, treatment toxicity, and patient-reported outcomes. The primary endpoints were activity and safety; secondary endpoints were identifying predictors of response and assessment of tumour control.
2.2. Participants
The InspECT database was queried for all BCC patients (any histological subtype) treated between 2008 and 2019. A multidisciplinary team agreed upon the indication to ECT; each participant received information regarding the treatment intent and then signed a consent form. The inclusion and exclusion criteria were derived from the European Standard Operative Procedures of ECT (ESOPE) [21,22]. In particular, the candidate patients had to have primary or recurrent BCC in which other treatment modalities had failed or were impracticable. In addition, normal lung and renal function were prerequisites to being suitable for the procedure. Absolute contraindications to the treatment were allergy to bleomycin or a previous cumulative dose of 400,000 IU/m2 due to the risk of lung fibrosis, pregnancy, or lactation [21,22]. Preoperative workup included a complete medical history, physical examination, and standard blood tests.
2.3. Procedure
2.3.1. Protocol
The procedure was initially performed following the 2006 ESOPE guidelines and, from 2018, their updated version [21,22], with no changes in chemotherapy doses or electroporation protocol.
2.3.2. Anaesthesia
Local anaesthesia, eventually associated with mild sedation, was used in patients with small (<3 cm) and few (n ≤ 7) lesions, whereas general sedation/anaesthesia was added in patients with tumours larger than 3 cm, mainly when located (a) close to the periosteum, (b) in sensitive regions (e.g., chin, cheek, lip), or (c) in patients needing airway protection.
2.3.3. Chemotherapy
Bleomycin was administered intravenously or intratumourally, depending on tumour features and patient preoperative assessment. The intravenous bolus was administered at 15,000 IU/m2 of body surface area, with a maximum dose capped at 30,000 IU. Dose adjustment for age or impaired renal function was used according to local guidelines. Bleomycin infusion lasted 2–5 min, and the electric pulses were applied after 8 min when the drug had diffused into tissues. As for the intratumoural route, the recommended concentration of bleomycin solution was 1000 IU/ml, and the injected volume was determined according to the lesion volume.
2.3.4. Electric Pulse Delivery
Electrode choice was based on BCC location, size, and morphology and included needle or plate electrodes of different sizes and geometries. The electric fields (eight pulses of 100 ms duration, with an amplitude of 1000 V/cm for needle or 1300 V/cm for plate electrodes) were delivered through a pulse generator certified for clinical application (CliniporatorTM, IGEA, Carpi, Italy). Intraoperatively, the timing of pulse delivery varied according to the route of bleomycin administration (immediately after intralesional injection, after 8 min following intravenous infusion).
2.3.5. Postprocedural Care
The treated lesions were covered with nonadherent dressings or alginates in cases of ulceration. Patients treated under general anaesthesia were monitored for 12/24 h.
2.4. Outcome Assessment
For each patient, the largest tumour diameter was measured using a millimetre ruler at baseline and one- and two-month follow-up, following the Response Evaluation Criteria in Solid Tumours (RECIST v1.1). Histological verification was not routinely performed but allowed according to clinical judgment. Complete response (CR) was defined as tumour disappearance, whereas partial response (PR) was a shrinkage of at least 30%, and progressive disease (PD) was an increase of at least 20%. The cases where inflammation or ulceration hampered response assessment were labelled as "not evaluable" (NE). Responses defined neither by PR nor PD criteria were considered stable disease (SD). Pain intensity was evaluated through a numerical rating scale (NRS) ranging from 0 to 10 (no pain and maximum pain, respectively). Toxicity and complications were graded from grade 1 (G1, mild adverse event) to grade 4 (G4, life-threatening/disabling adverse event) according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) v3.0. Except for the two-month visit to assess the response, as per RECIST, patient follow-up was in accordance with local institutional protocols. Local recurrence within the ECT field was diagnosed based on clinical and histological findings, as appropriate.
2.5. Statistical Analysis
Univariate response analysis was performed with contingency tables of frequency and Pearson’s chi-squared test with Yates’ continuity correction. Multivariate analysis of variance (MANOVA) was used to identify predictors of response. Local progression-free survival (LPFS) was calculated from the first ECT to the date of relapse or local progression or last follow-up. The survival curve was calculated by the Kaplan–Meier method. Pain scores were compared using coupled Student’s t-test analysis. p-value < 0.05 was considered statistically significant. Analyses were performed with NCSS 9 software (NCSS, LLC. Kaysville, UT, USA).
