Health Literacy and Clinical Trial Participation in French Cancer Patients: A National Survey
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
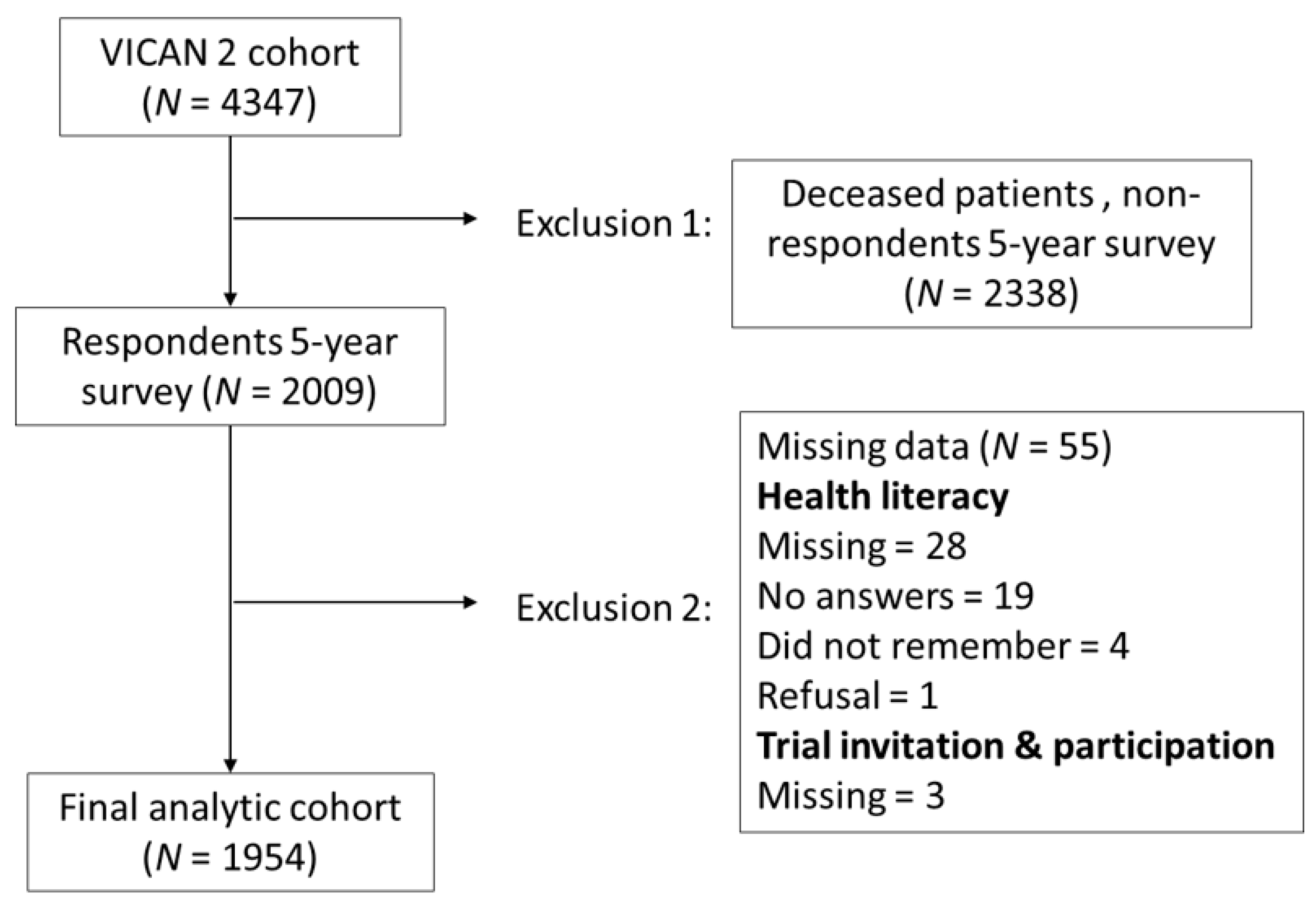
2.1. Study Design and Participants
2.2. Variables
2.2.1. Clinical Trial Invitation and Participation (Main Outcomes)
2.2.2. Independent Variables
2.3. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Djulbegovic, B.; Kumar, A.; Glasziou, P.P.; Perera, R.; Reljic, T.; Dent, L.; Raftery, J.; Johansen, M.; Di Tanna, G.L.; Miladinovic, B.; et al. New Treatments Compared to Established Treatments in Randomized Trials. Cochrane Database Syst. Rev. 2012, 10, MR000024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, I.; Chabannon, C.; Mancini, J.; Viret, F.; Vey, N.; Julian-Reynier, C. Contributing to Research via Biobanks: What It Means to Cancer Patients. Health Expect. Int. J. Public Particip. Health Care Health Policy 2014, 17, 523–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julian-Reynier, C.; Genève, J.; Dalenc, F.; Genre, D.; Monnier, A.; Kerbrat, P.; Largillier, R.; Serin, D.; Rios, M.; Roché, H.; et al. Assessment of Care by Breast Cancer Patients Participating or Not Participating in a Randomized Controlled Trial: A Report with the Patients’ Committee for Clinical Trials of the Ligue Nationale Contre Le Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 3038–3044. [Google Scholar] [CrossRef] [PubMed]
- Unger, J.M.; Cook, E.; Tai, E.; Bleyer, A. The Role of Clinical Trial Participation in Cancer Research: Barriers, Evidence, and Strategies. Am. Soc. Clin. Oncol. Educ. Book ASCO Am. Soc. Clin. Oncol. Meet. 2016, 35, 185–198. [Google Scholar] [CrossRef]
- Michaels, M.; Blakeney, N.; Langford, A.T.; Ford, M.E. Five Principles for Effective Cancer Clinical Trial Education within the Community Setting. J. Cancer Educ. Off. J. Am. Assoc. Cancer Educ. 2015, 30, 197–203. [Google Scholar] [CrossRef]
- Gross, C.P.; Filardo, G.; Mayne, S.T.; Krumholz, H.M. The Impact of Socioeconomic Status and Race on Trial Participation for Older Women with Breast Cancer. Cancer 2005, 103, 483–491. [Google Scholar] [CrossRef]
- Murthy, V.H.; Krumholz, H.M.; Gross, C.P. Participation in Cancer Clinical Trials: Race-, Sex-, and Age-Based Disparities. JAMA 2004, 291, 2720–2726. [Google Scholar] [CrossRef]
