Concomitant Bladder Tumor Is a Risk Factor for Bladder Recurrence but Not Upper Tract
Abstract
:1. Introduction
2. Patients and Method
2.1. Data Source
2.2. Statistical Methods
3. Results
3.1. Patient Demographics
3.2. Comparison between Concomitant and Non-Concomitant Bladder Tumor Patients
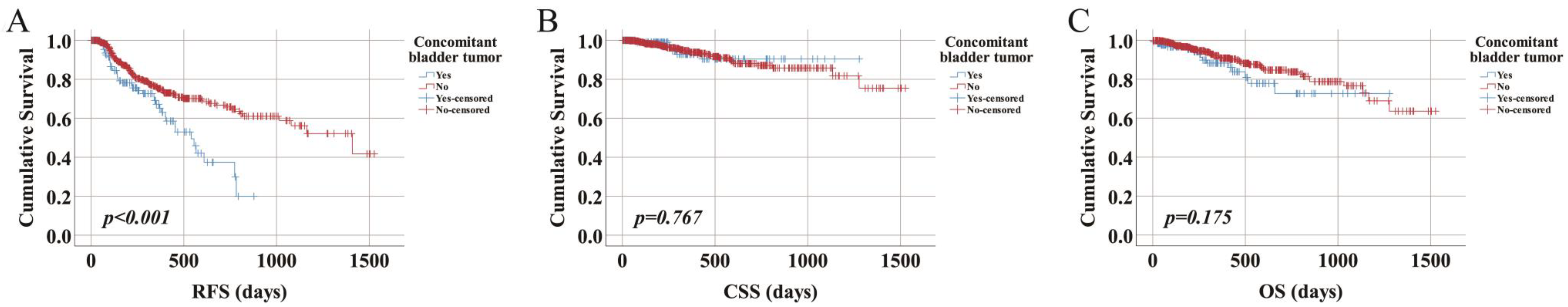
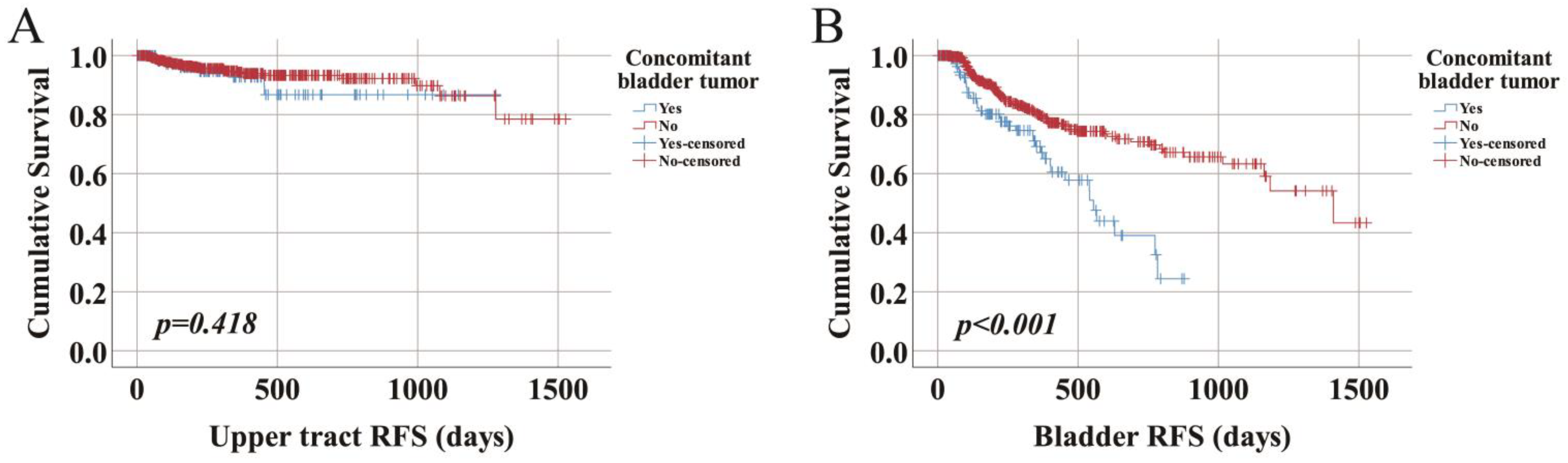
3.3. Recurrence-Free Survival
3.4. Cancer-Specific Survival
3.5. Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Liedberg, F.; Kjellström, S.; Lind, A.K.; Sherif, A.; Söderkvist, K.; Falkman, K.; Thulin, H.; Aljabery, F.; Papantonio, D.; Ströck, V.; et al. Swedish National Guidelines on Urothelial Carcinoma: 2021 update on non-muscle invasive bladder cancer and upper tract urothelial carcinoma. Scand. J. Urol. 2022, 56, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Soria, F.; Shariat, S.F.; Lerner, S.P.; Fritsche, H.M.; Rink, M.; Kassouf, W.; Spiess, P.E.; Lotan, Y.; Ye, D.; Fernández, M.I.; et al. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J. Urol. 2017, 35, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Carpinito, G.P.; Cook, G.S.; Tverye, A.N.; Gold, S.A.; Lotan, Y.; Margulis, V.; Howard, J.M. Outcomes of patients undergoing concurrent radical cystectomy and nephroureterectomy: A single-institution series. Can. Urol. Assoc. J. 2022, 16, 7. [Google Scholar] [CrossRef]
- Cosentino, M.; Palou, J.; Gaya, J.M.; Breda, A.; Rodriguez-Faba, O.; Villavicencio-Mavrich, H. Upper urinary tract urothelial cell carcinoma: Location as a predictive factor for concomitant bladder carcinoma. World J. Urol. 2013, 31, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.C.; Womack, S.; Sagalowsky, A.I.; Carmody, T.; Erickstad, M.D.; Roehrborn, C.G. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: A 30-year experience in 252 patients. Urology 1998, 52, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Olgac, S.; Mazumdar, M.; Dalbagni, G.; Reuter, V.E. Urothelial carcinoma of the renal pelvis: A clinicopathologic study of 130 cases. Am. J. Surg. Pathol. 2004, 28, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Teoh, J.Y.; Kamat, A.M.; Black, P.C.; Grivas, P.; Shariat, S.F.; Babjuk, M. Recurrence mechanisms of non-muscle-invasive bladder cancer—A clinical perspective. Nat. Rev. Urol. 2022, 19, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Baard, J.; Celebi, M.; de la Rosette, J.; Alcaraz, A.; Shariat, S.; Cormio, L.; Cavadas, V.; Laguna, M.P. Evaluation of Patterns of Presentation, Practice, and Outcomes of Upper Tract Urothelial Cancer: Protocol for an Observational, International, Multicenter, Cohort Study by the Clinical Research Office of the Endourology Society. JMIR Res. Protoc. 2020, 9, e15363. [Google Scholar] [CrossRef] [PubMed]
