Breast Cancer Surgery: New Issues
Abstract
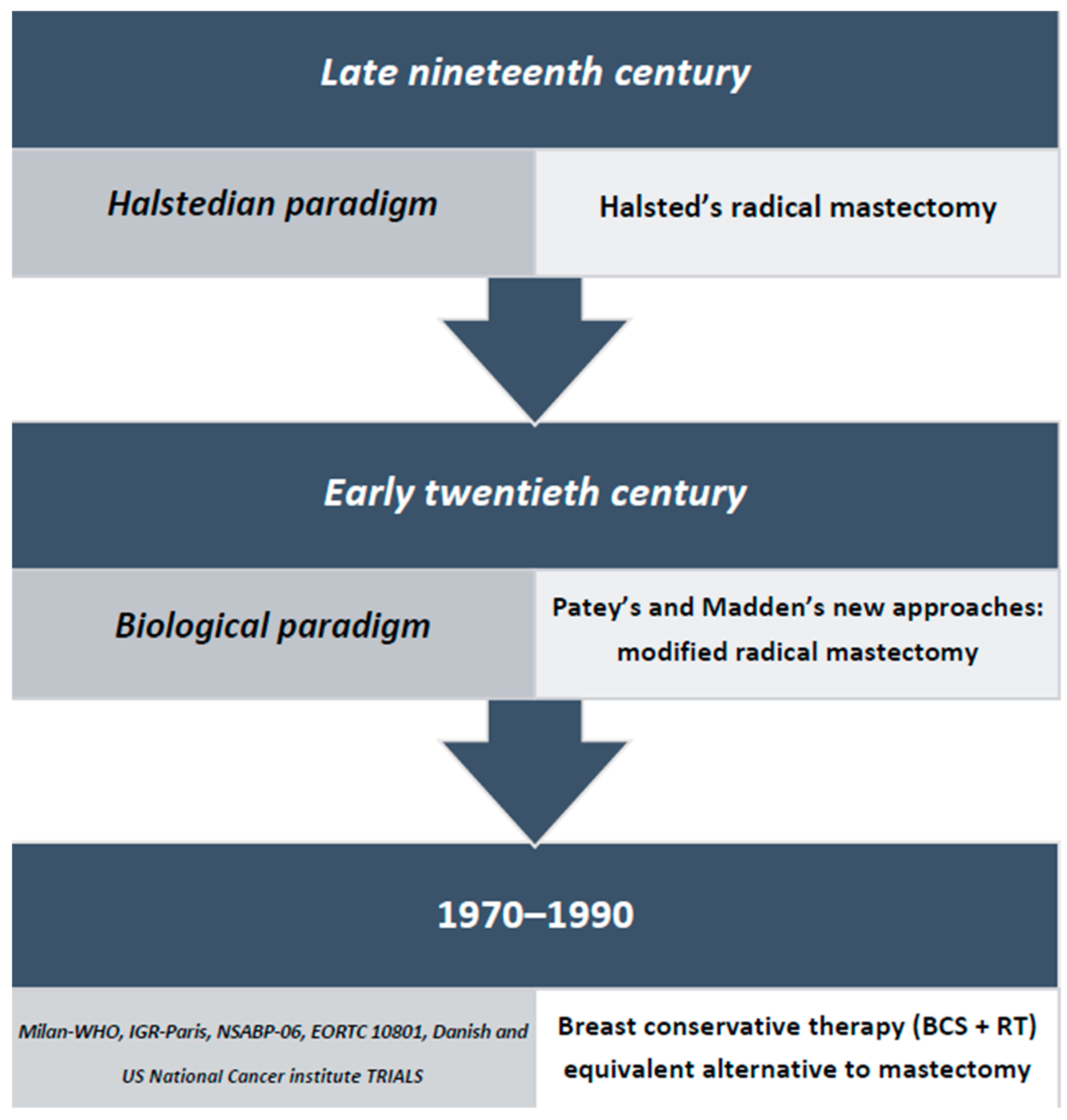
1. Introduction
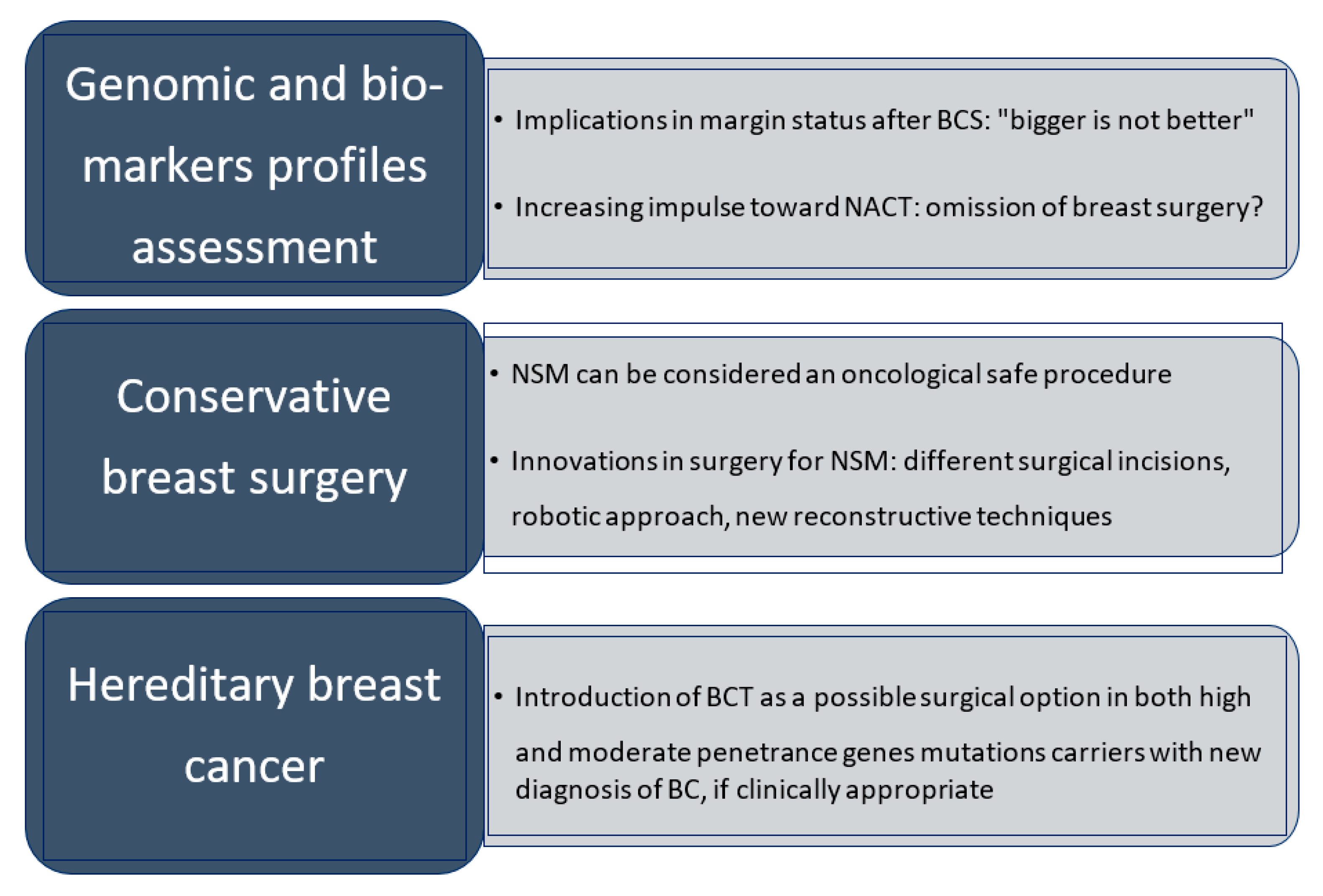
2. “Less Is More, Bigger Is Not Better”
3. Margin Status in Breast-Conserving Surgery and Molecular Subtypes Implication
4. Second Breast Conservative Surgery for Ipsilateral Breast Tumor Recurrence: The New Standard of Treatment
5. Neo-Adjuvant Treatments: The Challenge of Nonoperative Management
6. Conservative Mastectomy
7. Hereditary Breast Cancer and De-Escalation in Breast Surgery
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sakorafas, G.H.; Safioleas, M. Breast cancer surgery: An historical narrative. Part I. From prehistoric times to Renaissance. Eur. J. Cancer Care 2009, 18, 530–544. [Google Scholar] [CrossRef]
- Plesca, M.; Bordea, C.; El Houcheimi, B.; Ichim, E.; Blidaru, A. Evolution of radical mastectomy for breast cancer. J. Med. Life 2016, 9, 183–186. [Google Scholar]
- Bell, B. A System of Surgery; Charles Elliot: Edinburgh, UK, 1783. [Google Scholar]
- Sakorafas, G.H.; Safioleas, M. Breast cancer surgery: An historical narrative. Part II. 18th and 19th centuries. Eur. J. Cancer Care 2010, 19, 6–29. [Google Scholar] [CrossRef] [PubMed]
- Halsted, W.S.I. The Results of Operations for the Cure of Cancer of the Breast Performed at the Johns Hopkins Hospital from June 1889, to January 1894. Ann. Surg. 1894, 20, 497–555. [Google Scholar] [CrossRef]
- Sakorafas, G.H.; Safioleas, M. Breast cancer surgery: An historical narrative. Part III. From the sunset of the 19th to the dawn of the 21st century. Eur. J. Cancer Care 2010, 19, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Gentilini, O.D.; Cardoso, M.J.; Poortmans, P. Less is more. Breast conservation might be even better than mastectomy in early breast cancer patients. Breast 2017, 35, 32–33. [Google Scholar] [CrossRef]
- Morrow, M.; Harris, J.R.; Schnitt, S.J. Surgical margins in lumpectomy for breast cancer—Bigger is not better. N. Engl. J. Med. 2012, 367, 79–82. [Google Scholar] [CrossRef]
- Patey, D.H.; Dyson, W.H. The prognosis of carcinoma of the breast in relation to the type of operation performed. Br. J. Cancer 1948, 2, 7–13. [Google Scholar] [CrossRef]
- Madden, J.L. Modified radical mastectomy. Surg. Gynecol. Obstet. 1965, 121, 1221–1230. [Google Scholar] [CrossRef]
- Blichert-Toft, M.; Rose, C.; Andersen, J.A.; Overgaard, M.; Axelsson, C.K.; Andersen, K.W.; Mouridsen, H.T. Danish randomized trial comparing breast conservation therapy with mastectomy: Six years of life-table analysis. Danish Breast Cancer Cooperative Group. J. Natl. Cancer Inst. Monogr. 1992, 11, 19–25. [Google Scholar]
