Abstract
Background: The Canadian Blood Services Cord Blood Bank (CBS CBB) was created to improve access to stem cell products for transplantation for patients across ethnic groups. An analysis of distributed units is needed to assess the effectiveness of the bank to meet the needs of patients from different ethnic groups. Methods: A descriptive analysis was performed on all cord blood units distributed from the CBS’ CBB as of 30 June 2022. Results: Distribution of the first 60 units based on CBS’ CBB inventory has been linear over time. A similar proportion of cord blood unit (CBU) recipients were pediatric or adult. More than half of the cord blood units (56.7%) were distributed to recipients outside of Canada, and CBUs were used to treat a broad range of hematologic and immune disorders. 43.3% of distributed CBUs were of non-Caucasian ethnicity and 18% were from donors self-reporting as multi-ethnic. The mean total nucleated cell counts and total CD34+ cell counts were 1.9 ± 0.1 × 109 cells and 5.3 ± 0.5 × 106 CD34+ cells, respectively. CD34+ cells per kg (recipient weight) varied significantly between pediatric (age 0–4), adolescent (age 5–17) and adult recipients (age 18 and older) (3.1 ± 0.5, 1.4 ± 0.5 and 0.9 ± 0.07 × 105 CD34+ cells/kg, respectively). HLA matching was 6/6 (15%), 5/6 (47%) or 4/6 (38%). Conclusions: The CBS’ CBB has facilitated the utilization of banked units for patients across a broad range of ages, geographic distribution, ethnicity, and diseases. Distributed units were well matched for HLA alleles and contained robust cell counts, reflecting a high-quality inventory with significant utility.
1. Introduction
Allogeneic hematopoietic cell transplantation (HCT) remains a curative approach to treat patients with hematological malignancies and inherited immune and metabolic disorders [1]. With declining fertility rates, fewer and fewer patients have matched sibling donors [2] and accessing a human leukocyte antigen (HLA)-compatible unrelated donor remains a challenge for many patients. In particular, several non-Caucasian ethnic groups have more complexity in HLA haplotype frequencies which reduces match likelihoods and, furthermore, disproportional representation of some ethnic groups within the global inventory of unrelated donors and cord blood units further reduces the chances of finding a matched donor [3]. Given Canada’s broad ethnic diversity, Canadian Blood Services launched a public cord blood bank to complement donor options within the CBS Stem Cell Registry with a goal of improving access to stem cell donors for all patients and, in particular, patients of non-Caucasian ethnicity.
The Canadian Blood Services’ Cord Blood Bank (CBS’ CBB) was launched in 2013 and has been banking units with total mononuclear cell counts ≥1.5 × 109 and from donors representing a broad range of ethnicities (≥1.3 × 109 for non-Caucasian units). More than 4000 units from the CBS’ CBB are currently searchable in the global inventory of public banks and more than 60% of units are from donors who self-report as non-Caucasians, including many donors reporting multiple ethnic associations [4]. Encouraging HLA-match likelihoods were demonstrated within the bank’s inventory in a recent modelling analysis [4]. Despite reports of success in high-risk leukemia patients [5], cord blood use has fluctuated in recent years in particular due to the increasing use of haploidentical transplantation [6,7]. Usage patterns from the CBS’ CBB have not previously been reported. Moreover, the degree to which usage has been impacted by critical points in the evolution of the bank, such as accreditation by the Association for the Advancement of Blood & Biotherapies (AABB) and the Foundation for the Accreditation of Cellular Therapy (FACT), and utilization during the ongoing pandemic remains unknown. Understanding characteristics of units distributed from the CBS’ CBB with regard to ethnicity is important for the continued efforts to expand ethnic diversity in the bank. We sought to describe the pattern of usage of distributed CBUs from our bank to better understand trends, and modify recruitment accordingly.
2. Methods
2.1. Donor, Recipient and CBU Information
Donor, product and recipient information for cord blood transplants coordinated by the CBS Stem Cell Registry as of 30 June 2022 (n = 60) were extracted from the CBS electronic database. Inventory dates on all banked CBUs as of 30 June 2022 (n = 4197) were extracted from the CBS electronic database. All data were deidentified and aggregated prior to analysis.
Utilization scores (US) were calculated for the first 60 CBUs distributed by the CBS CBB as previously described [8]. In brief, the following equation was used to calculate the utilization score for each distributed CBU: Utilization Score = [exp(−6.736 + 0.192X + 0.040Y)]/[1 + exp(−6.736 + 0.192X + 0.040Y)], where X is the total nucleated cell count (×108) and Y is the total CD34+ count (×106).
2.2. Statistical Analyses
All descriptive statistical analyses were performed using Prism 9 (GraphPad) or Microsoft Excel software. Data are shown as means ± SEM when applicable. All graphs and visuals were generated using either Prism 9 (GraphPad) or Microsoft Excel software.