- Baard, J.; Cormio, L.; Cavadas, V.; Alcaraz, A.; Shariat, S.F.; de la Rosette, J.; Laguna, M.P. Contemporary patterns of presentation, diagnostics and management of upper tract urothelial cancer in 101 centres: The Clinical Research Office of the Endourological Society Global upper tract urothelial carcinoma registry. Curr. Opin. Urol. 2021, 31, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Kuroiwa, K.; Inokuchi, J.; Nishiyama, H.; Kojima, T.; Kakehi, Y.; Sugimoto, M.; Tanigawa, T.; Fujimoto, H.; Gotoh, M.; Masumori, N.; et al. Impact of Previous, Simultaneous or Subsequent Bladder Cancer on Prognosis after Radical Nephroureterectomy for Upper Urinary Tract Urothelial Carcinoma. J. Urol. 2019, 202, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Colin, P.; Koenig, P.; Ouzzane, A.; Berthon, N.; Villers, A.; Biserte, J.; Rouprêt, M. Environmental factors involved in carcinogenesis of urothelial cell carcinomas of the upper urinary tract. BJU Int. 2009, 104, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- Singla, N.; Fang, D.; Su, X.; Bao, Z.; Cao, Z.; Robyak, H.; Xiong, G.; Zhang, L.; Woldu, S.; Hutchinson, R.; et al. Preoperative predictors of nonorgan-confined disease in upper-tract urothelial carcinoma differ between China and the United States. Urol. Oncol. 2018, 36, 88.e11–88.e18. [Google Scholar] [CrossRef] [PubMed]
- Giudici, N.; Bonne, F.; Blarer, J.; Minoli, M.; Krentel, F.; Seiler, R. Characteristics of upper urinary tract urothelial carcinoma in the context of bladder cancer: A narrative review. Transl. Androl. Urol. 2021, 10, 4036–4050. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Ying, Y.; Yu, X.; Wang, L.; Zhang, Z.; Xu, C. Impact of previous, simultaneous or intravesical recurrence bladder cancer on prognosis of upper tract urothelial carcinoma after nephroureterectomy: A large population-based study. Transl. Androl. Urol. 2021, 10, 4365–4375. [Google Scholar] [CrossRef] [PubMed]
- Milojevic, B.; Djokic, M.; Sipetic-Grujicic, S.; Grozdic Milojevic, I.; Vuksanovic, A.; Nikic, P.; Vukovic, I.; Djordjevic, D.; Bumbasirevic, U.; Tulic, C. Prognostic significance of non-muscle-invasive bladder tumor history in patients with upper urinary tract urothelial carcinoma. Urol. Oncol. 2013, 31, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Pignot, G.; Colin, P.; Zerbib, M.; Audenet, F.; Soulié, M.; Hurel, S.; Delage, F.; Irani, J.; Descazeaud, A.; Droupy, S.; et al. Influence of previous or synchronous bladder cancer on oncologic outcomes after radical nephroureterectomy for upper urinary tract urothelial carcinoma. Urol. Oncol. 2014, 32, 23.e1–23.e8. [Google Scholar] [CrossRef]
