Real-World Adherence to Toxicity Management Guidelines for Immune-Related Adverse Events
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
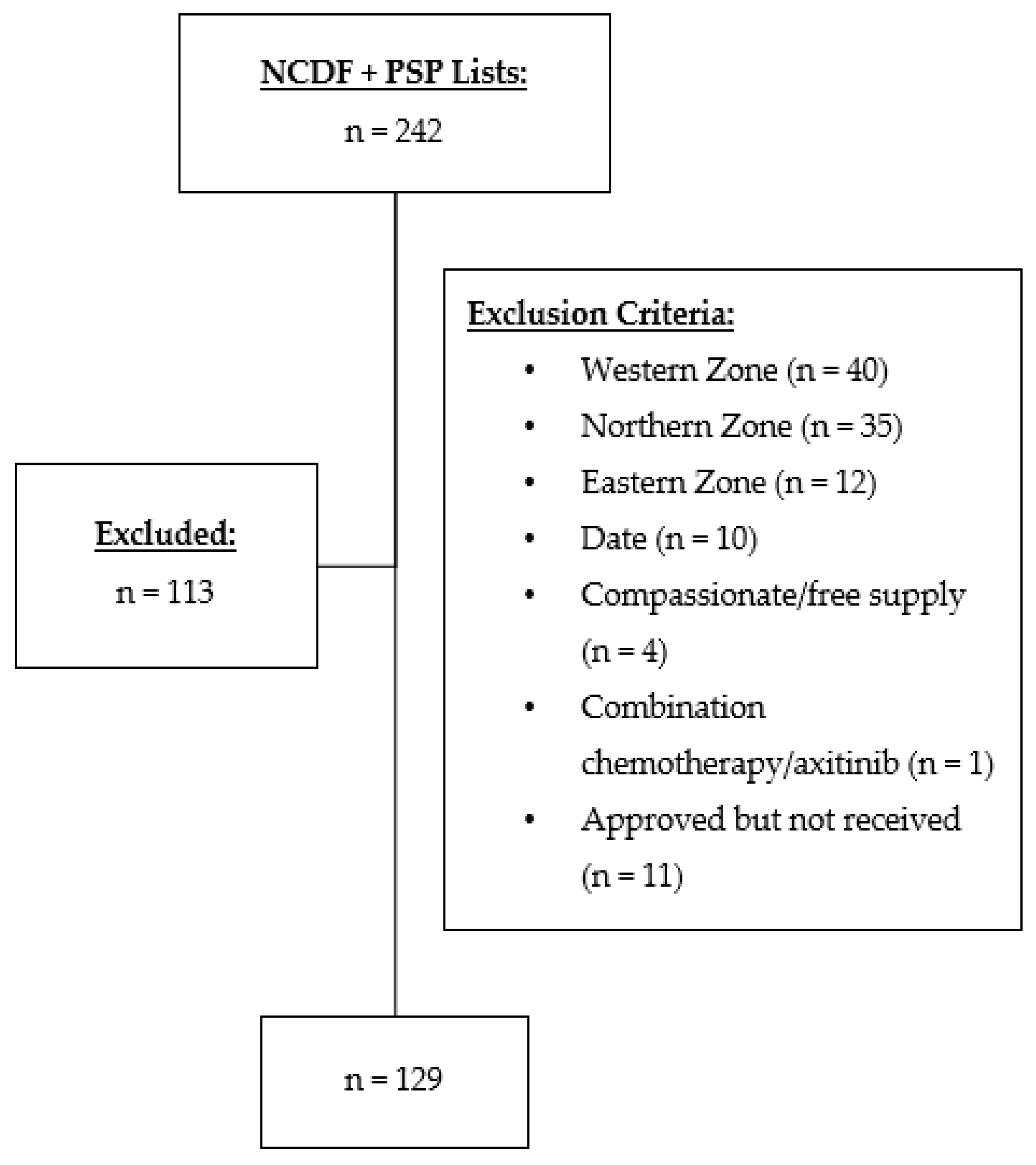
2.2. Participants
2.3. Outcomes
2.4. Data Collection and Analysis
3. Results
3.1. Patient Characteristics
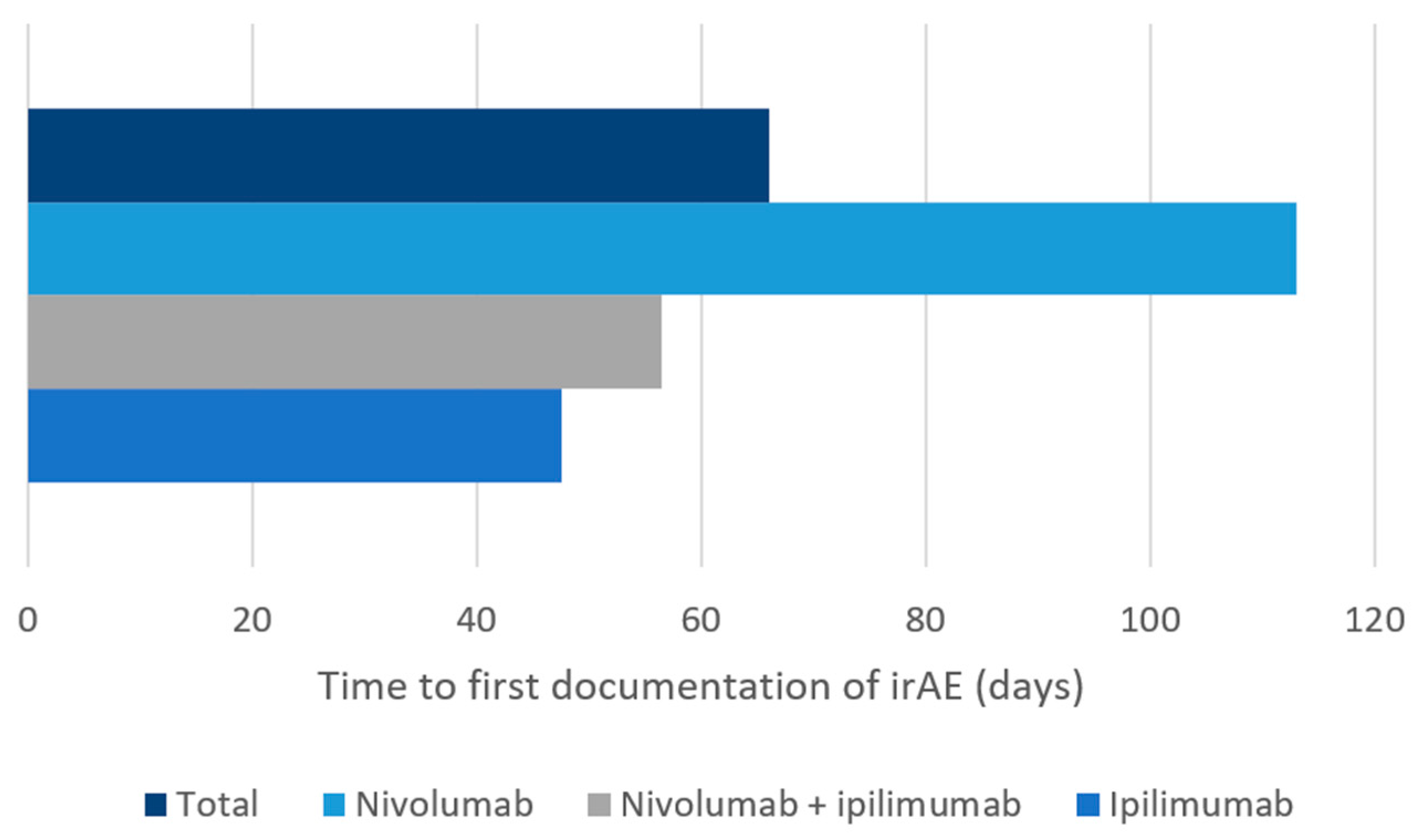
3.2. Characterization of Immune-Related Adverse Events
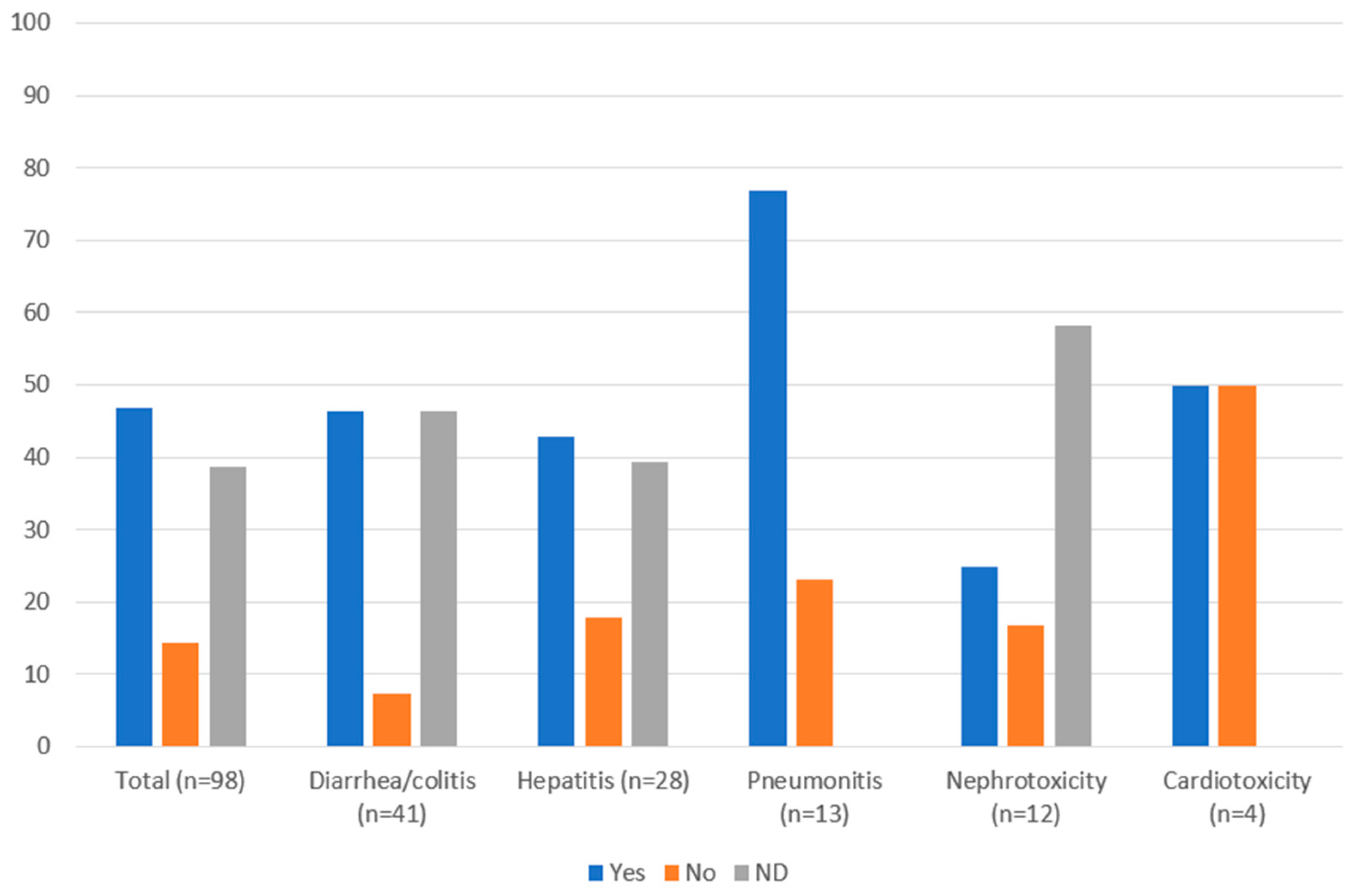
3.3. Management of Immune-Related Adverse Events
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| CCO [1] | ASCO [15] | ESMO [16] | NCCN [17] | BC Cancer [18] | |
|---|---|---|---|---|---|
| Publication Year | 2020 | 2018 | 2017 | 2019 | 2019 |
| Nationality | Canadian | American | European | American | Canadian |
| Guideline Type | Clinical Practice Guideline | Clinical Practice Guideline | Clinical Practice Guideline | Clinical Practice Guideline | Protocol Summary |
| Methodology | Multidisciplinary working group of oncology clinicians with immunotherapy experience. Based on best available evidence, current practice in Ontario, guidance from clinical experts, and working group consensus. | Multidisciplinary, multi-organizational panel of experts in medical oncology, dermatology, gastroenterology, rheumatology, pulmonology, endocrinology, urology, neurology, hematology, emergency medicine, nursing, trialist, and advocacy. A systematic review of the literature (focused on guidelines, systematic reviews and meta-analyses, randomized controlled trials, and case series published from 2000 through 2017) and an informal consensus process. | Developed in accordance with the ESMO standard operating procedures for Clinical Practice