3. Results
3.1. Patient Population
A total of 330 patients were consecutively treated at 15 European centres (Table 1); 30 subjects were excluded from the response analysis due to one of the following reasons: the inability or unwillingness to attend follow-up (n = 14), application of other treatments (n = 6), death from other cause (n = 5), or lost to follow-up (n = 5). As a result, 300 patients (188 males, 112 females) with 587 tumours were evaluable. The treatment intent was palliative in 39 cases (13%). The median BCC size was 13 mm (range 5–350 mm), and 90% of tumours (560/587) were smaller than 3 cm. Most (80%) were located in the head and neck, and 5% were ulcerated.

Table 1.
Patient and treatment characteristics (330 patients with 623 tumours).
3.2. Treatment
The procedure was performed under local anaesthesia in 67% of cases (Table 1). Bleomycin was administered intravenously or intratumourally in 57% and 43% of patients.
3.3. Toxicity
Adverse events included skin hyperpigmentation (7%, n = 22 patients; 2 with grade-2) and skin ulceration (6.7%, n = 20; 3 with grade-3). Other uncommon side effects included local oedema (n = 6), flu-like symptoms (n = 4), rash/allergic reaction (n = 3), skin atrophy (n = 1), hypopigmentation (n = 1). Pain scores increased in the immediate postprocedural assessment, although 96% of patients (96%) experienced no/mild discomfort. Local pain scores assessed at two months and the last follow-up (median 12 months [range, 2.3–78]) were significantly lower compared with baseline (Table 2). Finally, the proportion of patients reporting the consumption of analgesics decreased from 9% at baseline to 5% at the last follow-up (p = 0.04).

Table 2.
Assessment of pain (n = 300 patients).
3.4. Tumour Response
Results are presented in Table 3. Following the first ECT course, the per-tumour clearance rate was 83% (488/587 lesions). Out of the 300 evaluable patients, 242 (81%) achieved CR, 46 (15%) PR, 9 (3%) SD, whereas 3 (1%) were NE. Two representative cases are shown in Figure 1 and Figure S1.

Table 3.
Tumour response following the first ECT application (n = 300 patients with 587 tumours).
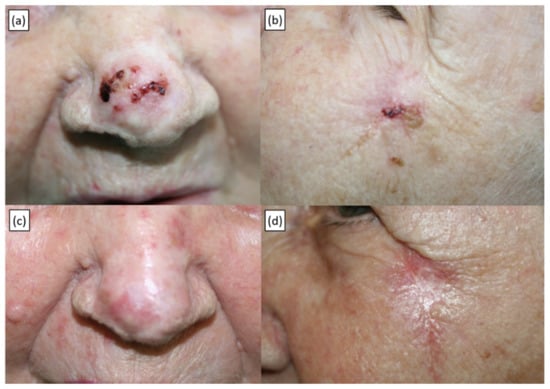
Figure 1.
Recurrent BCC of the tip of the nose and concomitant primary BCC of the left zygomatic area in an 89-year-old patient treated with a single ECT application under local anaesthesia. (a,b) Baseline presentation. (c,d) Twelve-month follow-up showing a complete response.
3.5. Predictors of Response
By univariate analysis, small (≤3 cm) tumour size (p = 0.002), absence of previous treatments (p < 0.001), no prior radiotherapy (p = 0.007), head and neck location (p = 0.008), and the coverage of deep (p = 0.001) and lateral margins (p = 0.045) were associated with tumour clearance, whereas only the coverage of deep tumour margin (odds ratio [O.R.] 5.44, p = 0.016) and the absence of previous treatments (O.R. 2.86, p = 0.001) remained independent predictors in multivariate analysis (Table 4). In multivariate analysis for the overall response, the significant variables were the absence of prior treatments, small tumour size, and head and neck location (Table S1).

Table 4.
Predictors of complete response.
3.6. Local Control and Patient Survival
Fifty-two patients (17%) underwent a second ECT after a median of 7 months (range, 1–47 months) for PR (n = 37) or recurrence (n = 15). With a median follow-up of 17 months (mean 22, range 2–103), 28 (9.3%) patients experienced local progression after a median interval of 13 months (range 3–46). Local progression included relapse after a previous CR (n = 26 patients) or progression after a previous PR (n = 27 patients). No significant characteristics emerged from the analysis of patients with recurrence (Table S2). Of these 28 subjects, 13 received a second ECT and 9 (69%) achieved CR. Overall, 1- and 2-year local progression-free survival (LPFS) was 96% (95% C.I. 93–98%) and 90% (95% C.I. 86–94%), respectively (Figure 2). Fifteen patients (5%) died of other causes after a median of 14 months (range 2–28 months).