- Behrendt, C.E.; Hurria, A.; Tumyan, L.; Niland, J.C.; Mortimer, J.E. Socioeconomic and Clinical Factors Are Key to Uncovering Disparity in Accrual onto Therapeutic Trials for Breast Cancer. J. Natl. Compr. Cancer Netw. JNCCN 2014, 12, 1579–1585. [Google Scholar] [CrossRef] [Green Version]
- Ford, J.G.; Howerton, M.W.; Lai, G.Y.; Gary, T.L.; Bolen, S.; Gibbons, M.C.; Tilburt, J.; Baffi, C.; Tanpitukpongse, T.P.; Wilson, R.F.; et al. Barriers to Recruiting Underrepresented Populations to Cancer Clinical Trials: A Systematic Review. Cancer 2008, 112, 228–242. [Google Scholar] [CrossRef]
- Simon, M.S.; Du, W.; Flaherty, L.; Philip, P.A.; Lorusso, P.; Miree, C.; Smith, D.; Brown, D.R. Factors Associated with Breast Cancer Clinical Trials Participation and Enrollment at a Large Academic Medical Center. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004, 22, 2046–2052. [Google Scholar] [CrossRef]
- Mc Grath-Lone, L.; Day, S.; Schoenborn, C.; Ward, H. Exploring Research Participation among Cancer Patients: Analysis of a National Survey and an in-Depth Interview Study. BMC Cancer 2015, 15, 618. [Google Scholar] [CrossRef] [Green Version]
- Mosconi, P.; Roberto, A.; Cerana, N.; Colombo, N.; Didier, F.; D’Incalci, M.; Lorusso, D.; Peccatori, F.A.; Network of Clinicians and Participants (1). Knowledge and Attitudes towards Clinical Trials among Women with Ovarian Cancer: Results of the ACTO Study. J. Ovarian Res. 2022, 15, 45. [Google Scholar] [CrossRef]
- Kearns, C.; Feighery, R.; Mc Caffrey, J.; Higgins, M.; Smith, M.; Murphy, V.; O’Reilly, S.; Horgan, A.M.; Walshe, J.; McDermott, R.; et al. Understanding and Attitudes toward Cancer Clinical Trials among Patients with a Cancer Diagnosis: National Study through Cancer Trials Ireland. Cancers 2020, 12, 1921. [Google Scholar] [CrossRef]
- Rudnas, B.; Montanari, E.; Dall’Agata, M.; Petracci, E.; Nanni, O. Patients’ Understanding of Clinical Research: An Italian Cancer Patient Survey. Tumori J. 2019, 105, 31–37. [Google Scholar] [CrossRef]
- Sørensen, K.; Van den Broucke, S.; Fullam, J.; Doyle, G.; Pelikan, J.; Slonska, Z.; Brand, H. Health Literacy and Public Health: A Systematic Review and Integration of Definitions and Models. BMC Public Health 2012, 12, 80. [Google Scholar] [CrossRef] [Green Version]