Guidelines development. The relevant literature has been selected by the expert authors. Levels of evidence and grades of recommendation have been applied. Statements without grading were considered justified standard clinical practice by the experts and the ESMO Faculty. | A statement of evidence and consensus of the authors regarding their views of currently accepted approaches to treatment. | A statement of concensus of BC Cancer professionals regarding their views of currently accepted approaches to treatment. |
| Diarrhea and Colitis | Management algorithm for diarrhea/colitis. Includes supportive therapy approaches. | Managament of colitis outlined in bullet point form. Diagnostic work-up and supportive therapy included. | Management algorithm for diarrhea/colitis. Other assessment and investigations included. | Management algorithm for diarrhea/colitis. Supportive therapy included. | Management algorithm for enterocolitis (diarrhea, abdominal pain, mucus, or blood in stools with or without fever, ileus, peritoneal signs). |
| Pneumonitisi | Management algorithm for pneumonitis. Includes supportive therapy approaches. | Managament of pneumonitis outlined in bullet point form. Diagnostic work-up and supportive therapy included. | Management algorithm for pneumonitis. Other assessment and investigations included. | Management algorithm for pneumonitis. Workup/supportive therapy included. | Management algorithm for pneumonitis (radiographic changes, new or worsening cough, chest pain, shortness of breath). |