- Seisen, T.; Peyronnet, B.; Dominguez-Escrig, J.L.; Bruins, H.M.; Yuan, C.Y.; Babjuk, M.; Böhle, A.; Burger, M.; Compérat, E.M.; Cowan, N.C.; et al. Oncologic Outcomes of Kidney-sparing Surgery Versus Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: A Systematic Review by the EAU Non-muscle Invasive Bladder Cancer Guidelines Panel. Eur. Urol. 2016, 70, 1052–1068. [Google Scholar] [CrossRef] [PubMed]
- EAU Guidelines. Edn. Presented at the EAU Annual Congress Amsterdam, 2022. ISBN 978-94-92671-16-5. Available online: http://uroweb.org/guidelines/compilations-of-all-guidelines/ (accessed on 10 May 2022).
- Chromecki, T.F.; Cha, E.K.; Fajkovic, H.; Margulis, V.; Novara, G.; Scherr, D.S.; Lotan, Y.; Raman, J.D.; Kassouf, W.; Bensalah, K.; et al. The impact of tumor multifocality on outcomes in patients treated with radical nephroureterectomy. Eur. Urol. 2012, 61, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Mbeutcha, A.; Rouprêt, M.; Kamat, A.M.; Karakiewicz, P.I.; Lawrentschuk, N.; Novara, G.; Raman, J.D.; Seitz, C.; Xylinas, E.; Shariat, S.F. Prognostic factors and predictive tools for upper tract urothelial carcinoma: A systematic review. World J. Urol. 2017, 35, 337–353. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Concomitant Bladder Tumor N = 218 (n, %) | Non-Concomitant Bladder Tumor N = 916 (n, %) | Total N = 1134 (n, %) | p-Value |
|---|---|---|---|---|
| Age | ||||
| ≤70 | 89 (40.8) | 441 (48.1) | 530 (46.7) | 0.047 |
| >70 | 129 (59.2) | 472 (51.5) | 601 (53.0) | |
| Male gender | 172 (78.9) | 642 (70.1) | 814 (71.8) | 0.010 |
| Smoking status | ||||
| No | 58 (26.6) | 301 (32.9) | 285 (25.1) | 0.047 |
| Yes, Present | 50 (22.9) | 235 (25.7) | 379 (33.4) | |
| Yes, Past | 87 (39.9) | 292 (31.9) | 359 (31.7) | |
| ASA | ||||
| I | 23 (10.6) | 119 (13.0) | 142 (12.5) | 0.115 |
| II | 115 (52.8) | 447 (48.8) | 562 (49.6) | |
| III | 67 (30.7) | 319 (34.8) | 386 (34.0) | |
| IV | 8 (3.7) | 20 (2.2) | 28 (2.5) | |
| V | 1 (0.5) | 0 (0) | 1 (0.1) | |
| CCI | ||||
| 0 | 47 (21.6) | 211 (23.0) | 258 (22.8) | 0.731 |
| 1 | 39 (17.9) | 140 (15.3) | 179 (15.8) | |
| 2 | 31 (14.2) | 143 (15.6) | 174 (15.3) | |
| 3 | 23 (10.6) | 74 (8.1) | 97 (8.6) | |
| 4 | 13 (6.0) | 59 (6.4) | 72 (6.3) | |