- Muscat, D.M.; Smith, S.; Dhillon, H.M.; Morony, S.; Davis, E.L.; Luxford, K.; Shepherd, H.L.; Hayen, A.; Comings, J.; Nutbeam, D.; et al. Incorporating Health Literacy in Education for Socially Disadvantaged Adults: An Australian Feasibility Study. Int. J. Equity Health 2016, 15, 84. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, C.P.; Nápoles, A.M.; Narine, S.; Gregorich, S.; Livaudais-Toman, J.; Nguyen, T.; Leykin, Y.; Roach, M.; Small, E.J. Knowledge and Attitudes Regarding Clinical Trials and Willingness to Participate among Prostate Cancer Patients. Contemp. Clin. Trials 2015, 45, 443–448. [Google Scholar] [CrossRef]
- Halverson, J.; Martinez-Donate, A.; Trentham-Dietz, A.; Walsh, M.C.; Strickland, J.S.; Palta, M.; Smith, P.D.; Cleary, J. Health Literacy and Urbanicity among Cancer Patients. J. Rural Health Off. J. Am. Rural Health Assoc. Natl. Rural Health Care Assoc. 2013, 29, 392–402. [Google Scholar] [CrossRef] [Green Version]
- Sudore, R.L.; Mehta, K.M.; Simonsick, E.M.; Harris, T.B.; Newman, A.B.; Satterfield, S.; Rosano, C.; Rooks, R.N.; Rubin, S.M.; Ayonayon, H.N.; et al. Limited Literacy in Older People and Disparities in Health and Healthcare Access. J. Am. Geriatr. Soc. 2006, 54, 770–776. [Google Scholar] [CrossRef]
- Huang, L.C.; Ma, Y.; Ngo, J.V.; Rhoads, K.F. What Factors Influence Minority Use of National Cancer Institute-Designated Cancer Centers? Cancer 2014, 120, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Bennett, C.L.; Ferreira, M.R.; Davis, T.C.; Kaplan, J.; Weinberger, M.; Kuzel, T.; Seday, M.A.; Sartor, O. Relation between Literacy, Race, and Stage of Presentation among Low-Income Patients with Prostate Cancer. J. Clin. Oncol Off. J. Am. Soc. Clin. Oncol. 1998, 16, 3101–3104. [Google Scholar] [CrossRef] [PubMed]
- Bouhnik, A.-D.; Bendiane, M.-K.; Cortaredona, S.; Sagaon Teyssier, L.; Rey, D.; Berenger, C.; Seror, V.; Peretti-Watel, P.; members of VICAN Group. The Labour Market, Psychosocial Outcomes and Health Conditions in Cancer Survivors: Protocol for a Nationwide Longitudinal Survey 2 and 5 Years after Cancer Diagnosis (the VICAN Survey). BMJ Open 2015, 5, e005971. [Google Scholar] [CrossRef] [PubMed]
- Mancini, J.; Pellegrini, I.; Viret, F.; Vey, N.; Daufresne, L.-M.; Chabannon, C.; Julian-Reynier, C. Consent for Biobanking: Assessing the Understanding and Views of Cancer Patients. J. Natl. Cancer Inst. 2011, 103, 154–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ousseine, Y.M.; Durand, M.-A.; Bouhnik, A.-D.; Smith, A.B.; Mancini, J. Multiple Health Literacy Dimensions Are Associated with Physicians’ Efforts to Achieve Shared Decision-Making. Patient Educ. Couns. 2019, 102, 1949–1956. [Google Scholar] [CrossRef]
