Recent Advances in Photodynamic Imaging and Therapy in Hepatobiliary Malignancies: Clinical and Experimental Aspects
Abstract
1. Introduction
1.1. Photomedicine and Photodynamic Reaction
1.2. Mechanism of Photodynamic Reaction and PS in Digestive Cancers
2. PDT
2.1. Effect on Tumors
2.2. PDT for Primary Liver Malignancies
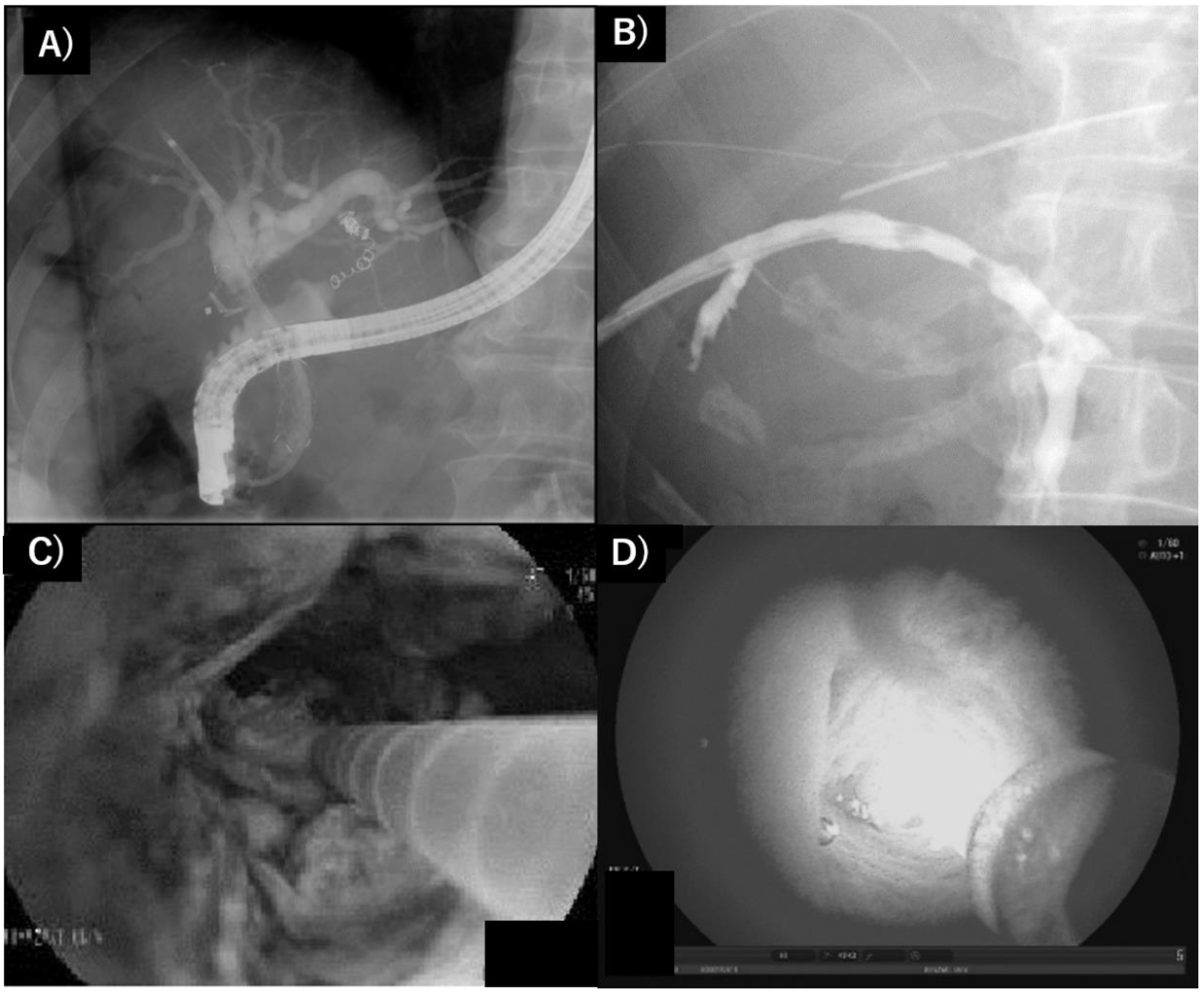
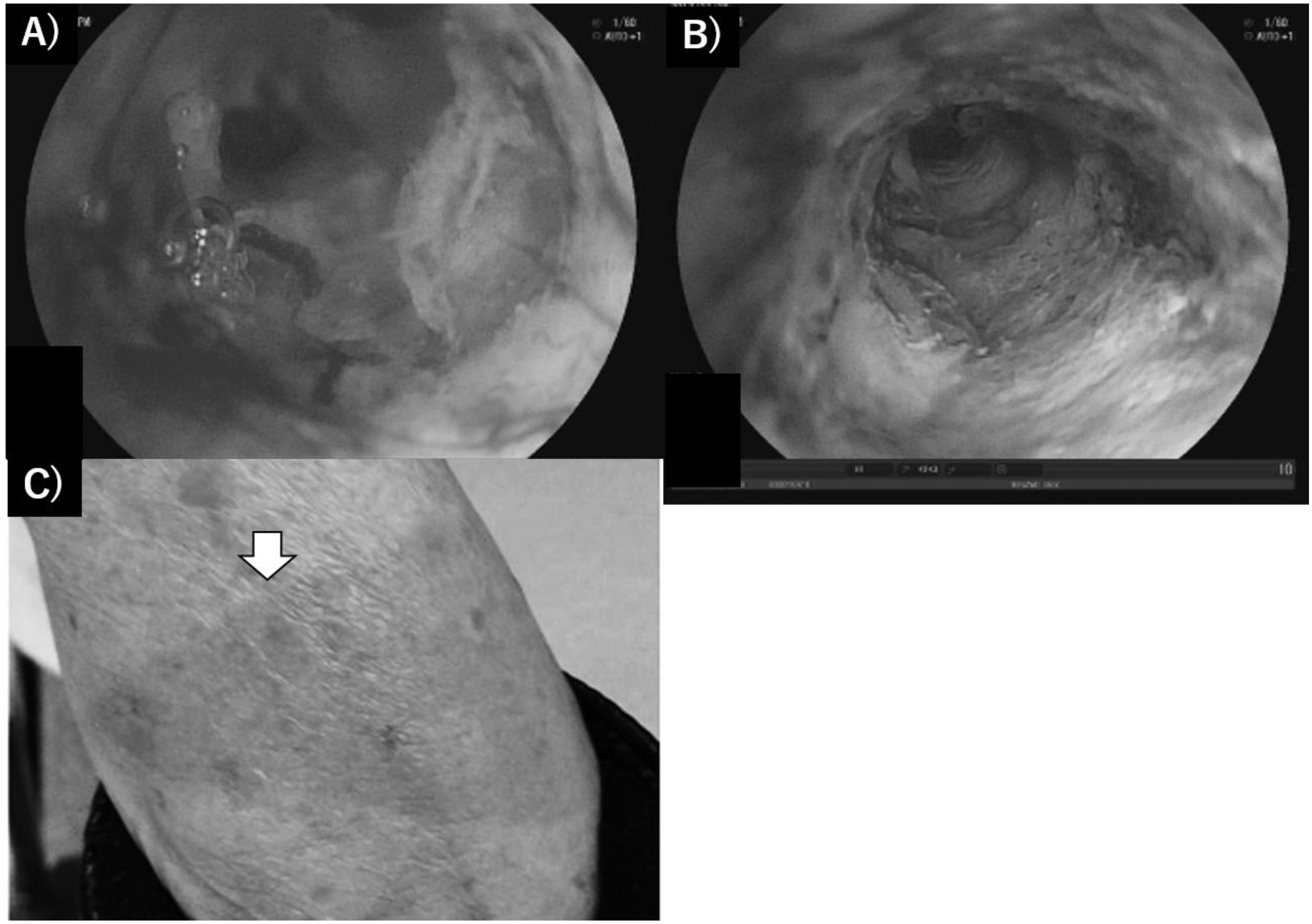
2.3. Extrahepatic Cholangiocarcinoma
2.4. Limitation and Disadvantages of PDT in Hepatobiliary Malignancies
3. PDD
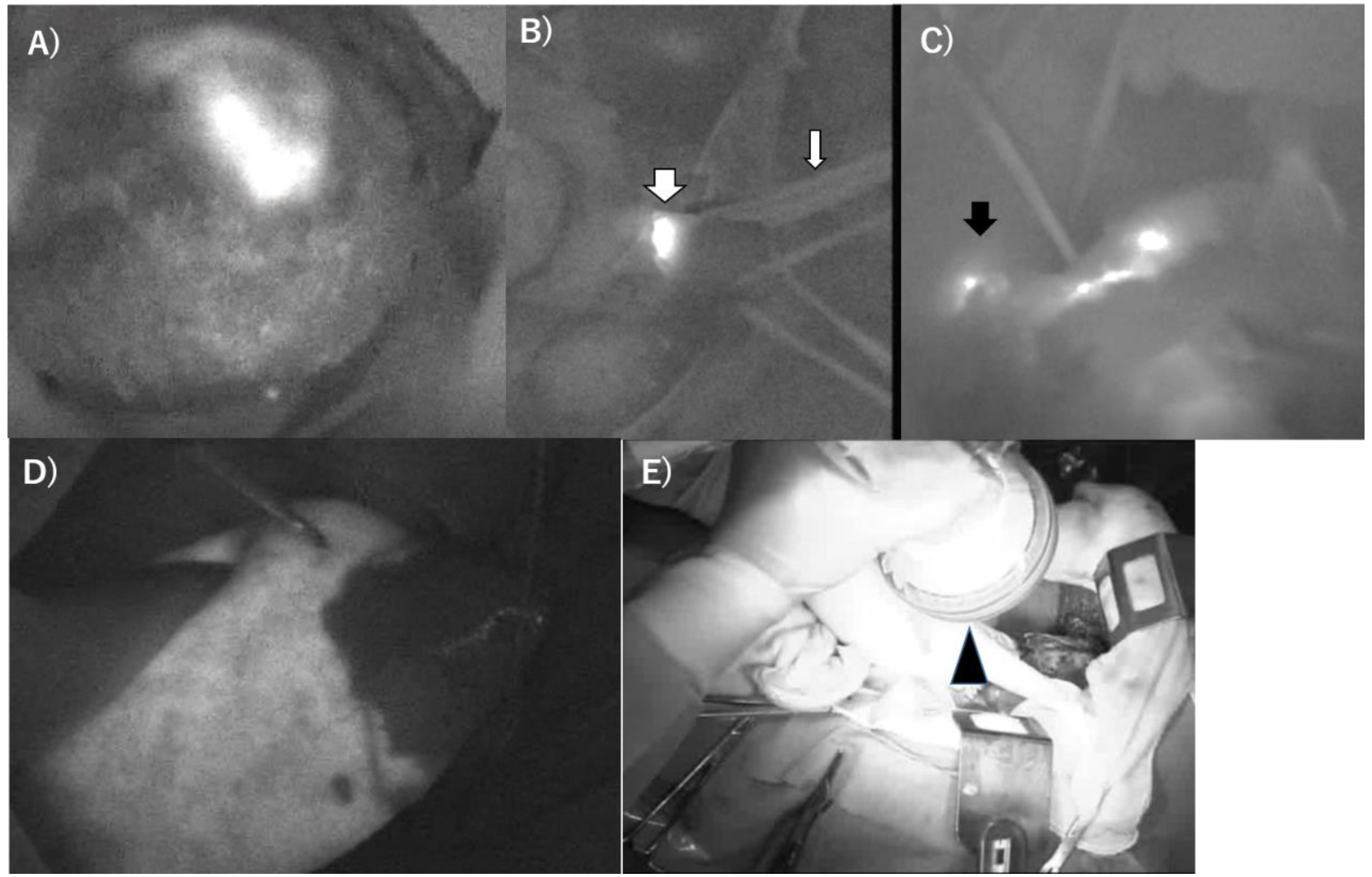
3.1. PDD during PDT
3.2. Recent PDD Using ALA and ICG-Based NIR Fluorescence Imaging
3.3. Limitation, Disadvantages of PDT in Hepatobiliary Malignancies
4. Problems and Debates
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grzybowski, A.; Pietrzak, K. From patient to discoverer–Niels Ryberg Finsen (1860–1904)—The founder of phototherapy in dermatology. Clin. Dermatol. 2012, 30, 451–455. [Google Scholar] [CrossRef]
- Daniell, M.D.; Hill, J.S. A history of photodynamic therapy. Aust. N. Z. J. Surg. 1991, 61, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The history of photodetection and photodynamic therapy. Photochem. Photobiol. 2001, 74, 656–669. [Google Scholar] [CrossRef]
- Dolmans, D.; Fukumura, D.; Jain, R. Photodynamic therapy for cancer. Nat. Rev. 2003, 3, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.Y.; Dai, T.; Hamblin, M.R. Photodynamic Therapy for Infections: Clinical Applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef]
- Agostinis, P.; Buytaert, E.; Breyssens, H.; Hendrickx, N. Regulatory pathways in photodynamic therapy induced apoptosis. Photochem. Photobiol. Sci. 2004, 3, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Pogue, B.W.; Ortel, B.; Chen, N.; Redmond, R.W.; Hasan, T. A photobiological and photophysical-based study of phototoxicity of two chlorins. Cancer Res. 2001, 61, 717–724. [Google Scholar]
- dos Santos, F.A.; Queiroz de Almeida, D.R.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic therapy in cancer treatment—An update review. J. Cancer Metastasis Treat. 2019, 5, 25–45. [Google Scholar] [CrossRef]
- Kataoka, H.; Hayashi, N.; Tanaka, M.; Kubota, E.; Yano, S.; Joh, T. Tumor affinity photosensitizers for photodynamic therapy. JJSLSM 2015, 36, 159–165. [Google Scholar] [CrossRef]
- Huang, Z. Photodynamic Diagnosis and Photodynamic Therapy Techniques. Optical Detection of Cancer. In Optical Detection Cancer; Meyers, A., Ed.; World Scientific Publishing Co., Pte., Ltd.: Singapore, 2011; pp. 79–98. [Google Scholar]
- Gomer, C.J.; Razum, N.J. Acute skin response in albino mice following porphyrin photosensitization under oxic and anoxic conditions. Photochem. Photobiol. 1984, 40, 435–439. [Google Scholar] [CrossRef]
- Moan, J.; Berg, K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem. Photobiol. 1991, 53, 549–553. [Google Scholar] [CrossRef]
- Epstein, J.H. Phototoxicity and photoallergy. Semin. Cutan. Med. Surg. 1999, 18, 274–284. [Google Scholar] [CrossRef]
- Nauta, J.M.; van Leengoed, H.L.; Star, W.M.; Roodenburg, J.L.; Witjes, M.J.; Vermey, A. Photodynamic therapy of oral cancer. A review of basic mechanisms and clinical applications. Eur. J. Oral Sci. 1996, 104, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Gollnick, S.O.; Liu, X.; Owczarczak, B.; Musser, D.A.; Henderson, B.W. Altered expression of interleukin 6 and interleukin 10 as a result of photodynamic therapy in vivo. Cancer Res. 1997, 57, 3904–3909. [Google Scholar] [PubMed]
- Oleinick, N.L.; Evans, H.H. The photobiology of photodynamic therapy: Cellular targets and mechanisms. Radiat. Res. 1998, 150, S146–S156. [Google Scholar] [CrossRef]
