Immune-Based Combinations versus Sorafenib as First-Line Treatment for Advanced Hepatocellular Carcinoma: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
2.4. Risk of Bias Assessment in Included Studies
2.5. Types of Outcomes Measures
2.6. Statistical Design
3. Results
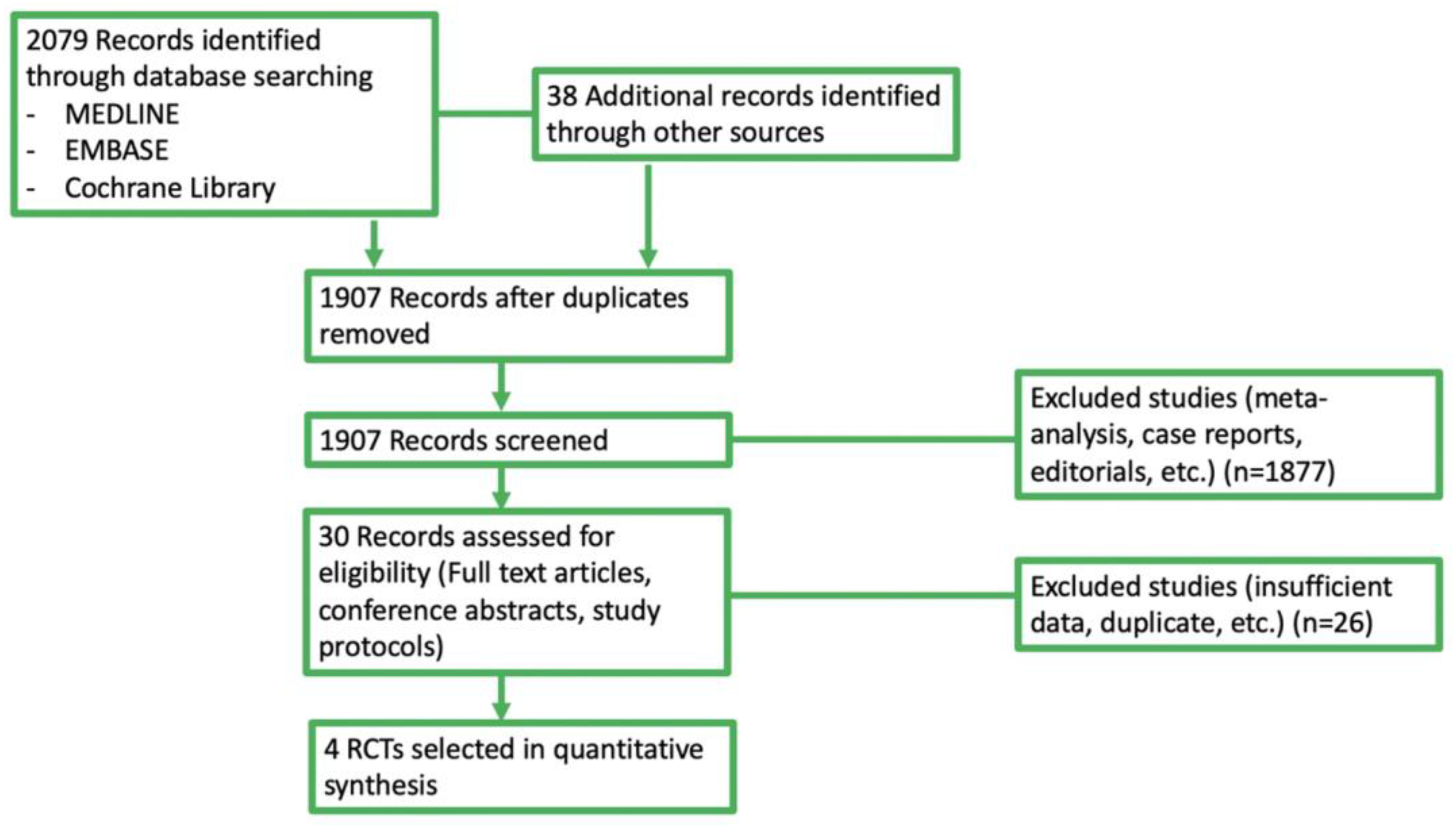
3.1. Selected Studies
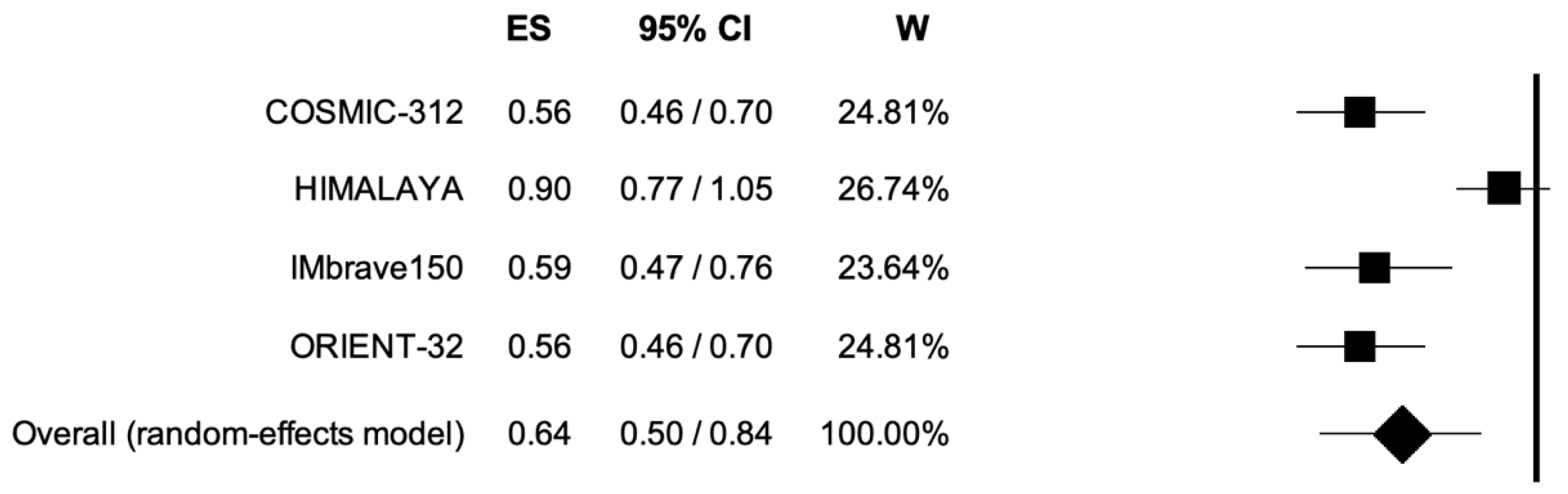
3.2. Overall Survival
3.3. Progression-Free Survival
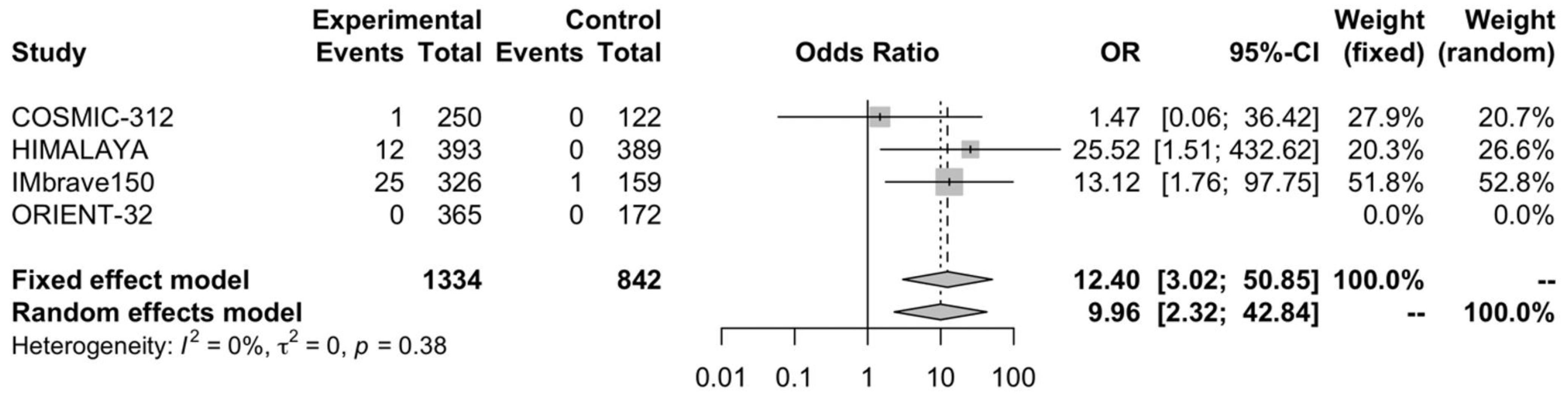
3.4. Complete Response Rate
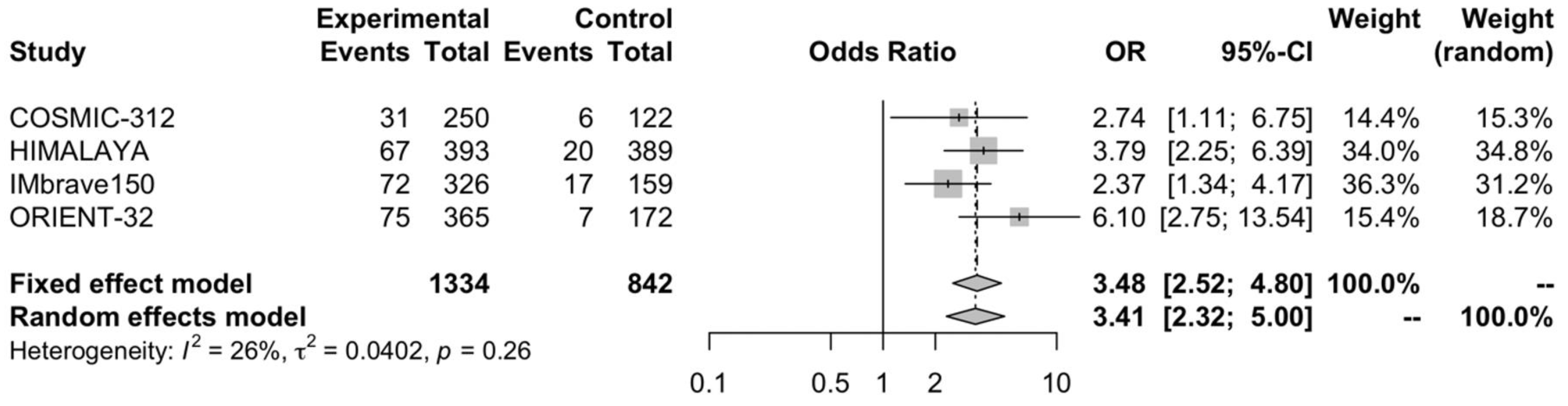
3.5. Partial Response Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Finn, R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2021, 19, 151–172. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H., 3rd; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Park, J.-W.; Finn, R.S.; Cheng, A.-L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Bouattour, M.; Lim, H.Y.; Breder, V.; Cheng, A.L. Pembrolizumab As Second-Line Therapy in Pa- tients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Gadaleta-Caldarola, G.; Brandi, G. First-line immune checkpoint inhibitor-based combinations in unresectable hepatocellular carcinoma: Current management and future challenges. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 1245–1251. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Lim, H.Y.; Kudo, M.; Breder, V.V.; Merle, P.; et al. IMbrave150: Updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J. Clin. Oncol. 2021, 39, 267. [Google Scholar] [CrossRef]