- Brice, J.H.; Foster, M.B.; Principe, S.; Moss, C.; Shofer, F.S.; Falk, R.J.; Ferris, M.E.; DeWalt, D.A. Single-Item or Two-Item Literacy Screener to Predict the S-TOFHLA among Adult Hemodialysis Patients. Patient Educ. Couns. 2014, 94, 71–75. [Google Scholar] [CrossRef]
- Powers, B.J.; Trinh, J.V.; Bosworth, H.B. Can This Patient Read and Understand Written Health Information? JAMA 2010, 304, 76–84. [Google Scholar] [CrossRef]
- Rey, G.; Jougla, E.; Fouillet, A.; Hémon, D. Ecological Association between a Deprivation Index and Mortality in France over the Period 1997–2001: Variations with Spatial Scale, Degree of Urbanicity, Age, Gender and Cause of Death. BMC Public Health 2009, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Huber, C.A.; Szucs, T.D.; Rapold, R.; Reich, O. Identifying Patients with Chronic Conditions Using Pharmacy Data in Switzerland: An Updated Mapping Approach to the Classification of Medications. BMC Public Health 2013, 13, 1030. [Google Scholar] [CrossRef] [Green Version]
- Baquet, C.R.; Ellison, G.L.; Mishra, S.I. Analysis of Maryland Cancer Patient Participation in National Cancer Institute-Supported Cancer Treatment Clinical Trials. J. Health Care Poor Underserved 2009, 20, 120–134. [Google Scholar] [CrossRef]
- Unger, J.M.; Hershman, D.L.; Fleury, M.E.; Vaidya, R. Association of Patient Comorbid Conditions with Cancer Clinical Trial Participation. JAMA Oncol. 2019, 5, 326–333. [Google Scholar] [CrossRef]
- Ousseine, Y.M.; Bouhnik, A.; Peretti-Watel, P.; Sarradon-Eck, A.; Memoli, V.; Bendiane, M.; Durand, M.; Mancini, J. The Impact of Health Literacy on Medico-social Follow-up Visits among French Cancer Survivors 5 Years after Diagnosis: The National VICAN Survey. Cancer Med. 2020, 9, 4185–4196. [Google Scholar] [CrossRef]
- The HLS19 Consortium of the WHO Action Network M-POHL. International Report on the Methodology, Results, and Recommendations of the European Health Literacy Population Survey 2019–2021 (HLS19) of M-POHL.; Austrian National Public Health Institute: Vienna, Austria, 2021; Available online: https://m-pohl.net/sites/m-pohl.net/files/inline-files/HLS19_International%20Report%20%28002%29_0.pdf (accessed on 3 March 2021).