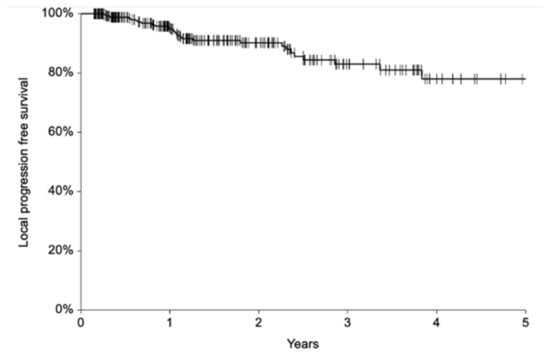
Figure 2.
Local progression-free survival.
4. Discussion
4.1. ECT Safety
The present registry-based study relies on the largest BCC series treated with ECT thus far and provides new critical insights into the application of this alternative nonsurgical therapy. Importantly, our findings reinforce the notion that ECT is a safe, although suboptimal, therapeutic option for BCC patients. Most participants reported no adverse events, and local toxicity ranged to 7%, mostly mild, consistent with outcomes reported with other skin-directed therapies [23]. In this regard, it is worth noting that preliminary evidence suggests that de-escalated doses of BLM or the use of calcium instead of chemotherapy may further reduce risk or even avert toxicity while preserving ECT efficacy [17].
4.2. Predictors of Response
Based on our results, ECT efficacy seems to be influenced by biological and technical factors. More than 80% of patients achieved a cure following a single course of treatment, and, interestingly, CR achievement correlated with BCC naïvety and the coverage of a deep tumour margin with electric pulses. These findings confirm the observations from a retrospective study in patients with head and neck cancers, where ECT demonstrated higher efficacy in chemo-naïve tumours [20]. In these patients, the absence of clone selection and preserved vasculature in radiotherapy-naïve tumours likely leads to unperturbed chemotherapy distribution and higher ECT efficacy.
As for the technical aspects, it should be acknowledged that electrode placement is operator-dependent and generally based only on clinical judgement; as such, uncertainty exists as to the depth and extension of electrode placement, also depending on BCC histological subtypes (Figure 3). This variation in treatment application needs to be addressed in future studies. Meanwhile, the predictive factors identified in this study can be helpful in clinical practice to select ECT candidates (i.e., patients with treatment-naïve BCC with tumours suitable for adequate electrode application) and inform clinicians regarding the need for retreatment based on intraoperative feedback (i.e., adequacy of electric fields on tumour margins). Additionally, they can support researchers in designing future studies in well-defined cohorts of patients.
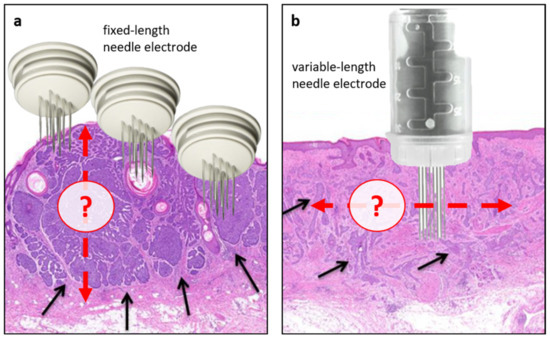
Figure 3.
Intraprocedural challenges of ECT in BCC. As a principle, effective ECT treatment delivery relies on adequate chemotherapy distribution within the tumour (following i.v. or i.t. administration) and complete coverage with electric fields (electroporation). In this regard, lower response rates are associated with previous radiotherapy (likely due to its effect on microcirculation) and the insufficient electroporation of tumour margins, an aspect closely associated with the modalities of electrode application. Generally, the extent and depth of electrode insertion are operator-dependent and based on clinical judgment. (a) An example of nodular BCC with well-circumscribed areas of cancer cells (black arrows) treated with a fixed-length electrode. In this case, the actual BCC depth may be underestimated, thus leaving deep portions of the tumour untreated. (b) An example of infiltrative BCC in which the actual extension of the tumour may be challenging to discriminate with potential repercussions on the coverage of lateral tumour margins. Red arrows indicate the potential depth (a) or extension (b) of electrode application.
In contrast to previous publications, the present analysis did not find that tumour size correlates with the response to ECT [8,18]. However, it should be noted that 90% of BCCs were <3 cm. Nonetheless, tumour size and head and neck location proved to be significant predictors of overall response (Table S1) and may still help refine patient selection and clinical decision-making in palliative cases.