- Wilson, B.C.; Olivo, M.; Singh, G. Subcellular localization of Photofrin and aminolevulinic acid and photodynamic cross-resistance in vitro in radiation-induced fibrosarcoma cells sensitive or resistant to photofrin-mediated photodynamic therapy. Photochem. Photobiol. 1997, 65, 166–176. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part three—Photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagn. Photodyn. Ther. 2005, 2, 91–106. [Google Scholar] [CrossRef]
- Tamada, K.; Sugano, K. Diagnosis and non-surgical treatment of bile duct carcinoma: Developments in the past decade. J. Gastroenterol. 2000, 35, 319–325. [Google Scholar] [CrossRef]
- Nanashima, A.; Nagayasu, T. Current status of photodynamic therapy in digestive tract carcinoma in Japan. Int. J. Mol. Sci. 2015, 16, 3434–3440. [Google Scholar] [CrossRef]
- Dougherty, T.J. Hematoporphyrin as a photosensitizer of tumors. Photochem. Photobiol. 1983, 38, 377–379. [Google Scholar] [CrossRef]
- Ortner, M.A.; Liebetruth, J.; Schreiber, S.; Hanft, M.; Wruck, U.; Fusco, V.; Müller, J.M.; Hörtnagl, H.; Lochs, H. Photodynamic therapy of nonresectable cholangiocarcinoma. Gastroenterology 1998, 114, 536–542. [Google Scholar] [CrossRef]
- Thunshelle, C.; Yin, R.; Chen, Q.; Hamblin, M.R. Current Advances in 5-aminolevulinic Acid Mediated Photodynamic Therapy. Curr. Dermatol. Rep. 2016, 5, 179–190. [Google Scholar] [CrossRef]
- Abo-Zeid, M.A.M.; Abo-Elfadl, M.T.; Mostafa, S.M. Photodynamic therapy using 5-aminolevulinic acid triggered DNA damage of adenocarcinoma breast cancer and hepatocellular carcinoma cell lines. Photodiagn. Photodyn. Ther. 2018, 21, 351–356. [Google Scholar] [CrossRef]
- Egger, N.G.; Schoenecker, J.A., Jr.; Gourley, W.K.; Motamedi, M.; Anderson, K.E.; Weinman, S.A. Photosensitization of experimental hepatocellular carcinoma with protoporphyrin synthesized from administered delta-aminolevulinic acid: Studies with cultured cells and implanted tumors. J. Hepatol. 1997, 26, 913–920. [Google Scholar] [CrossRef]
- Date, M.; Fukuchi, K.; Namiki, Y.; Okumura, A.; Morita, S.; Takahashi, H.; Ohura, K. Therapeutic effect of photodynamic therapy using PAD-S31 and diode laser on human liver cancer cells. Liver Int. 2004, 24, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.M.; Chan, J.Y.; Au, S.W.; Kong, S.K.; Tsui, S.K.; Waye, M.M.; Mak, T.C.; Fong, W.P.; Fung, K.P. Pheophorbide a, an active compound isolated from Scutellaria barbata, possesses photodynamic activities by inducing apoptosis in human hepatocellular carcinoma. Cancer Biol. Ther. 2006, 5, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Yow, C.M.; Wong, C.K.; Huang, Z.; Ho, R.J. Study of the efficacy and mechanism of ALA-mediated photodynamic therapy on human hepatocellular carcinoma cell. Liver Int. 2007, 27, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Fadel, M.; Kassab, K.; Youssef, T. Photodynamic efficacy of hypericin targeted by two delivery techniques to hepatocellular carcinoma cells. Lasers Med. Sci. 2010, 25, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Xue, J.; Dai, Y.; Liu, H.; Chen, N.; Jia, L.; Huang, J. Inhibition of human hepatocellular carcinoma HepG2 by phthalocyanine photosensitiser PHOTOCYANINE: ROS production, apoptosis, cell cycle arrest. Eur. J. Cancer 2012, 48, 2086–2096. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Djavid, G.E.; Hadizadeh, M.; Jahanshiri-Moghadam, M.; Hajian, P. The efficacy of Radachlorin-mediated photodynamic therapy in human hepatocellular carcinoma cells. J. Photochem. Photobiol. B Biol. 2015, 142, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, T.; Wang, S.; Zhou, W.; Li, Y.; Chen, X.; Li, W.; Huang, Z.; Li, T.; Yang, L.; et al. Anticancer effect of LS-HB-mediated photodynamic therapy on hepatocellular carcinoma in vitro and in vivo. Photodiagn. Photodyn. Ther. 2020, 30, 101718. [Google Scholar] [CrossRef] [PubMed]