- Jeppesen, K.M.; Coyle, J.D.; Miser, W.F. Screening Questions to Predict Limited Health Literacy: A Cross-Sectional Study of Patients with Diabetes Mellitus. Ann. Fam. Med. 2009, 7, 24–31. [Google Scholar] [CrossRef]
- Institut National Du Cancer. Bilan National Des Activités En Recherche Clinique 2003–2010; Collection Rapports & Synthèses; INCa: Boulogne-Billancourt, France, 2012. [Google Scholar]
- Chino, F.; Zafar, S.Y. Financial Toxicity and Equitable Access to Clinical Trials. Am. Soc. Clin. Oncol. Educ. Book Am. Soc. Clin. Oncol. Annu. Meet. 2019, 39, 11–18. [Google Scholar] [CrossRef]
- Colon-Otero, G.; Smallridge, R.C.; Solberg, L.A.; Keith, T.D.; Woodward, T.A.; Willis, F.B.; Dunn, A.N. Disparities in Participation in Cancer Clinical Trials in the United States. Cancer 2008, 112, 447–454. [Google Scholar] [CrossRef]
- Frérot, M.; Jooste, V.; Binquet, C.; Fournel, I.; Bedenne, L.; Bouvier, A.-M. Factors Influencing Inclusion in Digestive Cancer Clinical Trials: A Population-Based Study. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2015, 47, 891–896. [Google Scholar] [CrossRef]
- Kanarek, N.F.; Tsai, H.-L.; Metzger-Gaud, S.; Damron, D.; Guseynova, A.; Klamerus, J.F.; Rudin, C.M. Geographic Proximity and Racial Disparities in Cancer Clinical Trial Participation. J. Natl. Compr. Cancer Netw. 2010, 8, 1343–1351. [Google Scholar] [CrossRef]
- Vanderpool, R.C.; Kornfeld, J.; Mills, L.; Byrne, M.M. Rural-Urban Differences in Discussions of Cancer Treatment Clinical Trials. Patient Educ. Couns. 2011, 85, e69–e74. [Google Scholar] [CrossRef] [Green Version]
- Sharrocks, K.; Spicer, J.; Camidge, D.R.; Papa, S. The Impact of Socioeconomic Status on Access to Cancer Clinical Trials. Br. J. Cancer 2014, 111, 1684–1687. [Google Scholar] [CrossRef] [Green Version]
- Byrne, M.M.; Tannenbaum, S.L.; Glück, S.; Hurley, J.; Antoni, M. Participation in Cancer Clinical Trials: Why are Patients not Participating? Med. Decis. Mak. Int. J. Soc. Med. Decis. Mak. 2014, 34, 116–126. [Google Scholar] [CrossRef]
- Meropol, N.J.; Wong, Y.-N.; Albrecht, T.; Manne, S.; Miller, S.M.; Flamm, A.L.; Benson, A.B.; Buzaglo, J.; Collins, M.; Egleston, B.; et al. Randomized Trial of a Web-Based Intervention to Address Barriers to Clinical Trials. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 469–478. [Google Scholar] [CrossRef]