- Kelley, R.K.; Rimassa, L.; Cheng, A.L.; Kaseb, A.; Qin, S.; Zhu, A.X.; Chan, S.L.; Melkadze, T.; Sukeepaisarnjaroen, W.; Breder, V.; et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 995–1008. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Li, Q.; Lu, Y.; Chen, Y.; Guo, Y.; et al. ORIENT-32 study group. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2-3 study. Lancet Oncol. 2021, 22, 977–990, Erratum in Lancet Oncol. 2021, 22, e347. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Chan, S.L.; Kudo, M.; Lau, G.; Kelley, R.K.; Furuse, J.; Sangro, B. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepato- cellular carcinoma (uHCC): HIMALAYA. JCO 2022, 40, 379. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Hand-Book for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Sangro, B.; Sarobe, P.; Hervás-Stubbs, S.; Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef]
- Foerster, F.; Gairing, S.J.; Ilyas, S.I.; Galle, P.R. Emerging immunotherapy for HCC: A guide for hepatologists. Hepatology 2022, 75, 1604–1626. [Google Scholar] [CrossRef]
- Ouyang, T.; Kan, X.; Zheng, C. Immune Checkpoint Inhibitors for Advanced Hepatocellular Carcinoma: Monotherapies and Combined Therapies. Front. Oncol. 2022, 12, 898964. [Google Scholar] [CrossRef]
- Wen, W.; Zhang, Y.; Zhang, H.; Chen, Y. Clinical outcomes of PD-1/PD-L1 inhibitors in patients with advanced hepatocellular carcinoma: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Di Federico, A.; Frega, G.; Palloni, A.; Tavolari, S.; Brandi, G. Predictive Biomarkers for Checkpoint Inhibitor-Based Immunotherapy in Hepatocellular Carcinoma: Where Do We Stand? Front. Oncol. 2021, 11, 803133. [Google Scholar] [CrossRef]
- Pfister, D.; Núñez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef]