3. Results
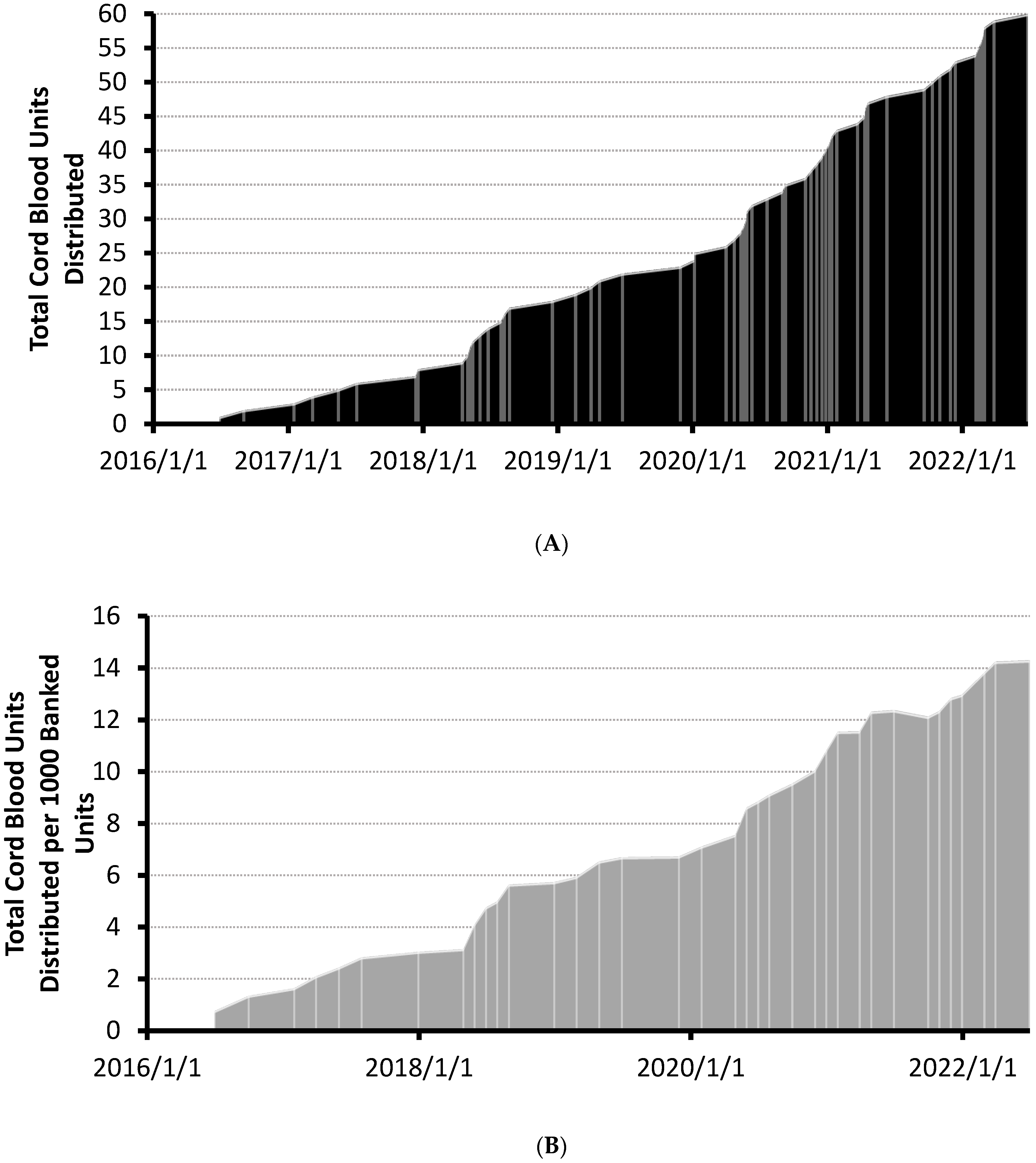
A total of 4197 units have been banked and were searchable in the CBS’ CBB at the time of analysis. The distribution of the first 60 units increased steadily since the first unit was released in 2016 (see Figure 1A). Cumulative distributions per 1000 banked units increased linearly over a 6 year period (see Figure 1B). No units were distributed before accreditation by the Association for the Advancement of Blood & Biotherapies (AABB) on 1 January 2016 and 19 of the first sixty units (32%) were distributed prior to the NetCord-FACT accreditation on 25 February 2019 (Figure 1A). No apparent change in utilization rates occurred following the FACT accreditation. The rate of total CBU reservation requests also increased linearly over the same time period (Supplementary Figure S1). Annualized gross usage of the CBS’ CBB has steadily increased to 1.4% as of June 2022 (Table 1).