4.3. Local Control
BCC is a slowly growing tumour; therefore, long-term patient outcome is essential in evaluating treatment efficacy. Critically, the recurrence rate at two years was 10%. This result is a suboptimal outcome compared with conventional treatments and necessitates the extended follow-up of our patients. The five-year recurrent rate in primary BCC is 1–3% following Mohs surgery and 8–10% following excision or radiotherapy. In recurrent BCC, 5-year recurrence rates are 5.6%, 17.4%, and 9.8%, respectively. However, the heterogeneity of our cohort (which included both primary and recurrent BCC, a small (10%) but not negligible proportion of tumours >3 cm, and 13% of patients treated with palliative intent due to frailty or comorbidities) makes it difficult to perform rigorous comparisons with other treatments.
Surprisingly, the variables associated with tumour clearance in multivariate analysis were not associated with local control (Table S2). Hence, we speculate that other factors may determine the sensitivity to bleomycin and ultimately dictate treatment outcomes in the intermediate term. These include (a) the low growth kinetics of BCC (bleomycin is selectively toxic to cells in the M and G2 phases of the cell cycle), (b) the fraction of hypoxic cells (oxygen is an essential substrate for bleomycin’s action), and (c) other biological determinants of bleomycin cytotoxicity (e.g., metabolic inactivation by bleomycin hydrolase, the integrity of the DNA repair systems). In this regard, the InspECT group is committed to investigating the biological determinants of response to ECT and has recently proposed a roadmap to pursue this goal [24].
4.4. Previous Clinical Experiences
Few studies have investigated ECT in BCC, broadly varying in design and patient characteristics (Table 5).

Table 5.
Published series on ECT in BCC (additional studies in unselected populations are available in Table S3).
4.5. Comparison with Other Treatments
Thus far, ECT has not been formally compared against other skin-directed nonsurgical therapies. However, the results from a 2014 meta-analysis in patients with cutaneous metastases (mainly melanoma and breast cancer) indicate similar efficacy and safety [23]. A recent trial randomised 99 patients with primary BCC between ECT with intratumoural bleomycin and standard surgical excision. At three years, the clearance rate was comparable (87.5% and 97.5%, respectively) [7]. Only two studies reported long-term outcomes, with 5-year local control rates of 70% and 87% [7,8]. Finally, some mixed series enrolled variable proportions of BCC patients (Table S3). Among these, the IMI-GIDO (n = 24 BCC patients), EURECA (n = 34), and InspECT (n = 298) study, which reported clearance rates of 67%, 91%, and 71%, respectively [7,18,19].
4.6. The Role of ECT in Locally Advanced BCC
Interestingly, ECT was also effective in cases with BCC larger than 3 cm, of which a representative patient is presented in Supplementary Figure S1. Advanced disease may be a novel investigational area of research for ECT users. Although relatively rare, locally advanced BCC is a therapeutic challenge, and radical surgical resection may result in substantial deformity or morbidity. In this context, oral hedgehog inhibitors are the standard of care, having demonstrated remarkable efficacy, with response rates ranging from 57% to 69% in the most recent pooled analyses [25]. Nonetheless, toxicity (e.g., muscle spasms, dysgeusia, alopecia, and gastrointestinal symptoms) often imposes adjustments to the treatment schedule [1]. Interestingly, although preliminary, the favourable results reported with ECT in locally advanced BCC provide the rationale for investigating combined strategies with systemic treatment, where local therapy may help consolidate response, manage recurrences, or maintain local control during ‘drug holidays’ [8,18].
4.7. The Role of ECT in an Ageing Population
Nowadays, an increasing proportion of BCC patients are part in the elderly population, where performance status, comorbidities and BCC multifocality may prevent demanding treatments. On this note, the patients included in the present study were deemed unfit for more invasive options, and 13% were treated with palliative intent, as reflected by their short survival. Interestingly, ECT is a well-tolerated and highly effective option, even in the oldest-old population [26].