| Hepatitis | Management algorithm for hepatitis. Includes supportive therapy approaches. | Managament for hepatitis outlined in bullet point form. Diagnostic work-up and supportive therapy included. | Management algorithm for hepatitis. Other assessment and investigations included. | Management algorithm for hepatic adverse events. Assessment/supportive therapy included. | Management algorithm for liver irAE (abnormal liver function test, jaundice, tiredness). |
| Nephrotoxicity | Management algorithm for renal toxicities. Includes supportive therapy approaches. | Management for nephritis and symptomatic nephritis outlined in bullet point form. Diagnostic work-up and supportive therapy included. | Management algorithm for nephritis. Other assessment and investigations included. | Management algorithm for renal adverse events (elevated serum creatinine/acute renal failure). Assessment/supportive therapy included. | Management algorithm for renal irAE (increase in serum creatinine, decreased urine output, hematuria, edema). |
| Cardiotoxicity | Brief review. | Management for cardiovascular toxicities outlined in bullet point form. Includes two sections: (1) myocarditis, pericarditis, arrhythmias, impaired ventricular function with heart failure and vasculitis, (2) venous thromboembolism. Diagnostic work-up and supportive therapy included. | Brief review. | Management algorithm for cardiovascular adverse events (myocarditis, pericarditis, arrhythmias, impaired ventricular function). Assessment/supportive therapy included. | Brief review of cardiovascular irAE (angiopathy, myositis, myocarditis, pericarditis, temporal arteritis, vasculitis). |
| Diarrhea /Colitis | Pneumonitis | Hepatitis | Nephrotoxicity | Cardiotoxicity | |
|---|---|---|---|---|---|