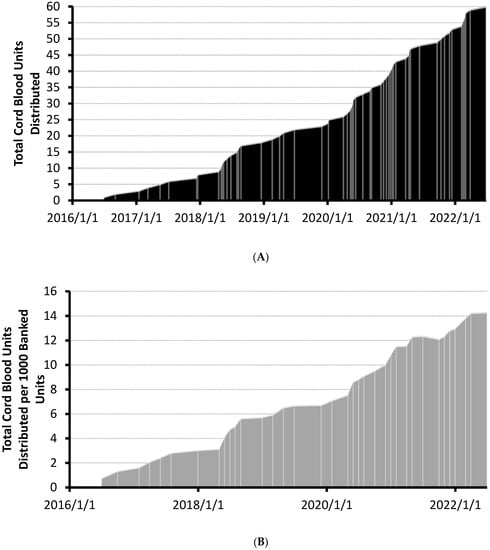
Figure 1.
(A) Cumulative plot of cord blood units distributed over time. Shipment dates of cord blood units are demarcated with lightly colored lines. Cord blood bank accreditation dates are indicated on the plot. (B) Cumulative plot of cord blood units distributed per 1000 banked CBUs over time. Total CBUs distributed were normalized to end-of-month total inventory counts of banked CBUs. Updated total of distributed units per thousand banked CBUs are demarcated with lightly colored lines. Cord blood bank accreditation dates are indicated on the plot. AABB accreditation (1 January 2016) and FACT accreditation (25 February 2019) received.

Table 1.
Usage of the CBS’ Cord Blood Bank.
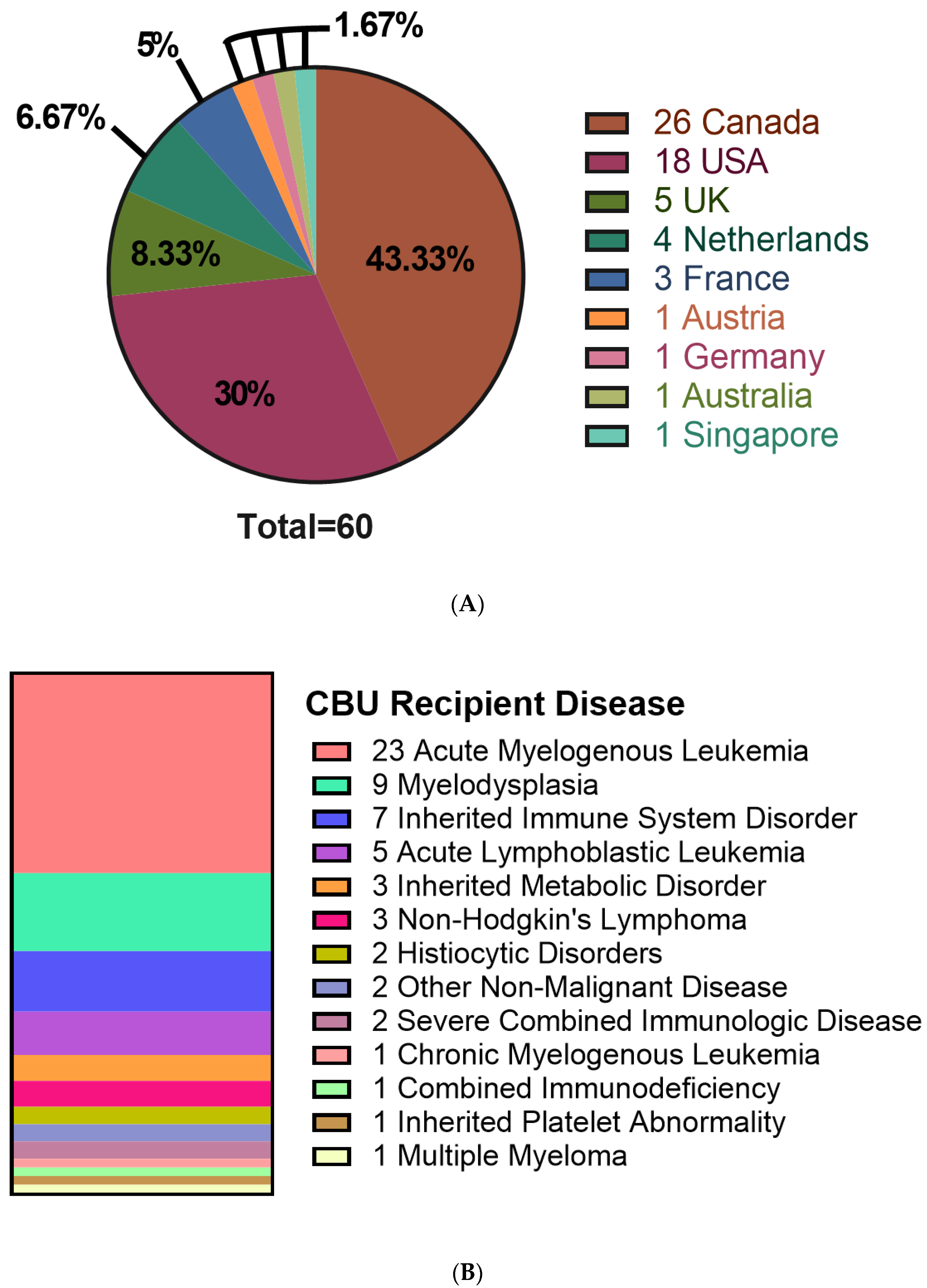
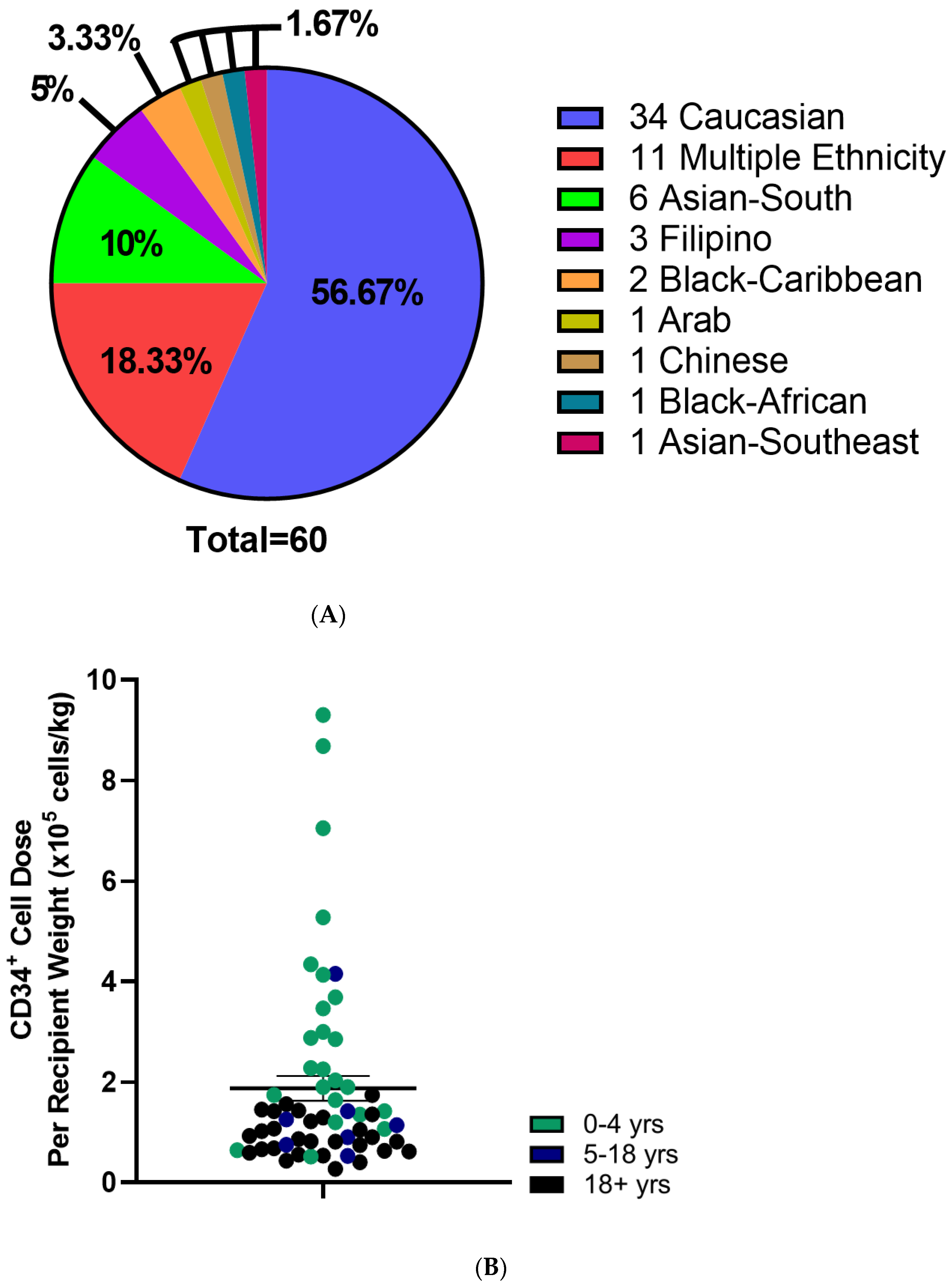
CBU recipients were 18 years or older (46.7%), aged 0–4 years (41.7%) or between 5–17 years of age (11.7%) (Table 2). Regarding the domestic use of units, 43.3% were transplanted into Canadian recipients. International recipients were from the United States (30%), Europe and the UK (23%), Australia (1.7%) and Singapore (1.7%) (Figure 2A). Recipients had a broad range of hematologic and inherited immune and metabolic disorders (Figure 2B). HLA matching was 6/6 (15%), 5/6 (47%) or 4/6 (38%) (Table 2). While the proportion of units from Caucasian donors that were 6/6 HLA matched (7 of 34 units, or 21%) was greater than for non-Caucasian units (2 of 26 units, or 7.9%), the difference was not significant (p = 0.28, Fisher’s exact test). Moreover, the effect of ethnicity on HLA match distribution was not significant in a two-way analysis of variance (p = 0.16). A total of 10 units (16.7%) were part of a double cord blood transplant and in all 10 cases, the recipients were adults with a hematologic malignancy (Table 2). Donors who self-identified as Caucasian comprised 56.7% of distributed units, while multiethnic donors accounted for 18.3% and other ethnicities were associated with 25% of units (Figure 3A). By contrast, the total inventory of banked units contained 63.5% non-Caucasian units (Figure 3B), including 26% of mothers who self-reported as multiethnic.