4.8. Study Limitations
This report has limitations. The first is the single-arm design. The second is a selection bias due to a lack of data from patients with worse outcomes. Third, the heterogeneity of patients limits the generalisation of findings. Another drawback is the lack of stratification according to BCC histological subtypes because aggressive histology has been reported to affect ECT efficacy [8]. Furthermore, no biopsy was performed for response verification nor the assessment of patient-rated cosmetic outcomes. Notwithstanding, we believe that a subset of well-selected subjects benefits from ECT. This seems to be a safe alternative when standard treatment is not practicable due to patient refusal/comorbidities or tumour characteristics (location, size, or multifocality) [8,26]. Notably, the ECT procedure is standardised and straightforward; unlike radiotherapy, outcomes can be compensated by salvage treatments. In the era of minimally invasive surgery, this treatment could be an ideal choice in the effort to minimise the extent of surgical intervention. At the same time, as reported by the National Institute for Health and Care Excellence (NICE) and a recent meta-analysis, the current level of evidence remains low in quantity and quality [27,28], and the role of ECT in current guidelines is marginal [29]. Hence, there is a need for standardisation and continuous rigorous evaluation through comprehensive data collection and the monitoring of indications, outcomes, and costs [30,31]. Additionally, the understaging of the disease is a potential risk with ECT because of the absence of a surgical specimen in most procedures; thus, judicious application and postoperative monitoring are mandatory. Finally, the cost to the health systems of treating BCC, mainly when located on the face, is high; multidisciplinary teams need to be aware of an increasing number of options, their results, and their cost [32].
5. Conclusions
Based on the illustrated data, the proposed ECT indications can be summarised as follows: (1) treatment of low-risk BCC in strictly selected subgroups (patients with multiple BCC (e.g., Gorlin–Goltz syndrome), unfit for surgical treatment because of their age or comorbidities; (2) organ-preserving treatment of small-sized high-risk BCC located in delicate anatomical areas (e.g., eyelid, nose, and auricle) in patients unfit/unwilling surgical treatment to preserve patient functioning (in this subgroup, however, recurrent status or aggressive histotype deserves a note of caution); (3) ancillary treatment of locally advanced BCC in conjunction with systemic treatment (treatment of patients with near-complete response, local recurrence, patients needing "drug holidays" from systemic treatment because of intolerance/toxicity).
Many clinical challenges and questions remain. Moving forward, we envision the following line of investigation and possible improvements: (1) de-escalation of the bleomycin dose to reduce dermatologic toxicity and consolidate tolerability [17]; (2) use of calcium instead of bleomycin (based on positive accumulating evidence on the efficacy and safety of calcium electroporation) [33]; (3) identification of the best strategies for ECT use in combination with immunotherapy or targeted therapy; (4) assessment of the quality of life and aesthetic outcomes.
In conclusion, there is no convincing evidence to indicate that ECT is associated with better outcomes when compared with standard treatment in BCC. Therefore, patients with localised disease should undergo excisional surgery whenever feasible. However, when excision is not viable, ECT is a safe and reasonably effective alternative with a higher chance of success in treatment-naïve individuals whose tumours are entirely covered with electric pulses. These predictive factors may help ECT users refine patient selection and design future studies. International databases and investigation of biological determinants of response will be mandatory to further advances.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/curroncol29080423/s1, Figure S1: Primary locally advanced BCC in a 96-year-old patient treated with ECT under general anaesthesia; Table S1: Predictors of objective response; Table S2: Patient characteristics according to development or not of local recurrence; Table S3: Clinical studies on ECT in unselected cancer populations, including patients with BCC [34,35,36,37].
Author Contributions
Conceptualisation, G.B., T.M. and A.G.; Patient treatment and follow-up, G.B., T.M., J.O., A.G., R.M., P.C., E.K., C.K.L., J.C., P.Q., C.K., V.S. and L.G.C.; methodology, F.d.T., R.S. and L.G.C.; formal analysis, G.B., T.M. and F.d.T.; data curation, F.d.T. and R.S.; writing—original draft preparation, G.B., T.M. and J.O.; writing—review and editing, A.G., R.M. and L.G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the National Medical Ethics Committee of the Republic of Slovenia (Protocol No. 102/09/14).
Informed Consent Statement
Written informed consent was obtained from the patient to publish this paper.
Data Availability Statement
The dataset generated during the current study is available from the corresponding author upon reasonable request.
Acknowledgments
InspECT BCC Working Group: Silvia Di Felice (University of Pavia, Italy), Graeme Moir (Barts Health NHS Trust, London, U.K.), Antonio Orlando (North Bristol NHS Trust, Bristol, U.K.), and Rowan Pritchard-Jones (Whiston Hospital, Prescot, U.K.). The authors would like to thank Alberto Tonelli for assisting with patient management. In the preparation of this manuscript, the authors followed the S-PRINT (Sustainable Paperless Reference Initiative Nourishes Trees) recommendation, printing 3 of 27 papers on the reference list (S-PRINT score: 11%) (https://twitter.com/LucaCampana_611/status/1140741813277483008 accessed on: 20 May 2022).