| Grade 1 | <4 stools/day above baseline | Asymptomatic; diagnostic radiological observations only; no intervention needed | AST/ALT up to 3 × ULN or total bilirubin up to 1.5 × ULN (for <2 × baseline) | Creatinine > 1–1.5 × ULN or 1.5 × baseline | Asymptomatic, creatine kinase or troponin elevation, abnormal ECG or findings consistent with pericarditis |
| Grade 2 | 4–6 stools above baseline, abdominal pain, mucus or blood in stool | Symptomatic; medical intervention indicated, limiting instrumental ADL | AST/ALT > 3–5 × ULN or total bilirubin > 1.5–3 × ULN (or >2 × baseline) | Creatinine > 1.5–3.0 × ULN or >1.5–3.0 × baseline | Mild symptoms or symptoms with moderate activity/ exertion, abnormal screening tests |
| Grade 3 | ≥7 stools/day above baseline, need for hospitalization for IV fluids ≥ 24 h | Severe symptoms; limiting self-care ADL; oxygen indicated | AST/ALT > 5–20 × ULN or total bilirubin > 3–10 × ULN | Creatinine > 3–6 × ULN or >3 × baseline | Symptoms at rest or minimal activity, cardiac biomarkers > ULN |
| Grade 4 | Grade 3 plus fever or peritoneal signs consistent with bowel perforation, or ileus, life-threatening | Life-threatening respiratory compromise; urgent intervention indicated (e.g., intubation and ventilation) | AST/ALT > 20 × ULN or total bilirubin > 10 × ULN | Creatinine > 6 × ULN | Moderate to severe decompensation, worsening signs and symptoms, cardiac biomarkers > 3 × ULN |
References
- Immune Checkpoint Inhibitor Toxicity Management: Clinical Practice Guideline. Toronto, (ON): Cancer Care Ontario. 2020. Available online: https://www.cancercareontario.ca/sites/ccocancercare/files/guidelines/full/ImmuneCheckpointInhibitor.pdf (accessed on 8 November 2020).