Table 2.
Characteristics of cord blood unit recipients.
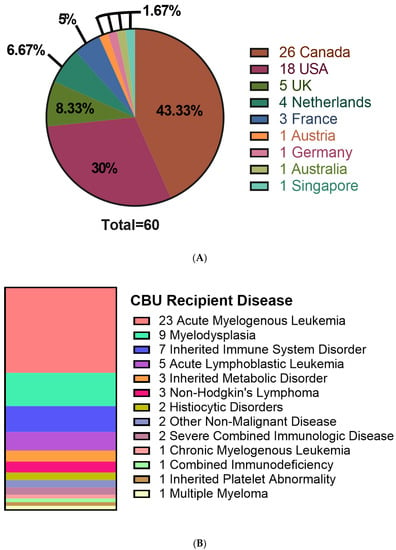
Figure 2.
Characteristics of recipients receiving cord blood units from the CBS cord blood bank. (A) Location of patients receiving distributed CBUs. (B) Disease of patients receiving CBU donations. N = 60.
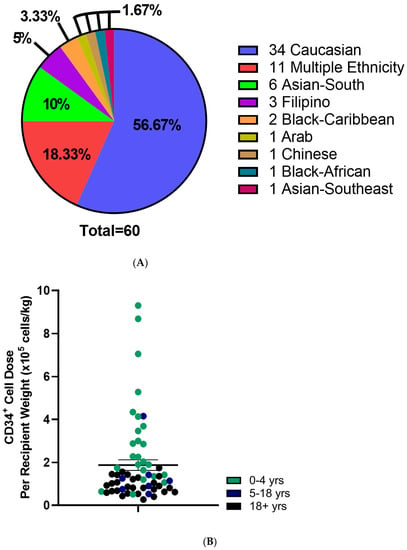
Figure 3.
Characteristics of Distributed Cord Blood Units. (A) Self reported Ethnicity of distributed CBUs. (B) CD34+ cell dose per kg recipient weight of distributed CBUs. Patient age indicated with color. N = 59. Means indicated ± SEM.
Mean total nucleated cell counts and total CD34+ cell counts were 1.9 ± 0.1 × 109 cells and 5.3 ± 0.5 × 106 CD34+ cells, respectively (Table 3 and Figure 3B). When adjusted to recipient weight, the mean CD34+ cell dose was 1.9 × 105 ± 0.2 cells/kg which varied by age (Table 3). Adult recipients (aged 18 and older) received 0.9 ± 0.07 × 105 CD34+ cells/kg, while pediatric (ages 0–4 years) and adolescent recipients (ages 5–17) received 3.1 ± 0.5 × 105 and 1.4 ± 0.5 × 105 CD34+ cells/kg, respectively (Table 3; Figure 3). TNC and CD34+ cell counts were not different between units from Caucasian compared with non-Caucasian ethnicity (Supplementary Figure S2). Utilization scores (US) were calculated for the distributed CBUs to evaluate their predicted utility (Supplementary Figure S3) and were >0.01 in all cases with 26.7% of units exceeding a UI of 0.1 (Table 3).

Table 3.
Characteristics of distributed cord blood units.
4. Discussion
Cord blood units distributed by the CBS’ CBB are well matched for HLA across a broad diversity of ethnic groups and have been used by adult and pediatric patients, both in Canada and abroad. Steady usage of cord blood units may reflect the overall high quality of units in the bank, early accreditation by the AABB and FACT, and the high proportion of units banked from non-Caucasian donors. In particular, usage of donors from multi-ethnic backgrounds may be of particular importance for patients who may not otherwise have compatible donors. Continued banking of units with high cell counts across a broad range of ethnicities appears worthwhile and important.