- Bahng, S.; Yoo, B.C.; Paik, S.W.; Koh, K.C.; Lee, K.T.; Lee, J.K.; Lee, J.H.; Choi, M.S.; Lee, K.H. Photodynamic therapy for bile duct invasion of hepatocellular carcinoma. Photochem. Photobiol. Sci. 2013, 12, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Renzulli, M.; Ramai, D.; Singh, J.; Sinha, S.; Brandi, N.; Ierardi, A.M.; Albertini, E.; Sacco, R.; Facciorusso, A.; Golfieri, R. Locoregional Treatments in Cholangiocarcinoma and Combined Hepatocellular Cholangiocarcinoma. Cancers 2021, 13, 3336. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, C.; Ding, Y.; Li, J.; Li, M.; Liang, X.; Zhou, J.; Wang, W. Biomimetic HDL nanoparticle mediated tumor targeted delivery of indocyanine green for enhanced photodynamic therapy. Colloids Surf. B Biointerfaces 2016, 148, 533–540. [Google Scholar] [CrossRef]
- Kaneko, J.; Kokudo, T.; Inagaki, Y.; Hasegawa, K. Innovative treatment for hepatocellular carcinoma (HCC). Transl. Gastroenterol. Hepatol. 2018, 3, 78. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Kaibori, M.; Hishikawa, H.; Nakatake, R.; Okumura, T.; Ozeki, E.; Hara, I.; Morimoto, Y.; Yoshii, K.; Kon, M. Near-infrared fluorescence imaging and photodynamic therapy with indocyanine green lactosome has antineoplastic effects for hepatocellular carcinoma. PLoS ONE 2017, 12, e0183527. [Google Scholar] [CrossRef]
- Hong, F.; Park, J.S.; Kim, S.W.; Park, S.J.; Kim, S.K. Near-infrared phototherapy for patient-derived orthotopic xenograft model of hepatocellular carcinoma in combination with indocyanine green. J. Photochem. Photobiol. B Biol. 2020, 209, 111938. [Google Scholar] [CrossRef]
- Abdel Fadeel, D.; Al-Toukhy, G.M.; Elsharif, A.M.; Al-Jameel, S.S.; Mohamed, H.H.; Youssef, T.E. Improved photodynamic efficacy of thiophenyl sulfonated zinc phthalocyanine loaded in lipid nano-carriers for hepatocellular carcinoma cancer cells. Photodiagn. Photodyn. Ther. 2018, 23, 25–31. [Google Scholar] [CrossRef]
- Gao, Y.; Zheng, Q.C.; Xu, S.; Yuan, Y.; Cheng, X.; Jiang, S.; Kenry, Y.Q.; Song, Z.; Liu, B.; Li, M. Theranostic Nanodots with Aggregation-Induced Emission Characteristic for Targeted and Image-Guided Photodynamic Therapy of Hepatocellular Carcinoma. Theranostics 2019, 9, 1264–1279. [Google Scholar] [CrossRef]
- van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic photodynamic therapy: Basic principles, current clinical status and future directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef]
- Matsumoto, J.; Suzuki, K.; Yasuda, M.; Yamaguchi, Y.; Hishikawa, Y.; Imamura, N.; Nanashima, A. Photodynamic therapy of human biliary cancer cell line using combination of phosphorus porphyrins and light emitting diode. Bioorg. Med. Chem. 2017, 25, 6536–6541. [Google Scholar] [CrossRef] [PubMed]
- Mai, N.N.H.; Yamaguchi, Y.; Choijookhuu, N.; Matsumoto, J.; Nanashima, A.; Takagi, H.; Sato, K.; Tuan, L.Q.; Hishikawa, Y. Photodynamic Therapy Using a Novel Phosphorus Tetraphenylporphyrin Induces an Anticancer Effect via Bax/Bcl-xL-related Mitochondrial Apoptosis in Biliary Cancer Cells. Acta Histochem. Cytochem. 2020, 53, 61–72. [Google Scholar] [CrossRef]
- Tanaka, M.; Kataoka, H.; Yano, S.; Ohi, H.; Moriwaki, K.; Akashi, H.; Taguchi, T.; Hayashi, N.; Hamano, S.; Mori, Y.; et al. Antitumor effects in gastrointestinal stromal tumors using photodynamic therapy with a novel glucose-conjugated chlorin. Mol. Cancer Ther. 2014, 13, 767–775. [Google Scholar] [CrossRef]