- Mancini, J.; Butow, P.N.; Julian-Reynier, C.; Dring, R.; Festy, P.; Fenaux, P.; Vey, N. Question Prompt List Responds to Information Needs of Myelodysplastic Syndromes Patients and Caregivers. Leuk. Res. 2015, 39, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kim, S.H. Simplification Improves Understanding of Informed Consent Information in Clinical Trials Regardless of Health Literacy Level. Clin. Trials Lond. Engl. 2015, 12, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Mancini, J.; Briggs, A.; Elkin, E.B.; Regan, J.; Hickey, C.; Targett, C.; Ager, R.; Masuda, S.; Bach, P.B.; Sabbatini, P.J. The Impact of Patient Education on Consideration of Enrollment in Clinical Trials. J. Community Support. Oncol. 2018, 16, e81–e88. [Google Scholar] [CrossRef]
- Torres, S.; de la Riva, E.E.; Tom, L.S.; Clayman, M.L.; Taylor, C.; Dong, X.; Simon, M.A. The Development of a Communication Tool to Facilitate the Cancer Trial Recruitment Process and Increase Research Literacy among Underrepresented Populations. J. Cancer Educ. Off. J. Am. Assoc. Cancer Educ. 2015, 30, 792–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Percac-Lima, S.; Ashburner, J.M.; Zai, A.H.; Chang, Y.; Oo, S.A.; Guimaraes, E.; Atlas, S.J. Patient Navigation for Comprehensive Cancer Screening in High-Risk Patients Using a Population-Based Health Information Technology System: A Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Hamel, L.M.; Penner, L.A.; Albrecht, T.L.; Heath, E.; Gwede, C.K.; Eggly, S. Barriers to Clinical Trial Enrollment in Racial and Ethnic Minority Patients with Cancer. Cancer Control 2016, 23, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Mancini, J.; Jansen, J.; Julian-Reynier, C.; Bechlian, D.; Vey, N.; Chabannon, C. Preferences of Older Adults with Cancer for Involvement in Decision-Making about Research Participation. J. Am. Geriatr. Soc. 2014, 62, 1191–1193. [Google Scholar] [CrossRef]
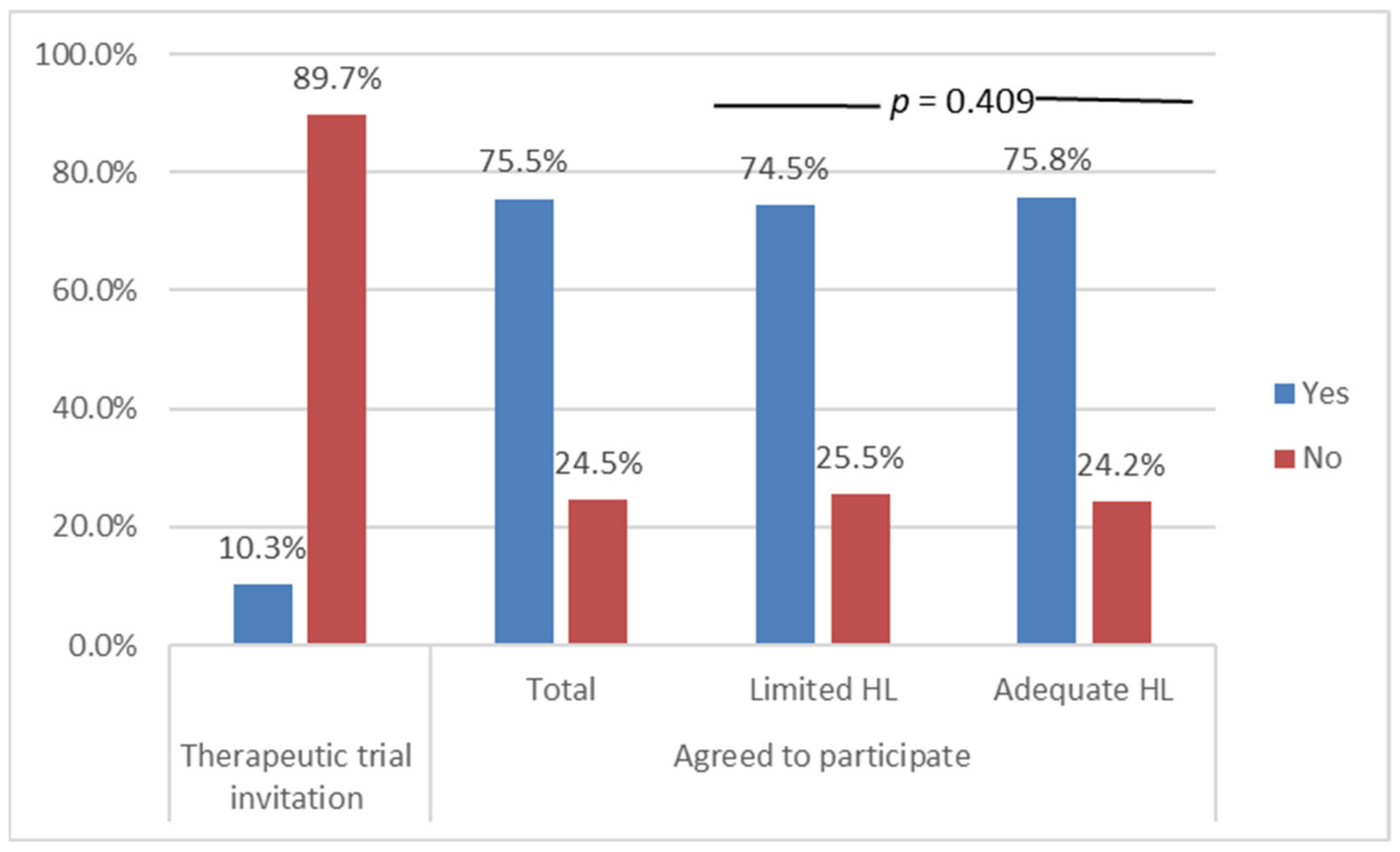
| Overall Population | Invited to Participate in a Clinical Trial | ||||
|---|---|---|---|---|---|
| % | No (89.7%)% | Yes (10.3%)% | p Value | ||
| Age at diagnosis (years) | ≤65 | 77.2 | 76.1 | 87.5 | <0.001 |
| >65 | 22.8 | 23.9 | 12.5 | ||
| Cancer management center | Private center | 50.8 | 52.5 | 36.0 | <0.001 |
| Comprehensive cancer center | 10.5 | 8.7 | 27.0 | ||
| Public academic or community center | 31.7 | 31.6 | 32.5 | ||
| Missing/unknown | 7.0 | 7.3 | 4.5 | ||
| Gender | Man | 35.9 | 37.4 | 23.0 | <0.001 |
| Woman | 65.1 | 62.6 | 77.0 | ||
| Education level | No diploma | 6.6 | 6.7 | 5.5 | 0.214 |
| <upper secondary school certificate | 43.0 | 43.5 | 38.2 | ||
| ≥upper secondary school certificate | 50.4 | 49.8 | 56.3 | ||
| Health literacy | Limited | 37.6 | 38.9 | 25.9 | 0.001 |
| Adequate | 62.4 | 61.1 | 74.1 | < | |
| Area of residence | Rural/Small town/city (<200,000 inhabitants | 65.9 | 67.0 | 56.5 | 0.005 |
| Large city (≥200,000 inhabitants) | 32.9 | 31.8 | 43.0 | ||
| Missing/unknown | 1.2 | 1.2 | 0.5 | ||
| Deprivation index | Low (<Q1) | 24.7 | 24.2 | 28.5 | 0.299 |
| Intermediate (Q1–Q3) | 50.3 | 50.3 | 50.5 | ||
| High (>Q3) | 24.5 | 24.9 | 21.0 | ||
| Missing/unknown | 0.5 | 0.6 | |||
| Financial resources | Low (<Q1) | 22.3 | 23.2 | 15.0 | 0.028 |
| Intermediate (Q1–Q3) | 46.5 | 46.4 | 47.0 | ||
| High (>Q3) | 24.6 | 23.9 | 31.0 | ||
| Missing/unknown | 6.6 | 6.5 | 7.0 | ||
| Cancer type | Breast | 43.4 | 41.9 | 56.2 | <0.001 |
| Lung | 3.6 | 3.2 | 7.5 | ||
| Prostate | 16.7 | 17.8 | 7.0 | ||
| Upper aero-digestive tract | 3.5 | 3.4 | 4.0 | ||
| Bladder | 2.9 | 3.3 | |||
| Kidney | 3.7 | 3.9 | 2.0 | ||
| Thyroid | 5.6 | 5.9 | 3.0 | ||
| Non-Hodgkin lymphoma | 3.3 | 3.1 | 5.0 | ||
| Melanoma | 4.8 | 4.7 | 5.5 | ||
| Cervical | 2.3 | 2.2 | 3.5 | ||
| Endometrial | 1.0 | 1.0 | 0.5 | ||
| Colorectal | 9.1 | 9.6 | 5.0 | ||
| Metastases at diagnosis | No | 98.3 | 98.4 | 97.5 | 0.266 |
| Yes | 1.7 | 1.6 | 2.5 | ||
| Individual chronic condition score | Mean (SD) | 0.73 (0.38) | 0.73 (0.38) | 0.76 (0.36) | 0.204 |
| Trial Invitation | |||||
|---|---|---|---|---|---|
| Adjusted Odds Ratio | 95% Confidence Interval | p Value | |||
| Lower | Upper | ||||
| Health literacy level | Limited | 0.55 | 0.39 | 0.77 | 0.001 |
| Adequate | 1 | ||||
| Age (years) | ≤65 | 1 | |||
| >65 | 0.60 | 0.37 | 0.97 | 0.039 | |
| Management Center | Private | 1 | |||
| Public academic or community center | 1.43 | 0.99 | 2.06 | 0.055 | |
| Comprehensive cancer center | 3.62 | 2.37 | 5.54 | <0.001 | |
| Missing/unknown | 0.82 | 0.39 | 1.69 | 0.594 | |
| Area of residence | Rural/Small town/city (<200,000 residents) | 0.67 | 0.49 | 0.92 | 0.014 |
| Large city (≥200,000 residents) | 1 | ||||
| Missing/unknown | 0.51 | 0.08 | 3.39 | 0.490 | |
| Financial resources | Low (<Q1) | 0.54 | 0.33 | 0.88 | 0.014 |
| Intermediate (Q1–Q3) | 0.82 | 0.57 | 1.18 | 0.301 | |
| High (>Q3) | 1 | ||||
| Missing/unknown | 1.03 | 0.53 | 2.02 | 0.918 | |
| Cancer type | Breast | 1.82 | 0.90 | 3.66 | 0.095 |
| Lung | 3.78 | 1.55 | 9.22 | 0.003 | |
| Prostate | 0.84 | 0.36 | 1.96 | 0.689 | |
| Upper aero-digestive tract | 2.16 | 0.82 | 5.64 | 0.117 | |
| Bladder | 0.09 | 0.00 | 4.56 | 0.232 | |
| Kidney | 0.94 | 0.28 | 3.18 | 0.921 | |
| Thyroid | 0.78 | 0.26 | 2.32 | 0.655 | |
| Non-Hodgkin lymphoma | 2.88 | 1.12 | 7.45 | 0.029 | |
| Melanoma | 1.86 | 0.73 | 4.75 | 0.195 | |
| Cervical | 2.60 | 0.90 | 7.47 | 0.076 | |
| Endometrial | 0.56 | 0.04 | 7.24 | 0.658 | |
| Colorectal | 1 | ||||
| Individual chronic condition score, per one-point increase | 1.31 | 0.86 | 2.00 | 0.212 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ousseine, Y.M.; Bouhnik, A.-D.; Mancini, J. Health Literacy and Clinical Trial Participation in French Cancer Patients: A National Survey. Curr. Oncol. 2022, 29, 3118-3129. https://doi.org/10.3390/curroncol29050253
Ousseine YM, Bouhnik A-D, Mancini J. Health Literacy and Clinical Trial Participation in French Cancer Patients: A National Survey. Current Oncology. 2022; 29(5):3118-3129. https://doi.org/10.3390/curroncol29050253
Chicago/Turabian StyleOusseine, Youssoufa M., Anne-Déborah Bouhnik, and Julien Mancini. 2022. "Health Literacy and Clinical Trial Participation in French Cancer Patients: A National Survey" Current Oncology 29, no. 5: 3118-3129. https://doi.org/10.3390/curroncol29050253
APA StyleOusseine, Y. M., Bouhnik, A.-D., & Mancini, J. (2022). Health Literacy and Clinical Trial Participation in French Cancer Patients: A National Survey. Current Oncology, 29(5), 3118-3129. https://doi.org/10.3390/curroncol29050253