- Baxi, S.; Yang, A.; Gennarelli, R.L.; Khan, N.; Wang, Z.; Boyce, L.; Korenstein, D. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis. BMJ 2018, 360, k793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Velasco, G.; Je, Y.; Bossé, D.; Awad, M.M.; Ott, P.A.; Moreira, R.B.; Schutz, F.; Bellmunt, J.; Sonpavde, G.P.; Hodi, F.S.; et al. Comprehensive Meta-analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol. Res. 2017, 5, 312–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Chen, Y.-P.; Du, X.-J.; Liu, J.-Q.; Huang, C.-L.; Chen, L.; Zhou, G.-Q.; Li, W.-F.; Mao, Y.-P.; Hsu, C.; et al. Comparative safety of immune checkpoint inhibitors in cancer: Systematic review and network meta-analysis. BMJ 2018, 363, k4226. [Google Scholar] [CrossRef]
- Khoja, L.; Day, D.; Chen, T.W.-W.; Siu, L.L.; Hansen, A.R. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Ann. Oncol. 2017, 28, 2377–2385. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yao, Z.; Bai, H.; Duan, J.; Wang, Z.; Wang, X.; Zhang, X.; Xu, J.; Fei, K.; Zhang, Z.; et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitor-based combination therapies in clinical trials: A systematic review and meta-analysis. Lancet Oncol. 2021, 22, 1265–1274. [Google Scholar] [CrossRef]
- Yang, Y.; Jin, G.; Pang, Y.; Huang, Y.; Wang, W.; Zhang, H.; Tuo, G.; Wu, P.; Wang, Z.; Zhu, Z. Comparative Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Advanced Cancer: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Da, L.; Teng, Y.; Wang, N.; Zaguirre, K.; Liu, Y.; Qi, Y.; Song, F. Organ-Specific Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitor Monotherapy versus Combination Therapy in Cancer: A Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2020, 10, 1671. [Google Scholar] [CrossRef] [Green Version]
- Park, R.; Lopes, L.; Cristancho, C.R.; Riano, I.M.; Saeed, A. Treatment-Related Adverse Events of Combination Immune Checkpoint Inhibitors: Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 10, 258. [Google Scholar] [CrossRef] [Green Version]
- Almutairi, A.R.; McBride, A.; Slack, M.; Erstad, B.L.; Abraham, I. Potential Immune-Related Adverse Events Associated with Monotherapy and Combination Therapy of Ipilimumab, Nivolumab, and Pembrolizumab for Advanced Melanoma: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 10, 91. [Google Scholar] [CrossRef]
- Gu, L.; Khadaroo, P.A.; Su, H.; Kong, L.; Chen, L.; Wang, X.; Li, X.; Zhu, H.; Zhong, X.; Pan, J.; et al. The safety and tolerability of combined immune checkpoint inhibitors (anti-PD-1/PD-L1 plus anti-CTLA-4): A systematic review and meta-analysis. BMC Cancer 2019, 19, 559. [Google Scholar] [CrossRef] [Green Version]
- Salem, J.E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.-P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raschi, E.; Mazzarella, A.; Antonazzo, I.C.; Bendinelli, N.; Forcesi, E.; Tuccori, M.; Moretti, U.; Poluzzi, E.; De Ponti, F. Toxicities with Immune Checkpoint Inhibitors: Emerging Priorities From Disproportionality Analysis of the FDA Adverse Event Reporting System. Target. Oncol. 2019, 14, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.; Peters, S.; Larkin, J.; Jordan, K. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. S4), iv119–iv142. [Google Scholar] [CrossRef]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Andrews, S.; Armand, P.; Bhatia, S.; Budde, L.E.; Costa, L.; Davies, M.; Dunnington, D.; et al. Management of Immunotherapy-Related Toxicities, Version 1.2019. J. Natl. Compr. Cancer Netw. 2019, 17, 255–289. [Google Scholar] [CrossRef] [Green Version]
- BC Cancer Protocol Summary for Management of Immune-Mediated Adverse Reactions to Checkpoint Inhibitors Immunotherapy. (BC): BC Cancer. 2019. Available online: http://www.bccancer.bc.ca/chemotherapy-protocols-site/Documents/Supportive%20Care/SCIMMUNE_Protocol.pdf (accessed on 8 November 2020).