- Nanashima, A.; Yamaguchi, H.; Shibasaki, S.; Ide, N.; Sawai, T.; Tsuji, T.; Hidaka, S.; Sumida, Y.; Nakagoe, T.; Nagayasu, T. Adjuvant photodynamic therapy for bile duct carcinoma after surgery: A preliminary study. J. Gastroenterol. 2004, 39, 1095–1101. [Google Scholar] [CrossRef]
- Wong Kee Song, L.M.; Wang, K.K.; Zinsmeister, A.R. Mono-l-aspartyl chlorine e6 (NPe6) and haematoporphyrin derivative (HpD) in photodynamic therapy administered to a human cholangiocarcinoma model. Cancer 1998, 82, 421–427. [Google Scholar] [CrossRef]
- Pahernik, S.A.; Dellian, M.; Berr, F.; Tannapfel, A.; Wittekind, C.; Goetz, A.E. Distribution and pharmacokinetics of Photofrin in human bile duct cancer. J. Photochem. Photobiol. B Biol. 1998, 47, 58–62. [Google Scholar] [CrossRef]
- Kiesslich, T.; Neureiter, D.; Alinger, B.; Alinger, B.; Jansky, G.L.; Berlanda, J.; Mkrtchyan, V.; Ocker, M.; Plaetzer, K.; Berr, F. Uptake and phototoxicity of meso-tetrahydroxyphenyl chlorine are highly variable in human biliary tract cancer cell lines and correlate with markers of differentiation and proliferation. Photochem. Photobiol. Sci. 2010, 9, 734–743. [Google Scholar] [CrossRef]
- Nonaka, T.; Nanashima, A.; Nonaka, M.; Uehara, M.; Isomoto, H.; Asahina, I.; Nagayasu, T. Analysis of apoptotic effects induced by photodynamic therapy in a human biliary cancer cell line. Anticancer Res. 2010, 30, 2113–2118. [Google Scholar] [PubMed]
- Nonaka, T.; Nanashima, A.; Nonaka, M.; Uehara, M.; Isomoto, H.; Nonaka, Y.; Nagayasu, T. Advantages of laserphyrin compared with photofrin in photodynamic therapy for bile duct carcinoma. J. Hepatobiliary Pancreat. Sci. 2011, 18, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, Y.; Nanashima, A.; Nonaka, T.; Uehara, M.; Isomoto, H.; Abo, T.; Nagayasu, T. Synergic effect of photodynamic therapy using talaporfin sodium with conventional anticancer chemotherapy for the treatment of bile duct carcinoma. J. Surg. Res. 2013, 181, 234–241. [Google Scholar] [CrossRef]
- Murakami, G.; Nanashima, A.; Nonaka, T.; Tominaga, T.; Wakata, K.; Sumida, Y.; Akashi, H.; Okazaki, S.; Kataoka, H.; Nagayasu, T. Photodynamic Therapy Using Novel Glucose-conjugated Chlorin Increases Apoptosis of Cholangiocellular Carcinoma in Comparison with Talaporfin Sodium. Anticancer Res. 2016, 36, 4493–4501. [Google Scholar] [CrossRef]
- Kiesslich, T.; Berlanda, J.; Plaetzer, K.; Krammer, B.; Berr, F. Comparative characterization of the efficiency and cellular pharmacokinetics of Foscan- and Foslip-based photodynamic treatment in human biliary tract cancer cell lines. Photochem. Photobiol. Sci. 2007, 6, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, K.; Shimazu, M.; Suzuki, M.; Kuroiwa, Y.; Usuda, J.; Itoi, T.; Tsuchida, A.; Aoki, T. Novel photodynamic therapy against biliary tract carcinoma using mono-L-aspartyl chlorine e6, basic evaluation for its feasibility and efficacy. J. Hepatobiliary Pancreat Sci. 2010, 17, 313–321. [Google Scholar] [CrossRef] [PubMed]
- McCaughan, J.S.; Mertens, B.F.; Cho, C.; Barabash, R.D.; Payton, H.W. Photodynamic therapy to treat tumors of the extrahepatic biliary ducts: A case report. Arch. Surg. 1991, 126, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Abulafi, A.M.; Allardice, J.T.; Williams, N.S.; Van Someren, N.; Swain, C.P.; Ainley, C. Photodynamic therapy for malignant tumours of the ampulla of Vater. Gut 1995, 36, 853–856. [Google Scholar] [CrossRef]
- Rumalla, A.; Baron, T.H.; Wang, K.K.; Gores, G.J.; Stadheim, L.M.; de Groen, P.C. Endoscopic application of photodynamic therapy for cholangiocarcinoma. Gastrointest. Endosc. 2001, 53, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Berr, F. Photodynamic therapy for cholangiocarcinoma. Semin. Liver Dis. 2004, 241, 77–187. [Google Scholar] [CrossRef] [PubMed]