| 5 | 4 (1.8) | 20 (2.2) | 24 (2.1) | |
| 6 | 6 (2.8) | 20 (2.2) | 26 (2.3) | |
| 7 | 4 (1.8) | 10 (1.1) | 14 (1.2) | |
| 8 | 2 (0.9) | 2 (0.2) | 4 (0.4) | |
| 9 | 0 (0) | 4 (0.4) | 4 (0.4) | |
| 10 | 0 (0) | 1 (0.1) | 1 (0.1) | |
| Procedure | ||||
| RNU performed | 193 (88.5) | 854 (93.2) | 1047 (92.3) | 0.019 |
| KSS performed | 25 (11.5) | 62 (6.8) | 87 (7.7) | |
| Tumor stage (2009) | ||||
| pTa | 44 (20.2) | 165 (18.0) | 209 (18.4) | 0.789 |
| pTis | 5 (2.3) | 13 (1.4) | 18 (1.6) | |
| pT1 | 39 (17.9) | 191 (20.9) | 230 (20.3) | |
| pT2 | 37 (17.0) | 166 (18.1) | 203 (17.9) | |
| pT3 | 56 (25.7) | 259 (28.3) | 315 (27.8) | |
| pT4 | 6 (2.8) | 31 (3.4) | 37 (3.3) | |
| Tumor grade | ||||
| G1 | 26 (11.9) | 107 (11.7) | 133 (11.7) | 0.342 |
| G2 | 42 (19.3) | 230 (25.1) | 272 (24.0) | |
| G3 | 108 (49.5) | 465 (50.8) | 573 (50.5) | |
| GX | 4 (1.8) | 8 (0.9) | 12 (1.1) | |
| Multifocal tumor | ||||
| No | 141 (64.7) | 650 (71.0) | 791 (69.8) | 0.004 |
| Yes | 60 (27.5) | 166 (18.1) | 226 (19.9) |
| Characteristics | HR (95% CI) | p-Value |
|---|---|---|
| Concomitant bladder tumor | ||
| No | Reference | |
| Yes | 1.562 (0.954–2.560) | 0.076 |
| Age | ||
| <70 | Reference | |
| ≥70 | 1.118 (0.728–1.718) | 0.611 |
| Gender | ||
| Female | Reference | |
| Male | 0.665 (0.383–1.154) | 0.147 |
| Smoking status | ||
| No | Reference | |
| Yes | 1.318 (0.778–2.234) | 0.305 |
| ASA | ||
| I-II | Reference | |
| III-V | 1.088 (0.706–1.676) | 0.703 |
| CCI | ||
| 0 | Reference | |
| 1–2 | 0.771 (0.465–1.278) | 0.313 |
| 3–10 | 1.302 (0.753–2.251) | 0.346 |
| Procedure | ||
| RNU | Reference | |
| KSS | 1.120 (0.547–2.293) | 0.756 |
| Tumor stage | ||
| <pT2 | Reference | |
| ≥pT2 | 1.276 (0.793–2.055) | 0.315 |
| Tumor grade | ||
| G1 | Reference | |
| G2 | 0.942 (0.490–1.812) | 0.859 |
| G3 | 0.600 (0.305–1.180) | 0.139 |
| Multifocal tumor | ||
| No | Reference | |
| Yes | 1.611 (1.017–2.550) | 0.042 |
| Characteristics | HR (95% CI) | p-Value |
|---|---|---|
| Concomitant bladder tumor | ||
| No | Reference | |
| Yes | 0.876 (0.292–2.625) | 0.812 |
| Age | ||
| <70 | Reference | |
| ≥70 | 0.811 (0.346–1.900) | 0.629 |
| Gender | ||
| Female | Reference | |
| Male | 0.930 (0.301–2.871) | 0.900 |
| Smoking status | ||
| No | Reference | |
| Yes | 1.473 (0.515–4.214) | 0.470 |
| ASA | ||
| I-II | Reference | |
| III-V | 0.749 (0.316–1.779) | 0.513 |
| CCI | ||
| 0 | Reference | |
| 1–2 | 0.352 (0.117–1.062) | 0.064 |
| 3–10 | 1.285 (0.487–3.390) | 0.613 |
| Procedure | ||
| RNU | Reference | |