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. U.S. Department of Health and Wellness. National Institutes of Health. National Cancer Institute. 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 15 September 2021).
- De Felice, K.M.; Gupta, A.; Rakshit, S.; Khanna, S.; Kottschade, L.A.; Finnes, H.D.; Papadakis, K.A.; Loftus, E.; Raffals, L.E.; Markovic, S.N. Ipilimumab-induced colitis in patients with metastatic melanoma. Melanoma Res. 2015, 25, 321–327. [Google Scholar] [CrossRef]
- Waljee, A.K.; Rogers, M.A.; Lin, P.; Singal, A.G.; Stein, J.; Marks, R.M.; Ayanian, J.Z.; Nallamothu, B.K. Short term use of oral corticosteroids and related harms among adults in the United States: Population based cohort study. BMJ 2017, 357, j1415. [Google Scholar] [CrossRef] [Green Version]
- Eggermont, A.M.M.; Kicinski, M.; Blank, C.U.; Mandala, M.; Long, G.; Atkinson, V.; Dalle, S.; Haydon, A.; Khattak, A.; Carlino, M.S.; et al. Association between Immune-Related Adverse Events and Recurrence-Free Survival among Patients with Stage III Melanoma Randomized to Receive Pembrolizumab or Placebo: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2020, 6, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Faje, A.T.; Lawrence, D.; Flaherty, K.; Rn, C.F.; Fadden, R.; Rubin, K.; Cohen, J.; Sullivan, R.J. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018, 124, 3706–3714. [Google Scholar] [CrossRef] [Green Version]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, K.; Elkrief, A.; Calabrese, C.; Lapointe, R.; Hudson, M.; Routy, B.; Miller, W.H., Jr.; Calabrese, L. Moving towards personalized treatments of immune-related adverse events. Nat. Rev. Clin. Oncol. 2020, 17, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Dougan, M.; Wang, Y.; Rubio-Tapia, A.; Lim, J.K. AGA Clinical Practice Update on Diagnosis and Management of Immune Checkpoint Inhibitor Colitis and Hepatitis: Expert Review. Gastroenterology 2021, 160, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Muntyanu, A.; Netchiporouk, E.; Gerstein, W.; Gniadecki, R.; Litvinov, I.V. Cutaneous Immune-Related Adverse Events (irAEs) to Immune Checkpoint Inhibitors: A Dermatology Perspective on Management [Formula: See text]. J. Cutan. Med. Surg. 2021, 25, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Higham, C.E.; Olsson-Brown, A.; Carroll, P.; Cooksley, T.; Larkin, J.; Lorigan, P.; Morganstein, D.; Trainer, P.J. Society for Endocrinology Endocrine Emergency Guidance: Acute management of the endocrine complications of checkpoint inhibitor therapy. Endocr. Connect. 2018, 7, G1–G7. [Google Scholar] [CrossRef]