- Ortner, M.A. Photodynamic therapy in cholangiocarcinomas. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 147–154. [Google Scholar] [CrossRef]
- Ortner, M.E.; Caca, K.; Berr, F.; Liebetruth, J.; Mansmann, U.; Huster, D.; Voderholzer, W.; Schachschal, G.; Mössner, J.; Lochs, H. Successful photodynamic therapy for nonresectable cholangiocarcinoma: A randomized prospective study. Gastroenterology 2003, 125, 1355–1363. [Google Scholar] [CrossRef]
- Zoepf, T.; Jakobs, R.; Arnold, J.C.; Apel, D.; Riemann, J.F. Palliation of Nonresectable Bile Duct Cancer: Improved Survival after Photodynamic Therapy. Am. J. Gastroenterol. 2005, 100, 2426–2430. [Google Scholar] [CrossRef]
- Witzigmann, H.; Berr, F.; Ringel, U.; Caca, K.; Uhlmann, D.; Schoppmeyer, K.; Tannapfel, A.; Wittekind, C.; Mossner, J.; Hauss, J.; et al. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: Palliative photodynamic therapy plus stenting is comparable to r1/r2 resection. Ann. Surg. 2006, 244, 230–239. [Google Scholar] [CrossRef]
- Kniebühler, G.; Pongratz, T.; Betz, C.S.; Göke, B.; Sroka, R.; Stepp, H.; Schirra, J. Photodynamic therapy for cholangiocarcinoma using low dose mTHPC (Foscan). Photodiagnosis Photodyn. Ther. 2013, 10, 220–228. [Google Scholar] [CrossRef]
- Ortner, M.A. Photodynamic therapy for cholangiocarcinoma: Overview and new developments. Curr. Opin. Gastroenterol. 2009, 25, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Bai, Y.; Ma, S.R.; Liu, F.; Li, Z.S. Systematic review: Photodynamic therapy for unresectable cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2010, 17, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Quyn, A.J.; Ziyaie, D.; Polignano, F.M.; Tait, I.S. Photodynamic therapy is associated with an improvement in survival in patients with irresectable hilar cholangiocarcinoma. HPB 2009, 11, 570–577. [Google Scholar] [CrossRef]
- Hong, M.J.; Cheon, Y.K.; Lee, E.J.; Lee, T.Y.; Shim, C. Long-term outcome of photodynamic therapy with systemic chemotherapy compared to photodynamic therapy alone in patients with advanced hilar cholangiocarcinoma. Gut Liver 2014, 8, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.P.; Aithal, G.P.; Ragunath, K.; Devlin, J.; Owen, F.; Meadows, H. Safety and long term efficacy of porfimer sodium photodynamic therapy in locally advanced biliary tract carcinoma. Photodiagn. Photodyn. Ther. 2012, 9, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Yoshitomi, H.; Miyakawa, S.; Uesaka, K.; Unno, M.; Endo, I.; Ota, T.; Ohtsuka, M.; Kinoshita, H.; Shimada, K.; et al. Clinical practice guidelines for management of biliary tract cancers 2015, the 2nd English edition. J. Hepato-Biliary-Pancreat. Sci. 2015, 22, 249–273. [Google Scholar] [CrossRef]
- Wiedmann, M.; Caca, K.; Berr, F.; Schiefke, I.; Tannapfel, A.; Wittekind, C.; Mössner, J.; Hauss, J.; Witzigmann, H. Neoadjuvant photodynamic therapy as a new approach to treating hilar cholangiocarcinoma: A phase II pilot study. Cancer 2003, 97, 2783–2790. [Google Scholar] [CrossRef]
- Wagner, A.; Wiedmann, M.; Tannapfel, A.; Mayr, C.; Kiesslich, T.; Wolkersdörfer, G.W.; Berr, F.; Hauss, J.; Witzigmann, H. Neoadjuvant Down-Sizing of Hilar Cholangiocarcinoma with Photodynamic Therapy--Long-Term Outcome of a Phase II Pilot Study. Int. J. Mol. Sci. 2015, 16, 26619–26628. [Google Scholar] [CrossRef]
- He, C.; Xia, J.; Gao, Y.; Chen, Z.; Wan, X. Chlorin A-mediated photodynamic therapy induced apoptosis in human cholangiocarcinoma cells via impaired autophagy flux. Am. J. Transl. Res. 2020, 12, 5080–5094. [Google Scholar]