Conflicts of Interest
The InspECT database is hosted by IGEA S.p.A. (Carpi, MO, Italy) but administered by an independent board. FdT is an IGEA employee. GB, TM, AG, PC, RC, EK, AJPC, GM, JO, PQ, AO, CK, and LGC have received previous travel grants from IGEA unrelated to the present study. SDF, RM, CKL, and SHL have no conflict of interest to declare.
References
- Migden, M.R.; Chang, A.L.S.; Dirix, L.; Stratigos, A.J.; Lear, J.T. Emerging trends in the treatment of advanced basal cell carcinoma. Cancer Treat. Rev. 2018, 64, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, C.M. Mohs’ micrographic surgery for basal cell carcinoma: Mohs’ surgery for BCC. Clin. Exp. Dermatol. 1999, 24, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Stratigos, A.J.; Sekulic, A.; Peris, K.; Bechter, O.; Prey, S.; Kaatz, M.; Lewis, K.D.; Basset-Seguin, N.; Chang, A.L.; Dalle, S.; et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2021, 22, 848–857. [Google Scholar] [CrossRef]
- Campana, L.G.; Miklavčič, D.; Bertino, G.; Marconato, R.; Valpione, S.; Imarisio, I.; Dieci, M.V.; Granziera, E.; Cemazar, M.; Alaibac, M.; et al. Electrochemotherapy of superficial tumours—Current status. Semin. Oncol. 2019, 46, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Jarm, T.; Cemazar, M.; Miklavcic, D.; Sersa, G. Antivascular effects of electrochemotherapy: Implications in treatment of metastases. Expert. Rev. Anticance. Ther. 2012, 10, 729–746. [Google Scholar] [CrossRef]
- Clover, A.J.P.; de Terlizzi, F.; Bertino, G.; Curatolo, P.; Odili, J.; Campana, L.G.; Kunte, C.; Muir, T.; Brizio, M.; Sersa, G. Electrochemotherapy in the treatment of cutaneous malignancy: Outcomes and subgroup analysis from the cumulative results from the pan-European International Network for Sharing Practice in Electrochemotherapy database for 2482 lesions in 987 patients (2008–2019). Eur. J. Cancer 2020, 138, 30–40. [Google Scholar] [CrossRef]
- Clover, A.J.P.; Salwa, S.P.; Bourke, M.G.; McKiernan, J.; Forde, P.F.; O’Sullivan, S.T.; Kelly, E.J.; Soden, D.M. Electrochemotherapy for the treatment of primary basal cell carcinoma; A randomised control trial comparing electrochemotherapy and surgery with five-year follow-up. Eur. J. Surg. Oncol. 2020, 46, 847–854. [Google Scholar] [CrossRef]
- Campana, L.G.; Marconato, R.; Valpione, S.; Galuppo, S.; Alaibac, M.; Rossi, C.R.; Mocellin, S. Basal cell carcinoma: 10-year experience with electrochemotherapy. J. Transl. Med. 2017, 15, 122. [Google Scholar] [CrossRef]
- Kis, E.G.; Baltás, E.; Ócsai, H.; Vass, A.; Németh, I.B.; Varga, E.; Oláh, J.; Kemény, L.; Tóth-Molnár, E. Electrochemotherapy in the treatment of locally advanced or recurrent eyelid-periocular basal cell carcinomas. Sci. Rep. 2019, 9, 4285. [Google Scholar] [CrossRef]
- Ruggeri, R.; Maurichi, A.; Tinti, M.C.; Cadenelli, P.; Patuzzo, R.; Gallino, G.; Santinami, M. Electrochemotherapy: A good idea in recurrent basal cell carcinoma treatment. Melanoma Manag. 2015, 2, 27–31. [Google Scholar] [CrossRef]
- Salwa, S.P.; Bourke, M.G.; Forde, P.F.; O’Shaughnessy, M.; O’Sullivan, S.T.; Kelly, E.J.; Soden, D.M.; Clover, A.J.P. Electrochemotherapy for the treatment of ocular basal cell carcinoma; a novel adjunct in the disease management. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 403–406. [Google Scholar] [CrossRef]
- Gatti, A.; Stinco, G.; di Meo, N.; Noal, C.; Maione, M.; Trevisan, G. Electrochemotherapy for a locally advanced basal cell carcinoma on the forehead. Indian J. Derm. Venereol. Leprol. 2014, 80, 378–380. [Google Scholar] [CrossRef]
- Kis, E.; Baltás, E.; Kinyó, Á.; Varga, E.; Nagy, N.; Gyulai, R.; Kemény, L.; Oláh, J. Successful Treatment of Multiple Basaliomas with Bleomycin-based Electrochemotherapy: A Case Series of Three Patients with Gorlin-Goltz Syndrome. Acta Derm. Venereol. 2012, 92, 648–651. [Google Scholar] [CrossRef]
