Efficacy and Safety of the “Trisection Method” Training System for Robot-Assisted Radical Cystectomy at a Single Institution in Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
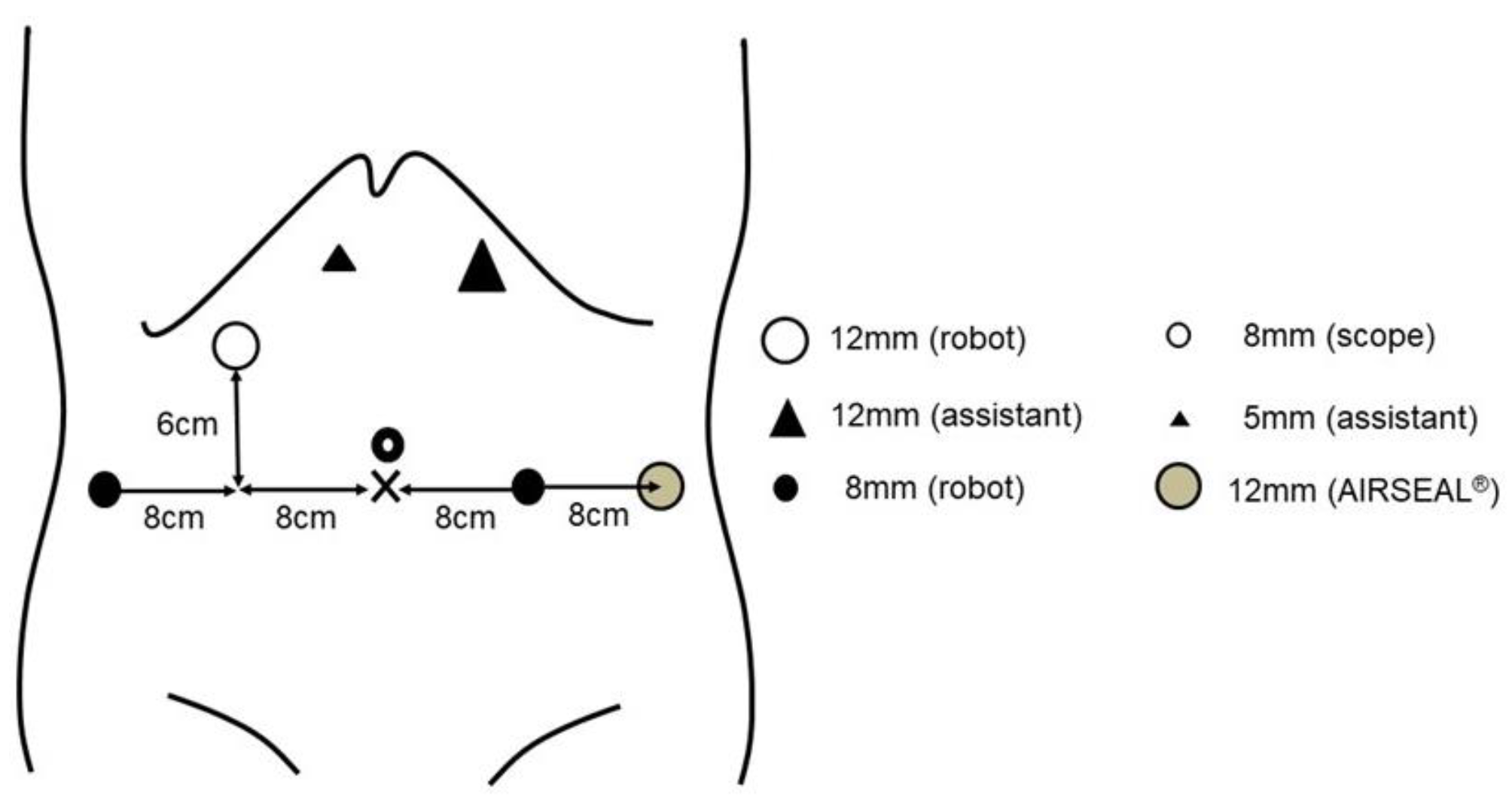
2.2. Surgical Procedure
2.3. Qualifications of the Surgeon Performing RARC
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Oncological Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef]
- Parekh, D.J.; Reis, I.M.; Castle, E.P.; Gonzalgo, M.L.; Woods, M.E.; Svatek, R.S.; Weizer, A.Z.; Konety, B.R.; Tollefson, M.; Krupski, T.L.; et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): An open-label, randomised, phase 3, non-inferiority trial. Lancet 2018, 391, 2525–2536. [Google Scholar] [CrossRef]
- Bochner, B.H.; Dalbagni, G.; Marzouk, K.H.; Sjoberg, D.D.; Lee, J.; Donat, S.M.; Coleman, J.A.; Vickers, A.; Herr, H.W.; Laudone, V.P. Randomized Trial Comparing Open Radical Cystectomy and Robot-assisted Laparoscopic Radical Cystectomy: Oncologic Outcomes. Eur. Urol. 2018, 74, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Sathianathen, N.J.; Kalapara, A.; Frydenberg, M.; Lawrentschuk, N.; Weight, C.J.; Parekh, D.; Konety, B.R. Robotic Assisted Radical Cystectomy vs Open Radical Cystectomy: Systematic Review and Meta-Analysis. J. Urol. 2019, 201, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Bochner, B.H.; Dalbagni, G.; Sjoberg, D.D.; Silberstein, J.; Keren Paz, G.E.; Donat, S.M.; Coleman, J.A.; Mathew, S.; Vickers, A.; Schnorr, G.C.; et al. Comparing Open Radical Cystectomy and Robot-assisted Laparoscopic Radical Cystectomy: A Randomized Clinical Trial. Eur. Urol. 2015, 67, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, A.S.; Santos, F.; Dragomir, A.; Tanguay, S.; Kassouf, W.; Aprikian, A.G. Postoperative mortality and complications after radical cystectomy for bladder cancer in Quebec: A population-based analysis during the years 2000–2009. Can Urol. Assoc. J. 2014, 8, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.P.; Al Hussein Al Awamlh, B.; Wu, X.; O’Malley, P.; Inoyatov, I.M.; Ayangbesan, A.; Faltas, B.M.; Christos, P.J.; Scherr, D.S. Recurrence patterns after open and robot-assisted radical cystectomy for bladder cancer. Eur. Urol. 2015, 68, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, A.S.; Gibson, S.; Jing, Z.; Wijburg, C.; Wagner, A.A.; Mottrie, A.; Dasgupta, P.; Peabody, J.; Hussein, A.A.; Guru, K.A. Rates and Patterns of Recurrences and Survival Outcomes after Robot-Assisted Radical Cystectomy: Results from the International Robotic Cystectomy Consortium. J. Urol. 2021, 205, 407–413. [Google Scholar] [CrossRef]