- Fisher, B.; Redmond, C.; Poisson, R.; Margolese, R.; Wolmark, N.; Wickerham, L.; Fisher, E.; Deutsch, M.; Caplan, R.; Pilch, Y. Eight-year results of a randomized clinical trial comparing total mastectomy and lumpectomy with or without irradiation in the treatment of breast cancer. N. Engl. J. Med. 1989, 320, 822–828. [Google Scholar] [CrossRef]
- Veronesi, U.; Salvadori, B.; Luini, A.; Banfi, A.; Zucali, R.; Del Vecchio, M.; Saccozzi, R.; Beretta, E.; Boracchi, P.; Farante, G. Conservative treatment of early breast cancer. Long-term results of 1232 cases treated with quadrantectomy, axillary dissection, and radiotherapy. Ann. Surg. 1990, 211, 250–259. [Google Scholar] [PubMed]
- Lichter, A.S.; Lippman, M.E.; Danforth, D.N., Jr.; d’Angelo, T.; Steinberg, S.M.; deMoss, E.; MacDonald, H.D.; Reichert, C.M.; Merino, M.; Swain, S.M. Mastectomy versus breast-conserving therapy in the treatment of stage I and II carcinoma of the breast: A randomized trial at the National Cancer Institute. J. Clin. Oncol. 1992, 10, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, J.A.; Bartelink, H.; Fentiman, I.S.; Lerut, T.; Mignolet, F.; Olthuis, G.; van der Schueren, E.; Sylvester, R.; Winter, J.; van Zijl, K. Randomized clinical trial to assess the value of breast-conserving therapy in stage I and II breast cancer, EORTC 10801 trial. J. Natl. Cancer Inst. Monogr. 1992, 11, 15–18. [Google Scholar]
- Sarrazin, D.; Lê, M.G.; Arriagada, R.; Contesso, G.; Fontaine, F.; Spielmann, M.; Rochard, F.; Le Chevalier, T.; Lacour, J. Ten-year results of a randomized trial comparing a conservative treatment to mastectomy in early breast cancer. Radiother. Oncol. 1989, 14, 177–184. [Google Scholar] [CrossRef]
- NIH Consensus Development Conference on the Treatment of Early-Stage Breast Cancer. Oncology (Williston Park) 1991, 5, 120–124. [PubMed]
- Consensus statement: Treatment of early-stage breast cancer. National Institutes of Health Consensus Development Panel. J. Natl. Cancer Inst. Monogr. 1992, 11, 1–5. [Google Scholar] [PubMed]
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.H.; Wolmark, N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, J.A.; Voogd, A.C.; Fentiman, I.S.; Legrand, C.; Sylvester, R.J.; Tong, D.; van der Schueren, E.; Helle, P.A.; van Zijl, K.; Bartelink, H. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J. Natl. Cancer Inst. 2000, 92, 1143–1150. [Google Scholar] [CrossRef]
- Veronesi, U.; Cascinelli, N.; Mariani, L.; Greco, M.; Saccozzi, R.; Luini, A.; Aguilar, M.; Marubini, E. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N. Engl. J. Med. 2002, 347, 1227–1232. [Google Scholar] [CrossRef]