- Fantini, F.; Gualdi, G.; Cimitan, A.; Giannetti, A. Metastatic Basal Cell Carcinoma with Squamous Differentiation: Report of a Case With Response of Cutaneous Metastases to Electrochemotherapy. Arch. Dermatol. 2008, 144, 1186–1188. [Google Scholar] [CrossRef][Green Version]
- Glass, L.F.; Jaroszeski, M.; Gilbert, R.; Reintgen, D.S.; Heller, R. Intralesional bleomycin-mediated electrochemotherapy in 20 patients with basal cell carcinoma. J. Am. Acad. Dermatol. 1997, 37, 596–599. [Google Scholar] [CrossRef]
- Glass, L.F.; Fenske, N.A.; Jaroszeski, M.; Perrott, R.; Harvey, D.T.; Reintgen, D.S.; Heller, R. Bleomycin-mediated electrochemotherapy of basal cell carcinoma. J. Am. Acad. Dermatol. 1996, 34, 82–86. [Google Scholar] [CrossRef]
- Rotunno, R.; Campana, L.G.; Quaglino, P.; de Terlizzi, F.; Kunte, C.; Odili, J.; Gehl, J.; Ribero, S.; Liew, S.H.; Marconato, R.; et al. Electrochemotherapy of unresectable cutaneous tumours with reduced dosages of intravenous bleomycin: Analysis of 57 patients from the International Network for Sharing Practices of Electrochemotherapy registry. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1147–1154. [Google Scholar] [CrossRef]
- Bertino, G.; Sersa, G.; de Terlizzi, F.; Occhini, A.; Plaschke, C.C.; Groselj, A.; Langdon, C.; Grau, J.J.; McCaul, J.A.; Heuveling, D.; et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: Results of the treatment of skin cancer. Eur. J. Cancer 2016, 63, 41–52. [Google Scholar] [CrossRef]
- Campana, L.G.; Testori, A.; Curatolo, P.; Quaglino, P.; Mocellin, S.; Framarini, M.; Borgognoni, L.; Ascierto, P.A.; Mozzillo, N.; Guida, M. Treatment efficacy with electrochemotherapy: A multi-institutional prospective observational study on 376 patients with superficial tumours. Eur. J. Surg. Oncol. 2016, 42, 1914–1923. [Google Scholar] [CrossRef]
- Campana, L.G.; Mali, B.; Sersa, G.; Valpione, S.; Giorgi, C.A.; Strojan, P.; Miklavcic, D.; Rossi, C.R. Electrochemotherapy in nonmelanoma head and neck cancers: A retrospective analysis of the treated cases. Br. J. Oral Maxillofac. Surg. 2014, 52, 957–964. [Google Scholar] [CrossRef]
- Mir, L.M.; Gehl, J.; Sersa, G.; Collins, C.G.; Garbay, J.R.; Billard, V.; Geertsend, P.F.; Rudolf, Z.; O’Sullivan, G.C.; Marty, M. Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses by the Cliniporator™ by means of invasive or non-invasive electrodes. Eur. J. Cancer Suppl. 2006, 4, 14–25. [Google Scholar] [CrossRef]
- Gehl, J.; Sersa, G.; Matthiessen, L.W.; Muir, T.; Soden, D.; Occhini, A.; Quaglino, P.; Curatolo, P.; Campana, L.G.; Kunte, C. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018, 57, 874–882. [Google Scholar] [CrossRef]
- Spratt, D.E.; Gordon Spratt, E.A.; Wu, S.; DeRosa, A.; Lee, N.Y.; Lacouture, M.E.; Barker, C.A. Efficacy of Skin-Directed Therapy for Cutaneous Metastases From Advanced Cancer: A Meta-Analysis. J. Clin. Oncol. 2014, 32, 3144–3155. [Google Scholar] [CrossRef]
- Sersa, G.; Ursic, K.; Cemazar, M.; Heller, R.; Bosnjak, M.; Campana, L.G. Biological factors of the tumour response to electrochemotherapy: Review of the evidence and a research roadmap. Eur. J. Surg. Oncol. 2021, 47, 1836–1846. [Google Scholar] [CrossRef]
- Jacobsen, A.A.; Aldahan, A.S.; Hughes, O.B.; Shah, V.V.; Strasswimmer, J. Hedgehog Pathway Inhibitor Therapy for Locally Advanced and Metastatic Basal Cell Carcinoma: A Systematic Review and Pooled Analysis of Interventional Studies. JAMA Dermatol. 2016, 152, 816–824. [Google Scholar] [CrossRef]
- Sersa, G.; Mascherini, M.; Di Prata, C.; Odili, J.; de Terlizzi, F.; McKenzie, G.A.G.; Clover, A.J.P.; Bertino, G.; Spina, R.; Groselj, A. Outcomes of older adults aged 90 and over with cutaneous malignancies after electrochemotherapy with bleomycin: A matched cohort analysis from the InspECT registry. Eur. J. Surg. Oncol. 2021, 47, 902–912. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Electrochemotherapy for Primary Basal Cell Carcinoma and Primary Squamous Cell Carcinoma. Guidance and Guidelines. 2014. Available online: https://www.nice.org.uk/guidance/ipg478/chapter/7-Further-information (accessed on 20 April 2022).