- Peng, Y.; Wong, C.C.; Yu, J. The paradox of immunotherapy in NASH-HCC. Signal Transduct. Target. Ther. 2021, 6, 228. [Google Scholar] [CrossRef] [PubMed]
- Schulze, K.; Imbeaud, S.; Letouzé, E.; Alexandrov, L.B.; Calderaro, J.; Rebouissou, S.; Couchy, G.; Meiller, C.; Shinde, J.; Soysouvanh, F.; et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet. 2015, 47, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Shimada, S.; Mogushi, K.; Akiyama, Y.; Furuyama, T.; Watanabe, S.; Ogura, T.; Ogawa, K.; Ono, H.; Mitsunori, Y.; Ban, D.; et al. Comprehensive molecular and immunological characterization of hepatocellular carcinoma. EBioMedicine 2019, 40, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Sia, D.; Jiao, Y.; Martinez-Quetglas, I.; Kuchuk, O.; Villacorta-Martin, C.; Castro de Moura, M.; Putra, J.; Camprecios, G.; Bassaganyas, L.; Akers, N.; et al. Identification of an Immune-specific Class of Hepatocellular Carcinoma, Based on Molecular Features. Gastroenterology 2017, 153, 812–826. [Google Scholar] [CrossRef]


| Trial | Study Design | Demographics (Arm A; Arm B) | No. pts Arm A/Arm B |
|---|---|---|---|
| IMbrave150 [9] | Phase 3 RCT | Median Age: 64; 66 Male sex: 82%; 83% Asian patients: 40%, 41% ECOG-PS 0: 62%; 62% HBV-positive: 49%; 46% HCV-positive: 21%; 22% | Atezolizumab plus bevacizumab: 326 Sorafenib: 159 |
| COSMIC-312 [10] | Phase 3 RCT | Median Age: 65; 64 Male sex: 86%; 88% Asian patients: 25%, 27% ECOG-PS 0: 65%; 61% HBV-positive: 30%; 29% HCV-positive: 28%; 28% | Atezolizumab plus cabozantinib: 250 Sorafenib: 122 |