- Poggi, M.M.; Danforth, D.N.; Sciuto, L.C.; Smith, S.L.; Steinberg, S.M.; Liewehr, D.J.; Menard, C.; Lippman, M.E.; Lichter, A.S.; Altemus, R.M. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: The National Cancer Institute Randomized Trial. Cancer 2003, 98, 697–702. [Google Scholar] [CrossRef]
- Arriagada, R.; Lê, M.G.; Rochard, F.; Contesso, G. Conservative treatment versus mastectomy in early breast cancer: Patterns of failure with 15 years of follow-up data. Institut Gustave-Roussy Breast Cancer Group. J. Clin. Oncol. 1996, 14, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, I.; Proschan, M.A. Randomized trials of breast-conserving therapy versus mastectomy for primary breast cancer: A pooled analysis of updated results. Am. J. Clin. Oncol. 2005, 28, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, J.A.; Danforth, D.N.; Cowan, K.H.; d’Angelo, T.; Steinberg, S.M.; Pierce, L.; Lippman, M.E.; Lichter, A.S.; Glatstein, E.; Okunieff, P. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N. Engl. J. Med. 1995, 332, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Saccozzi, R.; Del Vecchio, M.; Banfi, A.; Clemente, C.; De Lena, M.; Gallus, G.; Greco, M.; Luini, A.; Marubini, E.; et al. Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N. Engl. J. Med. 1981, 305, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Corso, G.; Veronesi, P.; Sacchini, V.; Galimberti, V.; Luini, A. The Veronesi quadrantectomy: An historical overview. Ecancermedicalscience 2017, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Vidya, R.; Leff, D.R.; Green, M.; McIntosh, S.A.; St John, E.; Kirwan, C.C.; Romics, L.; Cutress, R.I.; Potter, S.; Carmichael, A.; et al. Innovations for the future of breast surgery. Br. J. Surg. 2021, 108, znab147. [Google Scholar] [CrossRef] [PubMed]
- Halsted, C.P.; Benson, J.R.; Jatoi, I. A historical account of breast cancer surgery: Beware of local recurrence but be not radical. Future Oncol. 2014, 10, 1649–1657. [Google Scholar] [CrossRef]
- Morrow, M.; Strom, E.A.; Bassett, L.W.; Dershaw, D.D.; Fowble, B.; Giuliano, A.; Harris, J.R.; O’Malley, F.; Schnitt, S.J.; Singletary, S.E.; et al. Standard for breast conservation therapy in the management of invasive breast carcinoma. CA Cancer J. Clin. 2002, 52, 277–300. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group. Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. N. Engl. J. Med. 1995, 333, 1444–1455. [Google Scholar] [CrossRef]
- Clarke, M.; Collins, R.; Darby, S.; Davies, C.; Elphinstone, P.; Evans, V.; Godwin, J.; Gray, R.; Hicks, C.; James, S.; et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 366, 2087–2106. [Google Scholar] [CrossRef]