- Schmidt, J.; Kuzyniak, W.; Berkholz, J.; Steinemann, G.; Ogbodu, R.; Hoffmann, B.; Nouailles, G.; Gürek, A.G.; Nitzsche, B.; Höpfner, M. Novel zinc- and silicon-phthalocyanines as photosensitizers for photodynamic therapy of cholangiocarcinoma. Int. J. Mol. Med. 2018, 42, 534–546. [Google Scholar] [CrossRef]
- Kim, D.H.; Im, B.N.; Hwang, H.S.; Na, K. Gemcitabine-loaded DSPE-PEG-PheoA liposome as a photomediated immune modulator for cholangiocarcinoma treatment. Biomaterials 2018, 183, 139–150. [Google Scholar] [CrossRef]
- Nanashima, A.; Nakashima, K.; Kawakami, H.; Ashizuka, S.; Kubota, Y. Nursing care management of photodynamic therapy in digestive tract carcinomas at a single cancer center. Photodiagn. Photodyn. Ther. 2017, 17, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Nanashima, A.; Isomoto, H.; Abo, T.; Nonaka, T.; Morisaki, T.; Arai, J.; Takagi, K.; Ohnita, K.; Shoji, H.; Urabe, S.; et al. How to access photodynamic therapy for bile duct carcinoma. Ann. Transl. Med. 2014, 2, 23. [Google Scholar]
- Dolmans, D.E.; Kadambi, A.; Hill, J.S.; Flores, K.R.; Gerber, J.N.; Walker, J.P.; Borel Rinkes, I.H.; Jain, R.K.; Fukumura, D. Targeting tumor vasculature and cancer cells in orthotopic breast tumor by fractionated photosensitizer dosing photodynamic therapy. Cancer Res. 2002, 62, 4289–4294. [Google Scholar]
- Lipson, R.L.; Baldes, E.J.; Olsen, A.M. Hematoporphyrin derivative: A new aid for endoscopic detection of malignant disease. J. Thorac. Cardiovasc. Surg. 1961, 42, 623–629. [Google Scholar] [CrossRef]
- Namikawa, T.; Fujisawa, K.; Munekage, E.; Iwabu, J.; Uemura, S.; Tsujii, S.; Maeda, H.; Kitagawa, H.; Fukuhara, H.; Inoue, K.; et al. Clinical application of photodynamic medicine technology using light-emitting fluorescence imaging based on a specialized luminous source. Med. Mol. Morphol. 2018, 51, 187–193. [Google Scholar] [CrossRef]
- Gashev, A.A.; Nagai, T.; Bridenbaugh, E.A. Indocyanine green and lymphatic imaging: Current problems. Lymphat. Res. Biol. 2010, 8, 127–130. [Google Scholar] [CrossRef]
- Marshall, M.V.; Rasmussen, J.C.; Tan, I.C.; Aldrich, M.B.; Adams, K.E.; Wang, X.; Fife, C.E.; Maus, E.A.; Smith, L.A.; Sevick-Muraca, E.M. Near-Infrared Fluorescence Imaging in Humans with Indocyanine Green: A Review and Update. Open Surg. Oncol. J. 2010, 2, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Vonarx-Coinsman, V.; Foultier, M.T.; de Brito, L.X.; Morlet, L.; Gouyette, A.; Patrice, T. HepG2 human hepatocarcinoma cells: An experimental model for photosensitization by endogenous porphyrins. J. Photochem. Photobiol. B Biol. 1995, 30, 201–208. [Google Scholar] [CrossRef]
- Otake, M.; Nishiwaki, M.; Kobayashi, Y.; Baba, S.; Kohno, E.; Kawasaki, T.; Fujise, Y.; Nakamura, H. Selective accumulation of ALA-induced PpIX and photodynamic effect in chemically induced hepatocellular carcinoma. Br. J. Cancer 2003, 89, 730–736. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Inoue, Y.; Tanaka, R.; Komeda, K.; Hirokawa, F.; Hayashi, M.; Uchiyama, K. Fluorescence Detection of Malignant Liver Tumors using 5-Aminolevulinic Acid-Mediated Photodynamic Diagnosis: Principles, Technique, and Clinical Experience. World J. Surg. 2014, 38, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Takahara, N.; Tateishi, K.; Tanaka, M.; Kanai, S.; Kato, H.; Nakatsuka, T.; Yamamoto, K.; Kogure, H.; Arita, J.; et al. 5-Aminolevulinic acid-mediated photodynamic activity in patient-derived cholangiocarcinoma organoids. Surg. Oncol. 2020, 35, 484–490. [Google Scholar] [CrossRef]
- Ishizawa, T.; Fukushima, N.; Shibahara, J.; Masuda, K.; Tamura, S.; Aoki, T.; Hasegawa, K.; Beck, Y.; Fukayama, M.; Kokudo, N. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 2009, 115, 2491–2504. [Google Scholar] [CrossRef] [PubMed]