While the overall inventory size and usage of the bank is modest on a global scale, continued steady usage diverges from global patterns reported recently [6,7], although usage during the COVID-19 pandemic has been variable, with increased usage in 2020 reported by the EBMT [9], and modest decrease in usage reported by the WMDA [10]. Banking of units with high cell counts and enriching the inventory to >60% non-Caucasian units in the CBS’ CBB may have charted a course to greater interest and steady usage at the CBS’ CBB in comparison to some other banks around the world. Many banks did not introduce thresholds for banking until after many units were already banked, or continue to bank all units collected. However, the number of units collected for the CBS’ CBB that did not meet the threshold for banking was impacted by concomitant delayed clamping of the umbilical cord, a practice that became more widespread following the launch of the CBS’ CBB [11]
The World Marrow Donor Association has reported that cord blood banking is well established in the United States and Europe while banking infrastructure remains lacking in many other countries, skewing the global inventory of banked units towards units of Caucasian ethnicity [12]. The Anthony Nolan Cell Therapy Centre has reported that 73% of banked units are from Caucasian donors with 3.1% from mothers of “mixed race” [13]. They suggest that banking of units with lower cell counts from non-Caucasian donors could enrich ethnic diversity within cord bank inventories. Notably, the CBS’ CBB embraced this approach from the outset using a lower TNC threshold for the banking non-Caucasian units and has banked 63% of units from non-Caucasians with 26% of units donated from mothers of multiple ethnicity associations. Multiethnic donors represented a significant proportion of the units distributed from the CBS’ CBB and units from non-Caucasian donors represented nearly half of the units distributed for transplantation. Cord blood transplantation has increased the opportunities for transplantation in patients from non-Caucasian ethnicities [3,14], although certain ethnic groups continue to face challenges in finding suitable units for transplantation [15] and cord blood units donated by non-European donors may be used preferentially for pediatric patients [13]. The CBS’ CBB is committed to structuring the CBU inventory to better serve non-Caucasian patients who comprise an increasing proportion of the Canadian population [16] and face more challenges in finding HLA-matched unrelated adult donors [5].
Over 40% of the CBU transplantations observed were to recipients aged between 0 and 4 years. While cord blood transplantation has demonstrated success in adult populations [6], marrow and peripheral blood stem cells are often preferred for adult recipients due to the higher TNC and CD34+ cell counts in products isolated from these methods [6,7]. CBU selection guidelines suggest minimum CD34+ cell doses of 1.5 × 105 CD34+ cells/kg recipient weight [17] and higher levels for non-malignant conditions [18,19,20,21]. We observed high cell doses in CBUs selected for pediatric patients; however, cell doses for adult patients were more often below this recommended cut-off. In many cases where the cell dose was low, the unit was part of a double cord blood transplant.
The extent to which cord blood transplantation is preferred over other alternative donor sources for HCT remains under study. A recent real-world analysis using data from the CIBMTR [22] suggests that haploidentical peripheral blood transplants may be preferred over double cord blood transplantation in adults with high-risk hematologic malignancies. While an RCT was completed [23], it did not enroll sufficiently to detect a difference in the primary outcome of progression-free survival, but did observe improved overall survival with haploidentical bone marrow transplant compared to double cord blood transplantation using reduced-intensity regimens. With favorable results from cord blood expansion studies [24], more studies will be needed to clarify the precise role of cord blood transplantation in adults and children.
5. Conclusions
This first analysis of 60 units distributed by the CBS’ CBB confirms the ability to facilitate HCT for patients in Canada and worldwide, including adult and pediatric patients with a broad range of diseases. Continued banking of high-quality units will ensure the ongoing availability to support patients undergoing HCT. Moreover, as the diversity of Canada and the global population continues to expand and evolve, maintaining an inventory with an evolving ethnic diversity will be needed, and especially units donated from multiethnic donors, to ensure continued access to stem cell products for patients awaiting transplant.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/curroncol29120752/s1, Figure S1: Cumulative plot of cord blood unit reserve requests per 1000 banked CBUs over time; Figure S2: Analysis of total nucleated cells (TNC) and CD34+ cells in Caucasian (n = 34) and non-Caucasian units (n = 26); Figure S3: Utilization scores of the first 60 cord blood units distributed by the Canadian Blood Services Cord Blood Bank.
Author Contributions
Conceptualization, M.D.S. and D.S.A.; methodology, G.P., M.D.S. and D.S.A.; software, M.G. and J.W.; validation, G.P., M.G., K.M., T.L., N.D., K.G., J.W. and T.P.; formal analysis, G.P. and M.G.; investigation, G.P., M.G., M.D.S. and D.S.A.; resources, K.G.; data curation, M.G. and J.W.; writing—original draft preparation, G.P., M.D.S. and D.S.A.; writing—review and editing, all authors; visualization, G.P., T.P., M.D.S. and D.S.A.; supervision, M.D.S. and D.S.A.; project administration, K.G., M.D.S. and D.S.A.; funding acquisition, K.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Canadian Blood Services Foundation.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the use of anonymized data for the purposes of operational improvement.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
All authors are employed by or have received remuneration from Canadian Blood Services.