- Kostine, M.; Finckh, A.; Bingham, C.O.; Visser, K.; Leipe, J.; Schulze-Koops, H.; Choy, E.H.; Benesova, K.; Radstake, T.; Cope, A.P.; et al. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Ann. Rheum. Dis. 2021, 80, 36–48. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.T.; Molina, G.E.; Lo, J.A.; Durbin, S.; Cohen, J.V.; Reynolds, K.L.; Kroshinsky, D. Dermatology consultation reduces interruption of oncologic management among hospitalized patients with immune-related adverse events: A retrospective cohort study. J. Am. Acad. Dermatol. 2020, 82, 994–996. [Google Scholar] [CrossRef] [Green Version]
| Characteristic | n = 129 |
|---|---|
| Age (mean ± SD) | 64 ± 11 |
| Sex [n (%)] | |
| Male | 84 (65.1) |
| Female | 45 (34.9) |
| Cancer Type [n (%)] | |
| Melanoma | 32 (24.8) |
| Renal Cell Carcinoma | 38 (29.5) |
| Non-Small Cell Lung Cancer | 51 (39.5) |
| Squamous Cell Carcinoma of Head and Neck | 8 (6.2) |
| Drug [n (%)] | |
| Nivolumab + Ipilimumab | 41 (31.8) |
| Nivolumab | 78 (60.5) |
| Ipilimumab | 10 (7.7) |
| Dosing [n (%)] | |
| Nivolumab 1 mg/kg + Ipilimumab 3 mg/kg | 17 (13.2) |
| Nivolumab 3 mg/kg + Ipilimumab 1 mg/kg | 24 (18.6) |
| Nivolumab 3 mg/kg | 19 (14.7) |
| Nivolumab 6 mg/kg | 7 (5.4) |
| Nivolumab Fixed Dosing a | 52 (40.3) |
| Ipilimumab 3 mg/kg | 10 (7.8) |
| Number of Cycles (median ± IQR) b | |
| Nivolumab + Ipilimumab | 4 ± 10 |
| Nivolumab | 4 ± 5.8 |
| Ipilimumab | 3.5 ± 1 |
| Drug | irAE Likelihood (n [%]) | ER Visit (n [%]) | ||
|---|---|---|---|---|
| Definitely | Probably | Possibly | ||
| Total (n = 98) | 25 (25.5) | 8 (8.2) | 65 (66.3) | 33 (33.7) |
| Nivolumab + Ipilimumab (n = 42) | 20 (47.6) | 6 (14.3) | 16 (38.1) | 22 (52.4) |
| Nivolumab (n = 48) | 4 (8.3) | 2 (4.2) | 42 (87.5) | 10 (20.8) |
| Ipilimumab (n = 8) | 1 (12.5) | 0 | 7 (87.5) | 1 (12.5) |
| Drug | Grade n (%) | ER Visit n (%) | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Diarrhea/Colitis | |||||
| Total (n = 41) | 25 (60.9) | 10 (24.4) | 5 (12.2) | 1 (2.4) | 12 (29.3) |
| Nivolumab + Ipilimumab (n = 17) | 4 (23.5) | 7 (41.2) | 5 (29.4) | 1 (5.9) | 11 (64.7) |
| Nivolumab (n = 21) | 18 (85.7) | 3 (14.3) | 0 | 0 | 1 (4.8) |
| Ipilimumab (n = 3) | 3 (100) | 0 | 0 | 0 | 0 |
| Hepatitis | |||||
| Total (n = 28) | 18 (64.3) | 5 (17.8) | 4 (14.3) | 1 (3.6) | 6 (21.4) |
| Nivolumab + Ipilimumab (n = 15) | 7 (46.7) | 3 (20.0) | 4 (26.7) | 1 (6.6) | 5 (33.3) |
| Nivolumab (n = 9) | 8 (88.9) | 1 (11.1) | 0 | 0 | 1 (11.1) |
| Ipilimumab (n = 4) | 3 (75.0) | 1 (25.0) | 0 | 0 | 0 |
| Pneumonitis a | |||||
| Total (n = 13) | 2 (15.4) | 8 (61.5) | 3 (23.1) | 0 | 7 (53.8) |
| Nivolumab + Ipilimumab (n = 4) | 1 (25.0) | 2 (50.0) | 1 (25.0) | 0 | 1 (25.0) |
| Nivolumab (n = 9) | 1 (11.1) | 6 (66.7) | 2 (22.2) | 0 | 6 (66.6) |
| Nephrotoxicity | |||||
| Total (n = 12) | 7 (58.3) | 5 (41.7) | 0 | 0 | 4 (33.3) |
| Nivolumab + Ipilimumab (n = 4) | 1 (25.0) | 3 (75.0) | 0 | 0 | 3 (75.0) |
| Nivolumab (n = 7) | 6 (85.7) | 1 (14.3) | 0 | 0 | 0 |
| Ipilimumab (n = 1) | 0 | 1 (100) | 0 | 0 | 1 (100) |
| Cardiotoxicity a | |||||
| Total (n = 4) | 1 (25.0) | 0 | 0 | 3 (75.0) | 4 (100) |
| Nivolumab + Ipilimumab (n = 2) | 1 (50.0) | 0 | 0 | 1 (50.0) | 2 (100) |
| Nivolumab (n = 2) | 0 | 0 | 0 | 2 (100) | 2 (100) |
| n = 129 | |
|---|---|
| Discontinuation [n (%)] | 68 (52.7) |
| Inefficacy/Disease Progression | 38 (55.9) |
| Toxicity (irAE of interest) a | 16 (23.5) |
| Pneumonitis | 8 (50.0) |
| Colitis | 7 (43.8) |
| Nephrotoxicity | 1 (6.25) |
| Toxicity (other) | 4 (5.9) |
| Other | 10 (14.7) |
| Death [n (%)] | 57 (44.2) |
| Disease Progression | 51 (89.5) |
| Toxicity (irAE of interest) a | 2 (3.5) |
| Hepatitis, nephrotoxicity, cardiotoxicity | 1 (50.0) |
| Colitis | 1 (50.0) |
| Toxicity (other) | 0 |
| Other | 4 (7.0) |
| Management (n [%]) | Diarrhea/Colitis (n = 41) | Hepatitis (n = 28) | Pneumonitis (n = 13) | Nephrotoxicity (n = 12) | Cardiotoxicity (n = 4) |
|---|---|---|---|---|---|
| Therapy continued and monitored | 7 (17.1) | 3 (10.7) | 2 (15.4) | 2 (16.7) | 0 |
| Steroids: | 13 (31.7) a | 8 (28.6) | 9 (69.2) | 3 (25.0) | 2 (50.0) |
| Prednisone 0.5–1 mg/kg/day b | 9 (22.0) | 4 (14.3) | 8 (61.5) | 1 (8.3) | 0 |
| Prednisone 2 mg/kg/day b | 1 (2.4) | 2 (7.1) | 1 (7.7) | 1 (8.3) | 2 (50.0) |
| Methylprednisolone 1–2 (or 2–4) mg/kg/day c | 5 (12.2) | 2 (7.1) | 0 | 1 (8.3) | 0 |
| Loperamide | 7 (17.1) | -- | -- | -- | -- |
| Oral/IV hydration | 6 (14.6) | -- | -- | 4 (33.3) | -- |
| Admitted to hospital | 9 (22.0) | 3 (10.7) | 5 (38.5) | 4 (33.3) | 3 (75) |
| Empiric antibiotic therapy | 5 (12.2) | -- | -- | -- | -- |
| Infliximab 5 mg/kg IV | 1 (2.4) | -- | 0 | -- | -- |
| Mycophenolate mofetil | -- | 2 (7.1) | -- | -- | -- |
| Therapy held and monitored | 4 (9.8) | 6 (21.4) | 6 (46.2) | 0 | 4 (100) |
| Therapy held until Grade 0–1 and prednisone < 7.5 mg/day (anti-CTLA-4) or <10 mg/day (anti-PD-1) | 8 (19.5) | 6 (21.4) | 3 (23.1) | 1 (8.3) | 0 |
| Permanently discontinued therapy d | 5 (12.2) | 3 (10.7) | 6 (46.1) | 2 (16.7) | 1 (25.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teimouri, A.; Minard, L.V.; Scott, S.N.; Daniels, A.; Snow, S. Real-World Adherence to Toxicity Management Guidelines for Immune-Related Adverse Events. Curr. Oncol. 2022, 29, 3104-3117. https://doi.org/10.3390/curroncol29050252
Teimouri A, Minard LV, Scott SN, Daniels A, Snow S. Real-World Adherence to Toxicity Management Guidelines for Immune-Related Adverse Events. Current Oncology. 2022; 29(5):3104-3117. https://doi.org/10.3390/curroncol29050252
Chicago/Turabian StyleTeimouri, Arezou, Laura V. Minard, Samantha N. Scott, Amanda Daniels, and Stephanie Snow. 2022. "Real-World Adherence to Toxicity Management Guidelines for Immune-Related Adverse Events" Current Oncology 29, no. 5: 3104-3117. https://doi.org/10.3390/curroncol29050252
APA StyleTeimouri, A., Minard, L. V., Scott, S. N., Daniels, A., & Snow, S. (2022). Real-World Adherence to Toxicity Management Guidelines for Immune-Related Adverse Events. Current Oncology, 29(5), 3104-3117. https://doi.org/10.3390/curroncol29050252