- Fox, I.J.; Wood, E.H. Indocyanine green: Physical and physiologic properties. Proc. Staff. Meet. Mayo Clin. 1960, 35, 732–744. [Google Scholar]
- Rangaraj, A.T.; Ghanta, R.K.; Umakanthan, R.; Soltesz, E.G.; Laurence, R.G.; Fox, J.; Cohn, L.H.; Bolman, R.M., 3rd; Frangioni, J.V.; Chen, F.Y. Real-time visualization and quantification of retrograde cardioplegia delivery using near infrared fluorescent imaging. J. Card Surg. 2008, 23, 701–708. [Google Scholar] [CrossRef][Green Version]
- Nakaseko, Y.; Ishizawa, T.; Saiura, A. Fluorescence-guided surgery for liver tumors. J. Surg. Oncol. 2018, 118, 324–331. [Google Scholar] [CrossRef]
- Ishizawa, T.; Saiura, A.; Kokudo, N. Clinical application of indocyanine green-fluorescence imaging during hepatectomy. Hepatobiliary Surg. Nutr. 2016, 5, 322–328. [Google Scholar] [CrossRef]
- Rossi, G.; Tarasconi, A.; Baiocchi, G.; De’ Angelis, G.L.; Gaiani, F.; Di Mario, F.; Catena, F.; Dalla Valle, R. Fluorescence guided surgery in liver tumors: Applications and advantages. Acta Biomed. 2018, 89, 135–140. [Google Scholar]
- Yamada, Y.; Ohno, M.; Fujino, A.; Kanamori, Y.; Irie, R.; Yoshioka, T.; Miyazaki, O.; Uchida, H.; Fukuda, A.; Sakamoto, S.; et al. Fluorescence-Guided Surgery for Hepatoblastoma with Indocyanine Green. Cancers 2019, 11, 1215. [Google Scholar] [CrossRef]
- Satou, S.; Ishizawa, T.; Masuda, K.; Kaneko, J.; Aoki, T.; Sakamoto, Y.; Hasegawa, K.; Sugawara, Y.; Kokudo, N. Indocyanine green fluorescent imaging for detecting extrahepatic metastasis of hepatocellular carcinoma. J. Gastroenterol. 2013, 48, 1136–1143. [Google Scholar] [CrossRef]
- Nanashima, A.; Tominaga, T.; Sumida, Y.; Tobinaga, S.; Nagayasu, T. Indocyanine green identification for tumor infiltration or metastasis originating from hepatocellular carcinoma. Int. J. Surg. Case Rep. 2018, 46, 56–61. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Huang, T.; Fang, C.; Su, S.; Tian, J.; Xia, X.; Li, B. Identification of extrahepatic metastasis of hepatocellular carcinoma using indocyanine green fluorescence imaging. Photodiagn. Photodyn. Ther. 2019, 25, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Abo, T.; Nanashima, A.; Tobinaga, S.; Hidaka, S.; Taura, N.; Takagi, K.; Arai, J.; Miyaaki, H.; Shibata, H.; Nagayasu, T. Usefulness of intraoperative diagnosis of hepatic tumors located at the liver surface and hepatic segmental visualization using indocyanine green-photodynamic eye imaging. Eur. J. Surg. Oncol. 2015, 41, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; The, C.S.C.; Ishizawa, T.; Aoki, T.; Cavallucci, D.; Lee, S.Y.; Panganiban, K.M.; Perini, M.V.; Shah, S.R.; Wang, H.; et al. Consensus Guidelines for the Use of Fluorescence Imaging in Hepatobiliary Surgery. Ann. Surg. 2021, 274, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Delaey, E.; van Laar, F.; De Vos, D.; Kamuhabwa, A.; Jacobs, P.; de Witte, P. A comparative study of the photosensitizing characteristics of some cyanine dyes. J. Photochem. Photobiol. B Biol. 2000, 55, 27–36. [Google Scholar] [CrossRef]
- El-Daly, S.M.; Gamal-Eldeen, A.M.; Abo-Zeid, M.A.; Borai, I.H.; Wafay, H.A.; Abdel-Ghaffar, A.R. Photodynamic therapeutic activity of indocyanine green entrapped in polymeric nanoparticles. Photodiagn. Photodyn. Ther. 2013, 10, 173–185. [Google Scholar] [CrossRef]
- Li, S.; Yang, S.; Liu, C.; He, J.; Li, T.; Fu, C.; Meng, X.; Shao, H. Enhanced Photothermal-Photodynamic Therapy by Indocyanine Green and Curcumin-Loaded Layered MoS2 Hollow Spheres via Inhibition of P-Glycoprotein. Int. J. Nanomed. 2021, 16, 433–442. [Google Scholar] [CrossRef]
- Hishikawa, H.; Kaibori, M.; Tsuda, T.; Matsui, K.; Okumura, T.; Ozeki, E.; Yoshii, K. Near-infrared fluorescence imaging and photodynamic therapy with indocyanine green lactosomes has antineoplastic effects for gallbladder cancer. Oncotarget 2019, 10, 5622–5631. [Google Scholar] [CrossRef]
- Cai, H.; Dai, X.; Guo, X.; Zhang, L.; Cao, K.; Yan, F.; Ji, B.; Liu, Y. Ataxia telangiectasia mutated inhibitor-loaded copper sulfide nanoparticles for low-temperature photothermal therapy of hepatocellular carcinoma. Acta Biomater. 2021, 127, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Cai, H.; Yang, Y.; Peng, F.; Song, M.; Sun, K.; Yan, F.; Liu, Y. Hybrid membrane camouflaged copper sulfide nanoparticles for photothermal-chemotherapy of hepatocellular carcinoma. Acta Biomater. 2020, 111, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.H.; Li, M.Y.; Sajjad, F.; Wang, J.H.; Meharban, F.; Gadoora, M.A.; Yan, Y.J.; Nyokong, T.; Chen, Z.L. Synthesis and pharmacological evaluation of chlorin derivatives for photodynamic therapy of cholangiocarcinoma. Eur. J. Med. Chem. 2020, 189, 112049. [Google Scholar] [CrossRef] [PubMed]
- Matull, W.R.; Dhar, D.K.; Ayaru, L.; Sandanayake, N.S.; Chapman, M.H.; Dias, A.; Bridgewater, J.; Webster, G.J.; Bong, J.J.; Davidson, B.R.; et al. R0 but not R1/R2 resection is associated with better survival than palliative photodynamic therapy in biliary tract cancer. Liver Int. 2011, 31, 99–107. [Google Scholar] [CrossRef]
- Liang, S.; Sun, M.; Lu, Y.; Shi, S.; Yang, Y.; Lin, Y.; Feng, C.; Liu, J.; Dong, C. Cytokine-induced killer cells-assisted tumor-targeting delivery of Her-2 monoclonal antibody-conjugated gold nanostars with NIR photosensitizer for enhanced therapy of cancer. J. Mater. Chem. B 2020, 8, 8368–8382. [Google Scholar] [CrossRef]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef]
- Liu, H.; Daly, L.; Rudd, G.; Khan, A.P.; Mallidi, S.; Liu, Y.; Cuckov, F.; Hasan, T.; Celli, J.P. Development and evaluation of a low-cost, portable, LED-based device for PDT treatment of early-stage oral cancer in resource-limited settings. Lasers Surg. Med. 2019, 51, 345–351. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Murayama, Y.; Komatsu, S.; Shiozaki, A.; Kuriu, Y.; Ikoma, H.; Nakanishi, M.; Ichikawa, D.; Fujiwara, H.; Okamoto, K.; et al. Efficacy of 5-aminolevulinic acid-mediated photodynamic therapy using light-emitting diodes in human colon cancer cells. Oncol. Rep. 2013, 29, 911–916. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nanashima, A.; Hiyoshi, M.; Imamura, N.; Yano, K.; Hamada, T.; Kai, K. Recent Advances in Photodynamic Imaging and Therapy in Hepatobiliary Malignancies: Clinical and Experimental Aspects. Curr. Oncol. 2021, 28, 4067-4079. https://doi.org/10.3390/curroncol28050345
Nanashima A, Hiyoshi M, Imamura N, Yano K, Hamada T, Kai K. Recent Advances in Photodynamic Imaging and Therapy in Hepatobiliary Malignancies: Clinical and Experimental Aspects. Current Oncology. 2021; 28(5):4067-4079. https://doi.org/10.3390/curroncol28050345
Chicago/Turabian StyleNanashima, Atsushi, Masahide Hiyoshi, Naoya Imamura, Koichi Yano, Takeomi Hamada, and Kengo Kai. 2021. "Recent Advances in Photodynamic Imaging and Therapy in Hepatobiliary Malignancies: Clinical and Experimental Aspects" Current Oncology 28, no. 5: 4067-4079. https://doi.org/10.3390/curroncol28050345
APA StyleNanashima, A., Hiyoshi, M., Imamura, N., Yano, K., Hamada, T., & Kai, K. (2021). Recent Advances in Photodynamic Imaging and Therapy in Hepatobiliary Malignancies: Clinical and Experimental Aspects. Current Oncology, 28(5), 4067-4079. https://doi.org/10.3390/curroncol28050345