References
- Kanate, A.S.; Majhail, N.S.; Savani, B.N.; Bredeson, C.; Champlin, R.E.; Crawford, S.; Giralt, S.A.; LeMaistre, C.F.; Marks, D.I.; Omel, J.L.; et al. Indications for Hematopoietic Cell Transplantation and Immune Effector Cell Therapy: Guidelines from the American Society for Transplantation and Cellular Therapy. Biol. Blood Marrow Transplant. 2020, 26, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Allan, D.S.; Takach, S.; Smith, S.; Goldman, M. Impact of declining fertility rates in Canada on donor options in blood and marrow transplantation. Biol. Blood Marrow Transplant. 2009, 15, 1634–1637. [Google Scholar] [CrossRef][Green Version]
- Gragert, L.; Eapen, M.; Williams, E.; Freeman, J.; Spellman, S.; Baitty, R.; Hartzman, R.; Rizzo, J.D.; Horowitz, M.; Confer, D.; et al. HLA Match Likelihoods for Hematopoietic Stem-Cell Grafts in the U.S. Registry. N. Engl. J. Med. 2014, 371, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Allan, D.; Kiernan, J.; Gragert, L.; Dibdin, N.; Bartlett, D.; Campbell, T.; Mostert, K.; Halpenny, M.; Ganz, K.; Maiers, M.; et al. Reducing ethnic disparity in access to high-quality HLA-matched cord blood units for transplantation: Analysis of the Canadian Blood Services’ Cord Blood Bank inventory. Transfusion 2019, 59, 2382–2388. [Google Scholar] [CrossRef] [PubMed]
- Milano, F.; Gooley, T.; Wood, B.; Woolfrey, A.; Flowers, M.E.; Doney, K.; Witherspoon, R.; Mielcarek, M.; Deeg, J.H.; Sorror, M.; et al. Cord-Blood Transplantation in Patients with Minimal Residual Disease. N. Engl. J. Med. 2016, 375, 944–953. [Google Scholar] [CrossRef]
- Niederwieser, D.; Baldomero, H.; Bazuaye, N.; Bupp, C.; Chaudhri, N.; Corbacioglu, S.; Elhaddad, A.; Frutos, C.; Galeano, S.; Hamad, N.; et al. One and a half million hematopoietic stem cell transplants: Continuous and differential improvement in worldwide access with the use of non-identical family donors. Haematologica 2021, 107, 1045–1053. [Google Scholar] [CrossRef]
- Passweg, J.R.; Baldomero, H.; Chabannon, C.; Basak, G.W.; De La Camara, R.; Corbacioglu, S.; Dolstra, H.; Duarte, R.; Glass, B.; Greco, R.; et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: Monitoring of activities and trends over 30 years. Bone Marrow Transplant. 2021, 56, 1651–1664. [Google Scholar] [CrossRef]
- Magalon, J.; Maiers, M.; Kurtzberg, J.; Navarrete, C.; Rubinstein, P.; Brown, C.; Schramm, C.; Larghero, J.; Katsahian, S.; Chabannon, C.; et al. Banking or Bankrupting: Strategies for Sustaining the Economic Future of Public Cord Blood Banks. PLoS ONE 2015, 10, e0143440. [Google Scholar] [CrossRef]
- Passweg, J.R.; Baldomero, H.; Chabannon, C.; Corbacioglu, S.; de la Cámara, R.; Dolstra, H.; Glass, B.; Greco, R.; Mohty, M.; Neven, B.; et al. Impact of the SARS-CoV-2 pandemic on hematopoietic cell transplantation and cellular therapies in Europe 2020: A report from the EBMT activity survey. Bone Marrow Transplant. 2022, 57, 742–752. [Google Scholar] [CrossRef]
- Jöris, M.M.; Schmidt, A.H.; Bernas, S.N.; Feinberg, J.; Sacchi, N.; Elmoazzen, H.; Fournier, D.; Oguz, F.; Oliveira, D.; Yang, K.-L.; et al. Impact of COVID-19 pandemic on global unrelated stem cell donations in 2020—Report from World Marrow Donor Association. Bone Marrow Transplant. 2022, 57, 1021–1024. [Google Scholar] [CrossRef]
- Allan, D.S.; Scrivens, N.; Lawless, T.; Mostert, K.; Oppenheimer, L.; Walker, M.; Petraszko, T.; Elmoazzen, H. Delayed clamping of the umbilical cord after delivery and implications for public cord blood banking. Transfusion 2015, 56, 662–665. [Google Scholar] [CrossRef]
- WMDA Global Trends Report. 2021. Available online: https://wmda.info/wp-content/uploads/2022/07/CORRECTED-21042022-GTR-2021-Summary-slides-002.pdf (accessed on 22 November 2022).