- Anderson, S.J.; Wapnir, I.; Dignam, J.J.; Fisher, B.; Mamounas, E.P.; Jeong, J.H.; Geyer, C.E., Jr.; Wickerham, D.L.; Costantino, J.P.; Wolmark, N. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J. Clin. Oncol. 2009, 27, 2466–2473. [Google Scholar] [CrossRef]
- Galimberti, V.; Taffurelli, M.; Leonardi, M.C.; Aristei, C.; Trentin, C.; Cassano, E.; Pietribiasi, F.; Corso, G.; Munzone, E.; Tondini, C.; et al. Surgical resection margins after breast-conserving surgery: Senonetwork recommendations. Tumori 2016, 3, 284–289. [Google Scholar] [CrossRef]
- Corso, G.; Magnoni, F.; Provenzano, E.; Girardi, A.; Iorfida, M.; De Scalzi, A.M.; Invento, A.; Colleoni, M.; Cassano, E.; Trentin, C.; et al. Multicentric breast cancer with heterogeneous histopathology: A multidisciplinary review. Future Oncol. 2020, 16, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Curigliano, G.; Loibl, S.; Dubsky, P.; Gnant, M.; Poortmans, P.; Colleoni, M.; Denkert, C.; Piccart-Gebhart, M.; Regan, M.; et al. Estimating the benefits of therapy for early-stage breast cancer: The St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer. Ann. Oncol. 2019, 30, 1541–1557. [Google Scholar] [CrossRef] [PubMed]
- Brouwer de Koning, S.G.; Vrancken Peeters, M.; Jóźwiak, K.; Bhairosing, P.A.; Ruers, T. Tumor Resection Margin Definitions in Breast-Conserving Surgery: Systematic Review and Meta-analysis of the Current Literature. Clin. Breast Cancer 2018, 18, e595–e600. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1674. [Google Scholar] [CrossRef]
- Morrow, M. Margins in breast-conserving therapy: Have we lost sight of the big picture? Expert Rev. Anticancer Ther. 2008, 8, 1193–1196. [Google Scholar] [CrossRef][Green Version]
- Morrow, M.; Winer, E.P. De-escalating Breast Cancer Surgery-Where Is the Tipping Point? JAMA Oncol. 2020, 6, 183–184. [Google Scholar] [CrossRef]
- Nguyen, P.L.; Taghian, A.G.; Katz, M.S.; Niemierko, A.; Abi Raad, R.F.; Boon, W.L.; Bellon, J.R.; Wong, J.S.; Smith, B.L.; Harris, J.R. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J. Clin. Oncol. 2008, 26, 2373–2378. [Google Scholar] [CrossRef]
- Moran, M.S.; Schnitt, S.J.; Giuliano, A.E.; Harris, J.R.; Khan, S.A.; Horton, J.; Klimberg, S.; Chavez-MacGregor, M.; Freedman, G.; Houssami, N.; et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J. Clin. Oncol. 2014, 32, 1507–1515. [Google Scholar] [CrossRef]
- Marinovich, M.L.; Noguchi, N.; Morrow, M.; Houssami, N. Changes in Reoperation after Publication of Consensus Guidelines on Margins for Breast-Conserving Surgery: A Systematic Review and Meta-analysis. JAMA Surg. 2020, 155, e203025. [Google Scholar] [CrossRef] [PubMed]
- Morrow, M.; Van Zee, K.J.; Solin, L.J.; Houssami, N.; Chavez-MacGregor, M.; Harris, J.R.; Horton, J.; Hwang, S.; Johnson, P.L.; Marinovich, M.L.; et al. Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology Consensus Guideline on Margins for Breast-Conserving Surgery with Whole-Breast Irradiation in Ductal Carcinoma In Situ. J. Clin. Oncol. 2016, 34, 4040–4046. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Dignam, J.; Bryant, J.; DeCillis, A.; Wickerham, D.L.; Wolmark, N.; Costantino, J.; Redmond, C.; Fisher, E.R.; Bowman, D.M.; et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J. Natl. Cancer Inst. 1996, 88, 1529–1542. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Dignam, J.; Mamounas, E.P.; Costantino, J.P.; Wickerham, D.L.; Redmond, C.; Wolmark, N.; Dimitrov, N.V.; Bowman, D.M.; Glass, A.G.; et al. Sequential methotrexate and fluorouracil for the treatment of node-negative breast cancer patients with estrogen receptor-negative tumors: Eight-year results from National Surgical Adjuvant Breast and Bowel Project (NSABP) B-13 and first report of findings from NSABP B-19 comparing methotrexate and fluorouracil with conventional cyclophosphamide, methotrexate, and fluorouracil. J. Clin. Oncol. 1996, 14, 1982–1992. [Google Scholar] [CrossRef] [PubMed]
- Mamounas, E.P.; Tang, G.; Fisher, B.; Paik, S.; Shak, S.; Costantino, J.P.; Watson, D.; Geyer, C.E., Jr.; Wickerham, D.L.; Wolmark, N. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: Results from NSABP B-14 and NSABP B-20. J. Clin. Oncol. 1996, 28, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Morrow, M. Personalizing extent of breast cancer surgery according to molecular subtypes. Breast 2013, 22 (Suppl. S2), S106–S109. [Google Scholar] [CrossRef]
- Van Maaren, M.C.; de Munck, L.; de Bock, G.H.; Jobsen, J.J.; van Dalen, T.; Linn, S.C.; Poortmans, P.; Strobbe, L.; Siesling, S. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: A population-based study. Lancet Oncol. 2016, 17, 1158–1170. [Google Scholar] [CrossRef]
- Agarwal, S.; Pappas, L.; Neumayer, L.; Kokeny, K.; Agarwal, J. Effect of breast conservation therapy vs. mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg. 2014, 149, 267–274. [Google Scholar] [CrossRef]
- de Boniface, J.; Szulkin, R.; Johansson, A. Survival after Breast Conservation vs Mastectomy Adjusted for Comorbidity and Socioeconomic Status: A Swedish National 6-Year Follow-up of 48 986 Women. JAMA Surg. 2021, 156, 628–637. [Google Scholar] [CrossRef]
- Houvenaeghel, G.; de Nonneville, A.; Cohen, M.; Classe, J.M.; Reyal, F.; Mazouni, C.; Chopin, N.; Martinez, A.; Daraï, E.; Coutant, C.; et al. Isolated ipsilateral local recurrence of breast cancer: Predictive factors and prognostic impact. Breast Cancer Res. Treat. 2019, 173, 111–122. [Google Scholar] [CrossRef]
- Corso, G.; Maisonneuve, P.; Santomauro, G.I.; De Scalzi, A.M.; Toesca, A.; Bassi, F.D.; Farante, G.; Caldarella, P.; Intra, M.; Galimberti, V.; et al. Ipsilateral Breast Tumor Reappearance and Contralateral Breast Cancer after Primary Breast Cancer Treatment: A Comprehensive Retrospective Study of 15,168 Patients. Oncology 2018, 95, 147–155. [Google Scholar] [CrossRef]
- Corso, G.; Maisonneuve, P.; Massari, G.; Invento, A.; Pravettoni, G.; De Scalzi, A.; Intra, M.; Galimberti, V.; Morigi, C.; Lauretta, M.; et al. Validation of a Novel Nomogram for Prediction of Local Relapse after Surgery for Invasive Breast Carcinoma. Ann. Surg. Oncol. 2020, 27, 1864–1874. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network (NCCN) NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 14 August 2021).
- Alpert, T.E.; Kuerer, H.M.; Arthur, D.W.; Lannin, D.R.; Haffty, B.G. Ipsilateral breast tumor recurrence after breast conservation therapy: Outcomes of salvage mastectomy vs. salvage breast-conserving surgery and prognostic factors for salvage breast preservation. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Gentilini, O.; Botteri, E.; Rotmensz, N.; Santillo, B.; Peradze, N.; Saihum, R.C.; Intra, M.; Luini, A.; Galimberti, V.; Goldhirsch, A.; et al. When can a second conservative approach be considered for ipsilateral breast tumour recurrence? Ann. Oncol. 2007, 18, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Gentilini, O.; Botteri, E.; Veronesi, P.; Sangalli, C.; Del Castillo, A.; Ballardini, B.; Galimberti, V.; Rietjens, M.; Colleoni, M.; Luini, A.; et al. Repeating conservative surgery after ipsilateral breast tumor reappearance: Criteria for selecting the best candidates. Ann. Surg. Oncol. 2012, 19, 3771–3776. [Google Scholar] [CrossRef]
- Arthur, D.W.; Winter, K.A.; Kuerer, H.M.; Haffty, B.; Cuttino, L.; Todor, D.A.; Anne, P.R.; Anderson, P.; Woodward, W.A.; McCormick, B.; et al. Effectiveness of Breast-Conserving Surgery and 3-Dimensional Conformal Partial Breast Reirradiation for Recurrence of Breast Cancer in the Ipsilateral Breast: The NRG Oncology/RTOG 1014 Phase 2 Clinical Trial. JAMA Oncol. 2020, 6, 75–82. [Google Scholar] [CrossRef]
- Sagona, A.; Gentile, D.; Anghelone, C.; Barbieri, E.; Marrazzo, E.; Antunovic, L.; Franceschini, D.; Tinterri, C. Ipsilateral Breast Cancer Recurrence: Characteristics, Treatment, and Long-Term Oncologic Results at a High-Volume Center. Clin. Breast Cancer 2021, 21, 329–336. [Google Scholar] [CrossRef]
- Gentile, D.; Sagona, A.; Barbieri, E.; Antunovic, L.; Franceschini, D.; Losurdo, A.; Fernandes, B.; Tinterri, C. Breast conserving surgery versus salvage mastectomy for ipsilateral breast cancer recurrence: A propensity score matching analysis. Updates Surg. 2021, 1–11. [Google Scholar] [CrossRef]