- Koie, T.; Ohyama, C.; Makiyama, K.; Shimazui, T.; Miyagawa, T.; Mizutani, K.; Tsuchiya, T.; Kato, T.; Nakake, K. Utility of robot-assisted radical cystectomy with intracorporeal urinary diversion for muscle-invasive bladder cancer. Int. J. Urol. 2019, 26, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Nakane, K.; Enomoto, T.; Tomioka, M.; Taniguchi, T.; Kawase, M.; Kato, D.; Takai, M.; Iinuma, K.; Seike, K.; Hagiwara, N.; et al. Favorable surgical outcomes and perioperative complication rates after robotic radical cystectomy and intracorporeal urinary diversion at a single, low-volume center: Initial experience with 65 consecutive cases. World J. Clin. Surg. 2021, 4, 1–6. [Google Scholar] [CrossRef]
- Hussein, A.A.; May, P.R.; Jing, Z.; Ahmed, Y.E.; Wijburg, C.J.; Canda, A.E.; Dasgupta, P.; Shamim Khan, M.; Menon, M.; Peabody, J.O.; et al. Outcomes of Intracorporeal Urinary Diversion after Robot-Assisted Radical Cystectomy: Results from the International Robotic Cystectomy Consortium. J. Urol. 2018, 199, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.A.; Dibaj, S.; Hinata, N.; Field, E.; O’leary, K.; Kuvshinoff, B.; Mohler, J.L.; Wilding, G.; Guru, K.A. Development and Validation of a Quality Assurance Score for Robot-assisted Radical Cystectomy: A 10-year Analysis. Urology 2016, 97, 124–129. [Google Scholar] [CrossRef]
- Mastroianni, R.; Ferriero, M.; Tuderti, G.; Anceschi, U.; Bove, A.M.; Brassetti, A.; Misuraca, L.; Zampa, A.; Torregiani, G.; Ghiani, E.; et al. Open Radical Cystectomy versus Robot-Assisted Radical Cystectomy with Intracorporeal Urinary Diversion: Early Outcomes of a Single-Center Randomized Controlled Trial. J. Urol. 2022, 207, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.W.; Tyritzis, S.; Nyberg, T.; Schumacher, M.C.; Laurin, O.; Adding, C.; Jonsson, M.; Khazaeli, D.; Steineck, G.; Wiklund, P.; et al. Robot-assisted radical cystectomy (RARC) with intracorporeal neobladder—What is the effect of the learning curve on outcomes? BJU Int. 2014, 113, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Filson, C.P.; Tan, H.J.; Chamie, K.; Laviana, A.A.; Hu, J.C. Determinants of radical cystectomy operative time. Urol. Oncol. 2016, 34, 431.e17-24. [Google Scholar] [CrossRef] [PubMed]
- Nakane, K.; Muramatsu Maekawa, Y.; Iinuma, K.; Mizutani, K.; Makiyama, K.; Koie, T. Utility technique of a totally intracorporeal ileal conduit after robot-assisted radical cystectomy. Int. J. Urol. 2019, 26, 1083–1084. [Google Scholar] [CrossRef] [PubMed]
- Koie, T.; Ohyama, C.; Yoneyama, T.; Nagasaka, H.; Yamamoto, H.; Imai, A.; Hatakeyama, S.; Hashimoto, Y. Robotic cross-folded U-configuration intracorporeal ileal neobladder for muscle-invasive bladder cancer: Initial experience and functional outcomes. Int. J. Med. Robot. 2018, 14, e1955. [Google Scholar] [CrossRef] [PubMed]
- Koie, T.; Ohyama, C.; Yamamoto, H.; Imai, A.; Hatakeyama, S.; Yoneyama, T.; Hashimoto, Y.; Yoneyama, T.; Tobisawa, Y.; Yamauchi, A.; et al. The feasibility and effectiveness of robot-assisted radical cystectomy after neoadjuvant chemotherapy in patients with muscle-invasive bladder cancer. Jpn. J Clin Oncol. 2017, 47, 252–256. [Google Scholar] [CrossRef][Green Version]
- Ariyoshi, A.; Fusijawa, Y.; Ohshima, K.; Hiratsuka, Y.; Sakamoto, K. Catheterless cutaneous ureterostomy. J. Urol. 1975, 114, 533–535. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Hayn, M.H.; Hussain, A.; Mansour, A.M.; Andrews, P.E.; Carpentier, P.; Castle, E.; Dasgupta, P.; Rimington, P.; Thomas, R.; Khan, S.; et al. Learning curve of robot-assisted radical cystectomy: Results from the International Robotic Cystectomy Consortium. Eur. Urol. 2010, 58, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Wijburg, C.J.; Hannink, G.; Michels, C.T.J.; Weijerman, P.C.; Issa, R.; Tay, A.; Decaestecker, K.; Wiklund, P.; Hosseini, A.; Sridhar, A.; et al. Learning curve analysis for intracorporeal robot-assisted radical cystectomy: Results from the EAU Robotic Urology Section Scientific Working Group. Eur. Urol. Open Sci. 2022, 39, 55–61. [Google Scholar] [CrossRef]
- López-Molina, C.; Carrion, A.; Campistol, M.; Piñero, A.; Lozano, F.; Salvador, C.; Raventós, C.X.; Trilla, E. Evaluating the impact of the learning curve on the perioperative outcomes of robot-assisted radical cystectomy with intracorporeal urinary diversion. Actas Urol. Esp. 2022, 46, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Tuderti, G.; Mastroianni, R.; Brassetti, A.; Bove, A.M.; Misuraca, L.; Anceschi, U.; Ferriero, M.; Gallucci, M.; Simone, G. Robot-assisted radical cystectomy with intracorporeal neobladder: Impact of learning curve and long-term assessment of functional outcomes. Minerva Urol. Nephrol. 2021, 73, 754–762. [Google Scholar] [PubMed]
- Tanneru, K.; Jazayeri, S.B.; Kumar, J.; Alam, M.U.; Norez, D.; Nguyen, S.; Bazargani, S.; Ganapathi, H.P.; Bandyk, M.; Marino, R.; et al. Intracorporeal versus extracorporeal urinary diversion following robot-assisted radical cystectomy: A meta-analysis, cumulative analysis, and systematic review. J. Robot. Surg. 2021, 15, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Mortezavi, A.; Crippa, A.; Kotopouli, M.I.; Akre, O.; Wiklund, P.; Hosseini, A. Association of Open vs Robot-Assisted Radical Cystectomy With Mortality and Perioperative Outcomes Among Patients With Bladder Cancer in Sweden. JAMA Netw. Open. 2022, 5, e228959. [Google Scholar] [CrossRef] [PubMed]
- Bertolo, R.; Agudelo, J.; Garisto, J.; Armanyous, S.; Fergany, A.; Kaouk, J. Perioperative Outcomes and Complications after Robotic Radical Cystectomy With Intracorporeal or Extracorporeal Ileal Conduit Urinary Diversion: Head-to-head Comparison From a Single-Institutional Prospective Study. Urology 2019, 129, 98–105. [Google Scholar] [CrossRef]
- Tan, T.W.; Nair, R.; Saad, S.; Thurairaja, R.; Khan, M.S. Safe transition from extracorporeal to intracorporeal urinary diversion following robot-assisted cystectomy: A recipe for reducing operative time, blood loss and complication rates. World J. Urol. 2019, 37, 367–372. [Google Scholar] [CrossRef]
- Dell’Oglio, P.; Turri, F.; Larcher, A.; D’Hondt, F.; Sanchez-Salas, R.; Bochner, B.; Palou, J.; Weston, R.; Hosseini, A.; Canda, A.E.; et al. Definition of a Structured Training Curriculum for Robot-assisted Radical Cystectomy with Intracorporeal Ileal Conduit in Male Patients: A Delphi Consensus Study Led by the ERUS Educational Board. Eur. Urol. Focus. 2022, 8, 160–164. [Google Scholar] [CrossRef] [PubMed]


| Clinical Covariates | Total | First-Generation Surgeon | Second-Generation Surgeon | Third-Generation Surgeons | p-Value |
|---|---|---|---|---|---|
| Number | 100 | 19 | 38 | 43 | |
| Age, year [median (IQR)] | 73.0 (67.0–78.0) | 73.0 (65.5–78.5) | 74.0 (67.2–78.0) | 73.0 (68.5–78.0) | 0.900 |
| Gender [number (%)] | 0.085 | ||||
| Male | 78 (78.0) | 14 (73.7) | 26 (68.4) | 38 (88.4) | |
| Female | 22 (22.0) | 5 (26.3) | 12 (31.6) | 5 (11.6) | |
| BMI, kg/m2 [median (IQR)] | 23.1 (20.2–25.2) | 24.0 (19.9–26.6) | 21.8 (19.8–24.1) | 24.1 (21.9–25.6) | 0.070 |
| Clinical T-stage [number (%)] | 0.374 | ||||
| T0 | 7 (7.0) | 1 (5.3) | 3 (7.9) | 3 (7.1) | |
| Tis | 3 (3.0) | 1 (5.3) | 1 (2.6) | 1 (2.4) | |
| T1 | 7 (7.0) | 1 (5.3) | 1 (2.6) | 5 (11.9) | |
| T2 | 43 (43.0) | 4 (21.1) | 17 (44.7) | 22 (52.4) | |
| T3 | 27 (27.0) | 8 (42.1) | 11 (28.9) | 8 (19.0) | |
| T4 | 12 (12.0) | 4 (21.1) | 5 (13.2) | 3 (7.1) | |
| Tx | 1 (1.0) | 1 (5.3) | 0 (0.0) | 0 (0.0) | |
| Clinical N-stage, number (%) | 0.813 | ||||
| negative | 90 (90.0) | 16 (84.2) | 34 (89.5) | 40 (93.0) | |
| positive | 10 (10.0) | 3 (15.8) | 4 (10.5) | 3 (7.0) | |
| Clinical M-stage, number (%) | 0.512 | ||||
| M0 | 99 (99.0) | 19 (100.0) | 38 (100.0) | 42 (97.7) | |
| M1 | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (2.3) | |
| Neoadjuvant chemotherapy, number (%) | 69 (69.0) | 14 (73.7) | 27 (71.1) | 28 (65.1) | 0.751 |
| Follow-up period, months [median (IQR)] | 9.0 (3.0–24.0) | 14.0 (4.0–29.0) | 11.5 (4.0–25.0) | 6.0 (2.5–14.0) | 0.105 |
| Clinical Characteristics | Total | First-Generation Surgeon | Second-Generation Surgeon | Third-Generation Surgeon | p-Value |
|---|---|---|---|---|---|
| Operative time, minutes [median (IQR)] | 398.5 (296.7–484.2) | 457.0 (319.0–484.5) | 389.5 (281.7–461.2) | 400.0 (319.0–483.5) | 0.838 |
| Time for cystectomy, minutes [median (IQR)] | 122.0 (100.5–142.0) | 106.0 (96.0–133.0) | 110.0(98.0–134.2) | 130.5 (115.0–151.7) | 0.019 |
| Estimated blood loss, mL [median (IQR)] | 205.0 (100.0–396.2) | 240.0 (105.0–392.5) | 150.0(96.2–261.2) | 320.0 (137.5–420.0) | 0.137 |
| Blood transfusion [number (%)] | 8 (8.0) | 0 (0.0) | 3 (7.9) | 5 (11.6) | 0.298 |
| Intraoperativecomplications [number (%)] | 6 (6.0) | 2 (10.5) | 1 (2.6) | 3 (7.0) | 0.466 |
| Pathological T stage [number, %] | 0.079 | ||||
| pT0 | 19 (19.6) | 4 (21.1) | 8 (21.1) | 7 (17.5) | |
| pT1 | 8 (8.2) | 0 | 3 (7.9) | 5 (12.5) | |
| pT2 | 20 (20.6) | 3 (15.8) | 7 (18.4) | 10 (25.0) | |
| pT3 | 31 (32.0) | 3 (15.8) | 14 (36.8) | 14 (35.0) | |
| pT4 | 10 (10.3) | 6 (31.6) | 3 (7.9) | 1 (2.5) | |
| pTa/pTis | 9 (9.2) | 3 (15.8) | 3 (7.9) | 3 (7.9) | |
| Pathological N stage [number, %] | 0.191 | ||||
| pN0 | 35 (35.7) | 6 (31.6) | 10 (26.3) | 19 (46.3) | |
| pN1 | 5 (5.1) | 0 (0.0) | 4 (10.5) | 1 (2.4) | |
| pN2 | 2 (2.0) | 1 (5.3) | 0 (0.0) | 1 (2.4) | |
| pNx | 56 (57.1) | 12 (63.2) | 24 (63.2) | 20 (48.8) | |
| Surgical margin [number (%)] | 0.140 | ||||
| RM0 | 95 (95.0) | 16 (84.2) | 38 (100.0) | 41 (95.3) | |
| RM1 | 2 (2.0) | 1 (5.3) | 0 (0.0) | 1 (2.3) | |
| RMx | 3 (3.0) | 2 (10.5) | 0 (0.0) | 1 (2.3) | |
| Time-to-liquid intake, days [median (IQR)] | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | >0.999 |
| Time-to-solid intake, days [median (IQR)] | 3.0 (2.0–3.0) | 3.0 (2.0–3.0) | 2.0 (2.0–3.0) | 3.0 (2.0–4.0) | 0.495 |
| LOS, days [median (IQR)] | 18.0 (15.0–22.0) | 19.0 (16.5–21.0) | 18.5 (14.0–21.7) | 18.0 (15.0–22.5) | 0.583 |
| Total | First-Generation Surgeon | Second-Generation Surgeon | Third-Generation Surgeons | p-Value | |
|---|---|---|---|---|---|
| Number | 100 | 25 | 37 | 37 | |
| Urinary diversion type [number (%)] | |||||
| Ileal conduit | 35 (35.0) | 8 (32.0) | 10 (27.0) | 17 (45.9) | 0.031 |
| Neobladder | 25 (25.0) | 11 (44.0) | 10 (27.0) | 4 (10.8) | |
| Ureterocutaneostomy | 39 (39.0) | 6 (24.0) | 17 (45.9) | 16 (43.2) | |
| Urinary diversion operative time, minutes [median (interquartile range)] | |||||
| Ileal conduit | 104.0 (91.0–120.0) | 106.5 (88.7–126.7) | 106.5 (93.7–118.5) | 100.0 (94.0–114.0) | 0.961 |
| Neobladder | 182.0 (159.0–231.0) | 182.0 (165.5–218.5) | 219.0 (162.0–262.5) | 169.5 (153.0–185.0) | 0.421 |
| Ureterocutaneostomy | 28.0 (20.0–36.7) | 26.0 (25.2–29.0) | 28.0 (22.2–31.5) | 28.5 (20.0–48.5) | 0.829 |
| Complication [Number (%)] | Total | First-Generation Surgeon | Second-Generation Surgeon | Third-Generation Surgeons | p-Value |
|---|---|---|---|---|---|
| Any grade | |||||
| Ileus | 16 (16.0) | 2 (10.5) | 7 (18.4) | 7 (16.7) | 0.742 |
| Pyelonephritis | 24 (24.0) | 3 (15.8) | 11 (28.9) | 10 (23.3) | 0.542 |
| Sepsis | 4 (4.0) | 1 (5.3) | 1 (2.6) | 2 (4.7) | 0.856 |
| Pelvic abscess | 3 (3.0) | 0 | 1 (2.6) | 2 (4.7) | 0.604 |
| Surgical site infection | 2 (2.0) | 0 | 0 | 2 (4.7) | 0.259 |
| Lymphorrhea | 1 (1.0) | 1 (5.3) | 0 | 0 | 0.116 |
| Cardiac disorder | 2 (2.0) | 0 | 1 (2.6) | 1 (2.3) | 0.783 |
| Anastomotic leakage | 2 (2.0) | 1 (5.3) | 1 (2.6) | 0 | 0.370 |
| Anastomotic stricture | 4 (4.0) | 0 | 1 (2.6) | 3 (7.0) | 0.374 |
| Grade ≥3 | |||||
| Anastomotic stricture | 4 (4.0) | 0 | 1 (2.6) | 3 (7.0) | 0.374 |
| Pelvic abscess | 2 (2.0) | 0 | 1 (2.6) | 1 (2.3) | 0.783 |
| Sepsis | 2 (2.0) | 0 | 1 (2.6) | 1 (2.3) | 0.783 |
| Anastomotic leakage | 1 (1.0) | 0 | 1 (2.6) | 0 | 0.370 |
| Surgical site infection | 1 (1.0) | 0 | 0 | 1 (2.3) | 0.512 |
| Lymphorrhea | 1 (1.0) | 1 (5.3) | 0 | 0 | 0.116 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakane, K.; Yamada, T.; Tomioka-Inagawa, R.; Sugino, F.; Kumada, N.; Kawase, M.; Takeuchi, S.; Kawase, K.; Kato, D.; Takai, M.; et al. Efficacy and Safety of the “Trisection Method” Training System for Robot-Assisted Radical Cystectomy at a Single Institution in Japan. Curr. Oncol. 2022, 29, 9294-9304. https://doi.org/10.3390/curroncol29120728
Nakane K, Yamada T, Tomioka-Inagawa R, Sugino F, Kumada N, Kawase M, Takeuchi S, Kawase K, Kato D, Takai M, et al. Efficacy and Safety of the “Trisection Method” Training System for Robot-Assisted Radical Cystectomy at a Single Institution in Japan. Current Oncology. 2022; 29(12):9294-9304. https://doi.org/10.3390/curroncol29120728
Chicago/Turabian StyleNakane, Keita, Toyohiro Yamada, Risa Tomioka-Inagawa, Fumiya Sugino, Naotaka Kumada, Makoto Kawase, Shinichi Takeuchi, Kota Kawase, Daiki Kato, Manabu Takai, and et al. 2022. "Efficacy and Safety of the “Trisection Method” Training System for Robot-Assisted Radical Cystectomy at a Single Institution in Japan" Current Oncology 29, no. 12: 9294-9304. https://doi.org/10.3390/curroncol29120728
APA StyleNakane, K., Yamada, T., Tomioka-Inagawa, R., Sugino, F., Kumada, N., Kawase, M., Takeuchi, S., Kawase, K., Kato, D., Takai, M., Iinuma, K., & Koie, T. (2022). Efficacy and Safety of the “Trisection Method” Training System for Robot-Assisted Radical Cystectomy at a Single Institution in Japan. Current Oncology, 29(12), 9294-9304. https://doi.org/10.3390/curroncol29120728