| KSS | 3.940 (1.352–11.486) | 0.012 |
| Tumor stage | ||
| <pT2 | Reference | |
| ≥pT2 | 2.840 (1.039–7.763) | 0.042 |
| Tumor grade | ||
| G1 | Reference | |
| G2 | 1.542 (0.308–7.718) | 0.598 |
| G3 | 0.885 (0.165–4.737) | 0.886 |
| Multifocal tumor | ||
| No | Reference | |
| Yes | 1.741 (0.667–4.543) | 0.257 |
| Characteristics | HR (95% CI) | p-Value |
|---|---|---|
| Concomitant bladder tumor | ||
| No | Reference | |
| Yes | 1.874 (1.104–3.183) | 0.020 |
| Age | ||
| <70 | Reference | |
| ≥70 | 1.288 (0.796–2.084) | 0.303 |
| Gender | ||
| Female | Reference | |
| Male | 0.646 (0.353–1.180) | 0.155 |
| Smoking status | ||
| No | Reference | |
| Yes | 1.225 (0.690–2.175) | 0.488 |
| ASA | ||
| I-II | Reference | |
| III-V | 1.040 (0.646–1.673) | 0.872 |
| CCI | ||
| 0 | Reference | |
| 1–2 | 0.816 (0.465–1.430) | 0.477 |
| 3–10 | 1.357 (0.742–2.483) | 0.322 |
| Procedure | ||
| RNU | Reference | |
| KSS | 0.619 (0.242–1.580) | 0.315 |
| Tumor stage | ||
| <pT2 | Reference | |
| ≥pT2 | 1.043 (0.622–1.749) | 0.874 |
| Tumor grade | ||
| G1 | Reference | |
| G2 | 0.861 (0.424–1.749) | 0.679 |
| G3 | 0.615 (0.296–1.278) | 0.193 |
| Multifocal tumor | ||
| No | Reference | |
| Yes | 1.559 (0.941–2.581) | 0.085 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Zhao, H.; Alvarez-Maestro, M.; Gravas, S.; Van Renterghem, K.; Zeng, G.; Ng, C.-F.; Laguna, P.; Teoh, J.Y.-C.; De La Rosette, J. Concomitant Bladder Tumor Is a Risk Factor for Bladder Recurrence but Not Upper Tract. Curr. Oncol. 2022, 29, 9284-9293. https://doi.org/10.3390/curroncol29120727
Liu K, Zhao H, Alvarez-Maestro M, Gravas S, Van Renterghem K, Zeng G, Ng C-F, Laguna P, Teoh JY-C, De La Rosette J. Concomitant Bladder Tumor Is a Risk Factor for Bladder Recurrence but Not Upper Tract. Current Oncology. 2022; 29(12):9284-9293. https://doi.org/10.3390/curroncol29120727
Chicago/Turabian StyleLiu, Kang, Hongda Zhao, Mario Alvarez-Maestro, Stavros Gravas, Koen Van Renterghem, Guohua Zeng, Chi-Fai Ng, Pilar Laguna, Jeremy Yuen-Chun Teoh, and Jean De La Rosette. 2022. "Concomitant Bladder Tumor Is a Risk Factor for Bladder Recurrence but Not Upper Tract" Current Oncology 29, no. 12: 9284-9293. https://doi.org/10.3390/curroncol29120727
APA StyleLiu, K., Zhao, H., Alvarez-Maestro, M., Gravas, S., Van Renterghem, K., Zeng, G., Ng, C.-F., Laguna, P., Teoh, J. Y.-C., & De La Rosette, J. (2022). Concomitant Bladder Tumor Is a Risk Factor for Bladder Recurrence but Not Upper Tract. Current Oncology, 29(12), 9284-9293. https://doi.org/10.3390/curroncol29120727