- Van den Bruele, A.B.; Chen, I.; Sevilimedu, V.; Le, T.; Morrow, M.; Braunstein, L.Z.; Cody, H.S., 3rd. Management of ipsilateral breast tumor recurrence following breast conservation surgery: A comparative study of re-conservation vs. mastectomy. Breast Cancer Res. Treat. 2021, 187, 105–112. [Google Scholar] [CrossRef]
- Hannoun-Levi, J.M.; Gal, J.; Van Limbergen, E.; Chand, M.E.; Schiappa, R.; Smanyko, V.; Kauer-Domer, D.; Pasquier, D.; Lemanski, C.; Racadot, S.; et al. Salvage Mastectomy Versus Second Conservative Treatment for Second Ipsilateral Breast Tumor Event: A Propensity Score-Matched Cohort Analysis of the GEC-ESTRO Breast Cancer Working Group Database. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 452–461. [Google Scholar] [CrossRef]
- Hannoun-Levi, J.M.; Polgar, C.; Strnad, V.; Breast Cancer Working Group of the GEC-ESTRO. In regard to Van den Bruele AB et al. Management of ipsilateral breast tumor recurrence following breast conservation surgery: A comparative study of re-conservation vs mastectomy. Breast Cancer Res Treat. 2021 May;187(1):105–112, doi:10.1007/s10549-020-06080-9. Epub 2021 Jan 12. PMID: 33433775; PMCID: PMC8068641. Breast Cancer Res. Treat. 2021. [Google Scholar] [CrossRef]
- Burstein, H.J.; Curigliano, G.; Thürlimann, B.; Weber, W.P.; Poortmans, P.; Regan, M.M.; Senn, H.J.; Winer, E.P.; Gnant, M.; Aebi, S.; et al. Customizing local and systemic therapies for women with early breast cancer: The St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann. Oncol. 2021, 32, 1216–1235. [Google Scholar] [CrossRef]
- Magnoni, F.; Galimberti, V.; Corso, G.; Intra, M.; Sacchini, V.; Veronesi, P. Axillary surgery in breast cancer: An updated historical perspective. Semin. Oncol. 2020, 47, 341–352. [Google Scholar] [CrossRef]
- Özkurt, E.; Sakai, T.; Wong, S.M.; Tukenmez, M.; Golshan, M. Survival Outcomes for Patients with Clinical Complete Response after Neoadjuvant Chemotherapy: Is Omitting Surgery an Option? Ann. Surg. Oncol. 2019, 26, 3260–3268. [Google Scholar] [CrossRef] [PubMed]
- Van Ramshorst, M.S.; van der Voort, A.; van Werkhoven, E.D.; Mandjes, I.A.; Kemper, I.; Dezentjé, V.O.; Oving, I.M.; Honkoop, A.H.; Tick, L.W.; van de Wouw, A.J.; et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1630–1640. [Google Scholar] [CrossRef]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Hegg, R.; Tausch, C.; Seo, J.H.; Tsai, Y.F.; Ratnayake, J.; et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 2013, 24, 2278–2284. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Kahler-Ribeiro-Fontana, S.; Pagan, E.; Magnoni, F.; Vicini, E.; Morigi, C.; Corso, G.; Intra, M.; Canegallo, F.; Ratini, S.; