Temporary Reversal of Hepatoenteric Collaterals during 90Y Radioembolization Planning and Administration
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gray, B.; Van Hazel, G.; Hope, M.; Burton, M.; Moroz, P.; Anderson, J.; Gebski, V. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann. Oncol. 2001, 12, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.; Coldwell, D.; Sangro, B.; Wasan, H.; Salem, R. Integrating radioembolization ((90)Y microspheres) into current treatment options for liver tumors: Introduction to the international working group report. Am. J. Clin. Oncol. 2012, 35, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Lewandowski, R.J.; Sato, K.T.; Atassi, B.; Ryu, R.K.; Ibrahim, S.; Nemcek, A.A., Jr.; Omary, R.A.; Madoff, D.C.; Murthy, R. Technical aspects of radioembolization with 90Y microspheres. Tech. Vasc. Interv. Radiol. 2007, 10, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Rognoni, C.; Ciani, O.; Sommariva, S.; Facciorusso, A.; Tarricone, R.; Bhoori, S.; Mazzaferro, V. Trans-arterial radioembolization in intermediate-advanced hepatocellular carcinoma: Systematic review and meta-analyses. Oncotarget 2016, 7, 72343–72355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murthy, R.; Brown, D.B.; Salem, R.; Meranze, S.G.; Coldwell, D.M.; Krishnan, S.; Nunez, R.; Habbu, A.; Liu, D.; Ross, W.; et al. Gastrointestinal complications associated with hepatic arterial Yttrium-90 microsphere therapy. J. Vasc. Interv. Radiol. 2007, 18, 553–561, quiz 562. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, R.J.; Morrow, I.M.; Sutherland, J.B. Treatment of liver tumors with yttrium-90 microspheres alone. Can. Assoc. Radiol. J. 1989, 40, 206–210. [Google Scholar] [PubMed]
- Stubbs, R.S.; Cannan, R.J.; Mitchell, A.W. Selective internal radiation therapy with 90yttrium microspheres for extensive colorectal liver metastases. J. Gastrointest. Surg. 2001, 5, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Tanaka, M.; Oi, H. Hepatic embolization from the common hepatic artery using balloon occlusion technique. AJR Am. J. Roentgenol. 1985, 145, 115–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, J.C.; Walker, S.C.; Ackermann, R.J.; Cotton, L.A.; Ensminger, W.D.; Shapiro, B. Hepatic radioembolization with yttrium-90 containing glass microspheres: Preliminary results and clinical follow-up. J. Nucl. Med. 1994, 35, 1637–1644. [Google Scholar] [PubMed]
- Mahvash, A.; Zaer, N.; Shaw, C.; Chasen, B.; Avritscher, R.; Murthy, R. Temporary balloon occlusion of the common hepatic artery for administration of yttrium-90 resin microspheres in a patient with patent hepatoenteric collaterals. J. Vasc. Interv. Radiol. 2012, 23, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Bester, L.; Salem, R. Reduction of arteriohepatovenous shunting by temporary balloon occlusion in patients undergoing radioembolization. J. Vasc. Interv. Radiol. 2007, 18, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Meek, J.; Fletcher, S.; Gauss, C.H.; Bezold, S.; Borja-Cacho, D.; Meek, M. Temporary Balloon Occlusion for Hepatic Arterial Flow Redistribution during Yttrium-90 Radioembolization. J. Vasc. Interv. Radiol. 2019, 30, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Murthy, R.; Lahoti, A.; Odisio, B.; Avritscher, R.; Chasen, B.; Mahvash, A. Temporary balloon occlusion of the common hepatic artery for yttrium-90 glass microspheres administration in a patient with hepatocellular cancer and renal insufficiency. Case Rep. Radiol. 2013, 2013, 560758. [Google Scholar] [CrossRef] [PubMed]
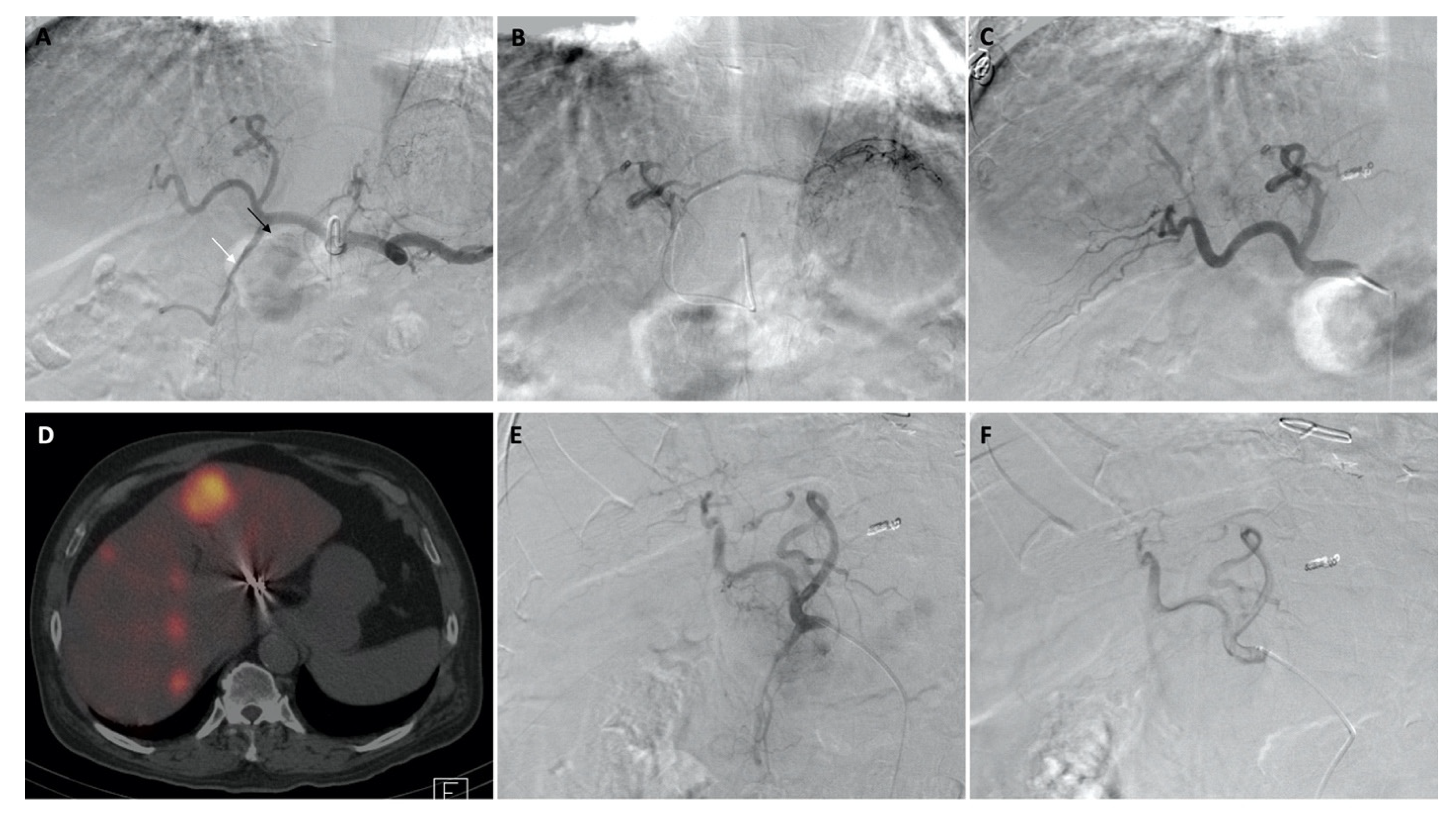
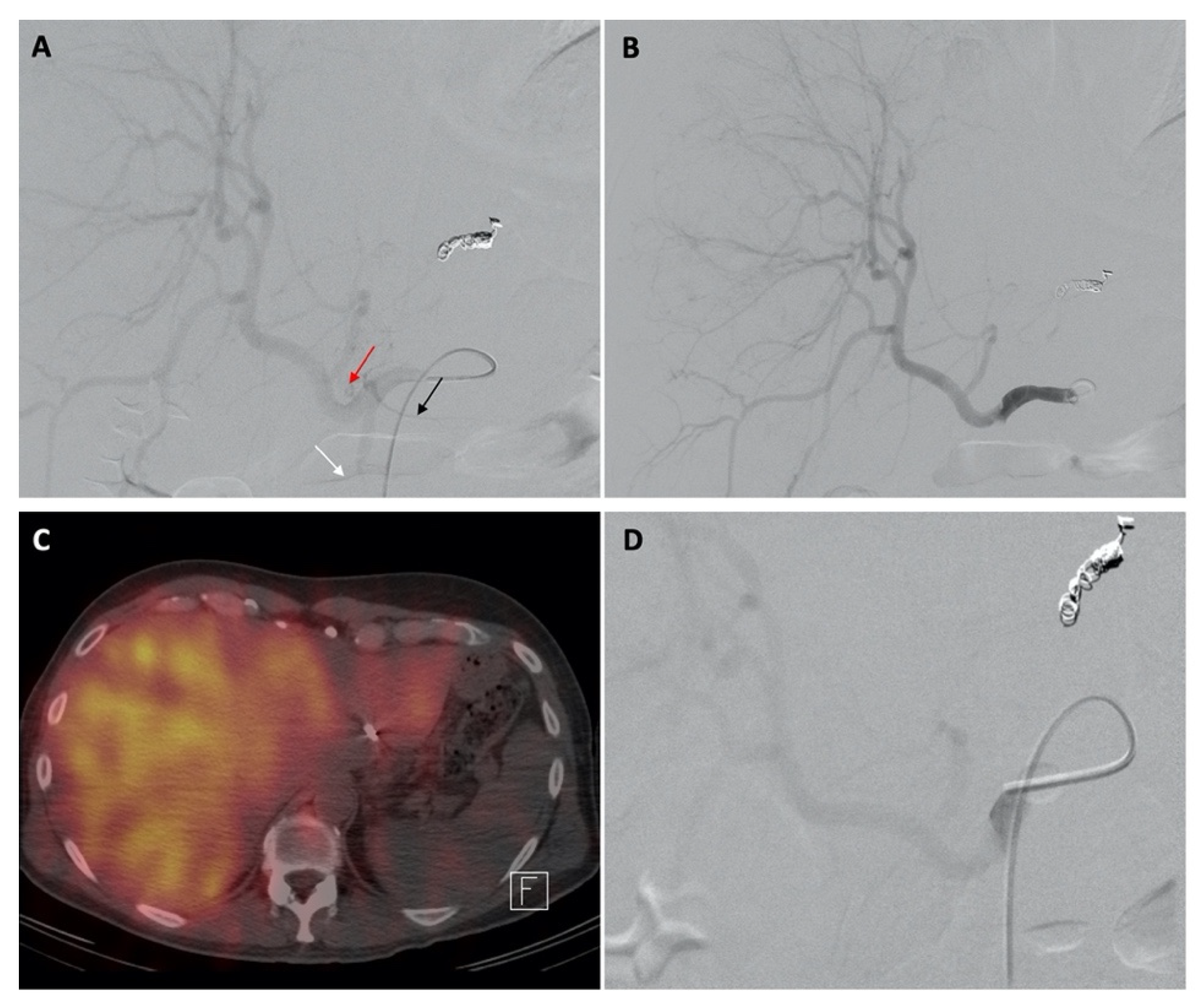

| Characteristic | n (%) | |
|---|---|---|
| Diagnostic Tc-99m MAA Administration (n = 66) | Therapeutic 90Y RE Administration (n = 72) | |
| Mean (±SD) age, years | 58.5 ± 15.1 | 57.9 ± 15.1 |
| Female (%) | 19 (29) | 25 (35) |
| Primary diagnosis | ||
| Hepatocellular carcinoma | 20 (30) | 22 (31) |
| Neuroendocrine tumor | 19 (29) | 25 (35) |
| Colorectal cancer | 18 (27) | 15 (21) |
| Carcinoid tumor | 4 (6) | 5 (1) |
| Medullary thyroid cancer | 1 (2) | 1 (1) |
| GI stromal tumor | 1 (2) | - |
| Adrenocortical carcinoma | 1 (2) | 1 (1) |
| Ameloblastoma | 1 (2) | 1 (1) |
| Small round cell tumor | 1 (2) | 1 (1) |
| Cholangiocarcinoma | - | 1 (1) |
| Michel’s classification of hepatic arterial anatomy | ||
| Type 1 | 53 (80) | 56 (78) |
| Type 2 | 3 (5) | 1 (1) |
| Type 3 | 2 (3) | 4 (6) |
| Type 4 | 1 (2) | 1 (1) |
| Type 5 | 4 (6) | 6 (8) |
| Type 6 | - | 1 (1) |
| Type 9 | 2 (3) | 2 (3) |
| LHA from CHA | 1 (2) | 1 (1) |
| Arteries with Flow Reversal | Number of Patients with Flow Reversal (%) | Arteries without Flow Reversal | Coil Embolization | Extrahepatic Uptake on SPECT/CT |
|---|---|---|---|---|
| GDA only | 15 (23) | In two patients, the RGA was not visualized; in three patients, the RGA flow did not reverse after balloon inflation; in one patient, flow in both the RGA and retroduodenal artery did not reverse | Flow redistribution: accessory LHA in one patient and segment IV branch in one patient; operator preference: RGA in one patient; hepatoenteric embolization: RGA in three patients | Pancreatic uptake in two patients |
| RGA only | 2 (3) | In one of the patients, the GDA flow was reversed before balloon inflation; in the other patient, the GDA flow did not reverse | - | - |
| GDA and RGA | 34 (52) | - | Flow redistribution: accessory LHA in two patients and replaced RHA in one patient; hepatoenteric embolization: accessory LGA in two patients | Gastric uptake in one patient, pancreatic uptake in one patient, and porta hepatic uptake through a small RHA branch in one patient |
| GDA and supraduodenal artery | 2 (3) | In one patient, the RGA flow did not reverse after balloon inflation | Hepatoenteric embolization: the RGA was embolized in one patient | Falciform ligament uptake in one patient |
| GDA and retroportal artery | 1 (2) | - | Operator preference: the RGA was embolized | - |
| GDA, RGA, and supraduodenal artery | 8 (12) | In one patient, the left inferior phrenic artery was identified, and its blood flow did not reverse after balloon inflation | - | Duodenal uptake through a proximal GDA branch and paraumbilical uptake in one patient and left diaphragmatic and falciform ligament uptake in the patient without flow reversal in the left inferior phrenic artery |
| GDA, RGA, and cystic artery | 1 (2) | - | - | - |
| GDA, RGA, and retroduodenal artery | 1 (2) | - | - | - |
| GDA, RGA, and segment 4b of the hepatic artery | 1 (2) | - | - | - |
| None | 1 (2) | - | Operator preference: the RGA was embolized | - |
| Total | 66 (100) | - | n = 14 (21%) | n = 8 (12%) |
| Arteries with Flow Reversal | Number of Patients with Flow Reversal (%) | Arteries without Flow Reversal | Coil Embolization during Treatment | Prior Coil Embolization during Planning | Complications |
|---|---|---|---|---|---|
| GDA only | 23 (32) | In one patient, the RGA was not visualized; in three patients, the RGA blood flow did not reverse after balloon inflation; in one patient, both the RGA and retroportal artery flow did not reverse; in one patient, the supraduodenal artery flow did not reverse; in one patient, the retroportal artery flow did not reverse | Flow redistribution: LHA in one patient; operator preference: RGA in two patients; hepatoenteric embolization: RGA and retroportal artery in one patient, retroportal artery in one patient, and RGA in one patients | Flow redistribution: segment IV branch in one patient, middle and left hepatic arteries in one patient *, accessory LHA in two patients, accessory RHA in one patient, and right phrenic artery in one patient; hepatoenteric embolization: RGA in six patients * and accessory left gastric artery in one patient | Microcatheter-induced arterial dissection in one patient |
| GDA and RGA | 31 (43) | A small pancreatic branch from the GDA in one patient and the falciform artery in one patient | Flow redistribution: accessory LHA in one patient; hepatoenteric embolization: RGA in one patient, and accessory LGA in one patient; extrahepatic: cystic artery in one patient | Flow redistribution: replaced RHA in one patient and accessory LHA in two patients; hepatoenteric embolization: accessory LGA in one patient | Duodenal ulcer in one patient, right CFA pseudoaneurysm in one patient managed conservatively, and minor contrast extravasation during RGA catheterization in one patient |
| GDA and retroduodenal artery | 1 (1) | - | - | - | Transient pain during the procedure |
| GDA and supraduodenal artery | 2 (3) | - | - | Hepatoenteric embolization: the RGA was embolized in both patients | - |
| Retroportal artery | 1 (1) | GDA | - | Hepatoenteric embolization: RGA | Transient pain during the procedure before administration |
| GDA, RGA, and supraduodenal artery | 7 (10) | - | The right phrenic artery was bland embolized as adjunct treatment | - | - |
| GDA, RGA, and cystic artery | 1 (1) | - | - | - | - |
| GDA, RGA, and retroduodenal artery | 2 (3) | - | - | - | - |
| GDA, RGA, and segment 4b of the hepatic artery | 1 (1) | - | - | - | - |
| None | 3 (4) | - | - | - | Mild spasm at the site of balloon inflation in one patient |
| Total | 72 (100) | - | n = 12 (17%) | n = 20 (28%) | n = 7 (10%) |
| Number | Authors | Year Published | Technique | Patients (Number) | Technical Success (%) | Gastrointestinal Complications |
|---|---|---|---|---|---|---|
| 1 | Nakamura, et al. [8] | 1985 | Gelfoam embolization | 12 | 100% | None |
| 2 | Andrews, et al. [9] | 1994 | Glass microspheres | 24 | 100% | 4 (two patients had previously diagnosed gastritis, all biopsy negative for microspheres) |
| 3 | Mahvash, et al. [10] | 2012 | Resin microspheres | 1 | successful | None |
| 4 | Smith, et al. [13] | 2013 | Glass microspheres | 1 | successful | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habibollahi, P.; Odisio, B.C.; Gurusamy, V.; Kuban, J.D.; Avritscher, R.; Abdelsalam, M.E.; Chasen, B.A.; Murthy, R.; Mahvash, A. Temporary Reversal of Hepatoenteric Collaterals during 90Y Radioembolization Planning and Administration. Curr. Oncol. 2022, 29, 9582-9592. https://doi.org/10.3390/curroncol29120753
Habibollahi P, Odisio BC, Gurusamy V, Kuban JD, Avritscher R, Abdelsalam ME, Chasen BA, Murthy R, Mahvash A. Temporary Reversal of Hepatoenteric Collaterals during 90Y Radioembolization Planning and Administration. Current Oncology. 2022; 29(12):9582-9592. https://doi.org/10.3390/curroncol29120753
Chicago/Turabian StyleHabibollahi, Peiman, Bruno C. Odisio, Varshana Gurusamy, Joshua D. Kuban, Rony Avritscher, Mohamed E. Abdelsalam, Beth A. Chasen, Ravi Murthy, and Armeen Mahvash. 2022. "Temporary Reversal of Hepatoenteric Collaterals during 90Y Radioembolization Planning and Administration" Current Oncology 29, no. 12: 9582-9592. https://doi.org/10.3390/curroncol29120753
APA StyleHabibollahi, P., Odisio, B. C., Gurusamy, V., Kuban, J. D., Avritscher, R., Abdelsalam, M. E., Chasen, B. A., Murthy, R., & Mahvash, A. (2022). Temporary Reversal of Hepatoenteric Collaterals during 90Y Radioembolization Planning and Administration. Current Oncology, 29(12), 9582-9592. https://doi.org/10.3390/curroncol29120753