- Hendel, K.; Jemec, G.B.E.; Haedersdal, M.; Wiegell, S.R. Electrochemotherapy with bleomycin for basal cell carcinomas: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2208–2215. [Google Scholar] [CrossRef]
- Nasr, I.; McGrath, E.J.; Harwood, C.A.; Botting, J.; Buckley, P.; Budny, P.G.; Fairbrother, P.; Fife, K.; Gupta, G.; Hashme, H.; et al. British Association of Dermatologists guidelines for the management of adults with basal cell carcinoma 2021. Br. J. Dermatol. 2021, 185, 899–920. [Google Scholar] [CrossRef]
- Campana, L.G.; Clover, A.J.; Valpione, S.; Quaglino, P.; Gehl, J.; Kunte, C.; Snoj, M.; Cemazar, M.; Rossi, C.R.; Miklavcic, D.; et al. Recommendations for improving the quality of reporting clinical electrochemotherapy studies based on qualitative systematic review. Radiol. Oncol. 2016, 50, 1–13. [Google Scholar] [CrossRef]
- Lear, J.T.; Corner, C.; Dziewulski PFife, K.; Ross, G.L.; Varma, S.; Harwood, C.A. Challenges and new horizons in the management of advanced basal cell carcinoma: A UK perspective. Br. J. Cancer 2021, 14, 1476–1481. [Google Scholar] [CrossRef]
- Baxter, J.M.; Patel, A.N.; Varma, S. Facial basal cell carcinoma. BMJ 2012, 21, e5342. [Google Scholar] [CrossRef] [PubMed]
- Ágoston, D.; Baltás, E.; Ócsai, H.; Rátkai, S.; Lázár, P.G.; Korom, I.; Varga, E.; Németh, I.B.; Dósa-Rácz Viharosné, E.; Gehl, J.; et al. Evaluation of Calcium Electroporation for the Treatment of Cutaneous Metastases: A Double Blinded Randomised Controlled Phase II Trial. Cancers 2020, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, V.; Scarfì, F.; Saqer, E.; Gori, A.; Tomassini, G.M.; Covarelli, P. The use of cisplatin electrochemotherapy in nonmelanoma skin cancers: A single-center study. Dermatol. Ther. 2020, 33, e13547. [Google Scholar] [CrossRef] [PubMed]
- Montuori, M.; Santurro, L.; Feliziani, A.; Ricciardi, E.; Gaudio, D.; Campione, E.; Bianchi, L.; Silvi, M.B.; Rossi, P. Electrochemotherapy for basocellular and squamocellular head and neck cancer: Preliminary experience in Day Surgery Unit. G. Ital. Dermatol. Venereol. 2016, 153, 19–25. [Google Scholar] [CrossRef]
- Groselj, A.; Bosnjak, M.; Strojan, P.; Krzan, M.; Cemazar, M.; Sersa, G. Efficiency of electrochemotherapy with reduced bleomycin dose in the treatment of nonmelanoma head and neck skin cancer: Preliminary results. Head Neck 2018, 40, 120–125. [Google Scholar] [CrossRef]
- Rodríguez-Cuevas, S.; Barroso-Bravo, S.; Almanza-Estrada, J.; Cristóbal-Martínez, L.; González-Rodríguez, E. Electrochemotherapy in primary and metastatic skin tumors: Phase II trial using intralesional bleomycin. Arch. Med. Res. 2001, 32, 273–276. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).