| ORIENT-32 [11] | Phase 2/3 RCT | Median Age: 53; 654 Male sex: 82%; 83% Asian patients: 40%, 41% ECOG-PS 0: 62%; 62% HBV-positive: 49%; 46% HCV-positive: 21%; 22% | Sintilimab plus IBI305: 365 Sorafenib: 172 |
| HIMALAYA [12] | Phase 3 RCT | Median Age: 65; 64 Male sex: 83.2%; 86.6% Asian patients: 39.7%, 40.1% ECOG-PS 0: 62.1%; 62.0% HBV-positive: 31.0%; 30.6% HCV-positive: 28.0%; 26.7% | Durvalumab plus tremelimumab: 393 Sorafenib: 389 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, A.; Ricci, A.D.; Fanizzi, A.; Massafra, R.; De Luca, R.; Brandi, G. Immune-Based Combinations versus Sorafenib as First-Line Treatment for Advanced Hepatocellular Carcinoma: A Meta-Analysis. Curr. Oncol. 2023, 30, 749-757. https://doi.org/10.3390/curroncol30010057
Rizzo A, Ricci AD, Fanizzi A, Massafra R, De Luca R, Brandi G. Immune-Based Combinations versus Sorafenib as First-Line Treatment for Advanced Hepatocellular Carcinoma: A Meta-Analysis. Current Oncology. 2023; 30(1):749-757. https://doi.org/10.3390/curroncol30010057
Chicago/Turabian StyleRizzo, Alessandro, Angela Dalia Ricci, Annarita Fanizzi, Raffaella Massafra, Raffaele De Luca, and Giovanni Brandi. 2023. "Immune-Based Combinations versus Sorafenib as First-Line Treatment for Advanced Hepatocellular Carcinoma: A Meta-Analysis" Current Oncology 30, no. 1: 749-757. https://doi.org/10.3390/curroncol30010057
APA StyleRizzo, A., Ricci, A. D., Fanizzi, A., Massafra, R., De Luca, R., & Brandi, G. (2023). Immune-Based Combinations versus Sorafenib as First-Line Treatment for Advanced Hepatocellular Carcinoma: A Meta-Analysis. Current Oncology, 30(1), 749-757. https://doi.org/10.3390/curroncol30010057