- Wynn, L.A.; Horton, R.; Gibson, D. Ethnic diversity and cord blood banking: Satisfying the unmet need. Cytotherapy 2022, 24, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.N.; Byam, C.E.; Kernan, N.A.; Lee, S.S.; Hawke, R.M.; Doshi, K.A.; Wells, D.S.; Heller, G.; Papadopoulos, E.B.; Scaradavou, A.; et al. Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. Biol. Blood Marrow Transplant. 2010, 16, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Kosuri, S.; Wolff, T.; Devlin, S.M.; Byam, C.; Mazis, C.M.; Naputo, K.; Davis, E.; Paulson, J.; Nhaissi, M.; Wells, D.S.; et al. Prospective Evaluation of Unrelated Donor Cord Blood and Haploidentical Donor Access Reveals Graft Availability Varies by Patient Ancestry: Practical Implications for Donor Selection. Biol. Blood Marrow Transplant. 2017, 23, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Statistics Canada. Table 98-400-X2016189 Ethnic Origin (101), Age (15A), Sex (3) and Selected Demographic, Cultural, Labour Force, Educational and Income Characteristics (651) for the Population in Private Households of Canada, Provinces and Territories, Census Metropolitan. 2018. Available online: https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/dt-td/Rp-eng.cfm?TABID=2&LANG=E&A=R&APATH=3&DETAIL=0&DIM=0&FL=A&FREE=0&GC=01&GL=- (accessed on 17 June 2022).
- Politikos, I.; Davis, E.; Nhaissi, M.; Wagner, J.E.; Brunstein, C.G.; Cohen, S.; Shpall, E.J.; Milano, F.; Scaradavou, A.; Barker, J.N. Guidelines for Cord Blood Unit Selection. Biol. Blood Marrow Transplant. 2020, 26, 2190–2196. [Google Scholar] [CrossRef]
- Hough, R.; Danby, R.; Russell, N.; Marks, D.; Veys, P.; Shaw, B.; Wynn, R.; Vora, A.; Mackinnon, S.; Peggs, K.S.; et al. Recommendations for a standard UK approach to incorporating umbilical cord blood into clinical transplantation practice: An update on cord blood unit selection, donor selection algorithms and conditioning protocols. Br. J. Haematol. 2016, 172, 360–370. [Google Scholar] [CrossRef]
- Boelens, J.J.; Aldenhoven, M.; Purtill, D.; Ruggeri, A.; DeFor, T.; Wynn, R.; Wraith, E.; Cavazzana-Calvo, M.; Rovelli, A.; Fischer, A.; et al. Outcomes of transplantation using various hematopoietic cell sources in children with Hurler syndrome after myeloablative conditioning. Blood 2013, 121, 3981–3987. [Google Scholar] [CrossRef]
- Fernandes, J.F.; Rocha, V.; Labopin, M.; Neven, B.; Moshous, D.; Gennery, A.R.; Friedrich, W.; Porta, F.; Diaz de Heredia, C.; Wall, D.; et al. Transplantation in patients with SCID: Mismatched related stem cells or unrelated cord blood? Blood J. Am. Soc. Hematol. 2012, 119, 2949–2955. [Google Scholar] [CrossRef]
- de Latour, R.P.; Purtill, D.; Ruggeri, A.; Sanz, G.; Michel, G.; Gandemer, V.; Maury, S.; Kurtzberg, J.; Bonfim, C.; Aljurf, M.; et al. Influence of nucleated cell dose on overall survival of unrelated cord blood transplantation for patients with severe acquired aplastic anemia: A study by eurocord and the aplastic anemia working party of the European group for blood and marrow transplantation. Biol. Blood Marrow Transplant. 2011, 17, 78–85. [Google Scholar]
- O’Donnell, P.V.; Brunstein, C.G.; Fuchs, E.J.; Zhang, M.J.; Allbee-Johnson, M.; Antin, J.H.; Leifer, E.S.; Elmariah, H.; Grunwald, M.R.; Hashmi, H.; et al. Umbilical cord blood or HLA-haploidentical transplantation: Real-world outcomes versus randomized trial outcomes. Transplant. Cell. Ther. 2022, 28, 109-e1. [Google Scholar] [CrossRef]
- Fuchs, E.J.; O’Donnell, P.V.; Eapen, M.; Logan, B.; Antin, J.H.; Dawson, P.; Devine, S.; Horowitz, M.M.; Horwitz, M.E.; Karanes, C.; et al. Double unrelated umbilical cord blood vs HLA-haploidentical bone marrow transplantation: The BMT CTN 1101 trial. Blood 2021, 137, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Saiyin, T.; Kirkham, A.M.; Bailey, A.J.; Shorr, R.; Pineault, N.; Maganti, H.B.; Allan, D.S. Clinical outcomes of umbilical cord blood transplantation utilizing ex vivo expansion: A systematic review and meta-analysis of controlled studies. Transplant. Cell. Ther. 2022. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).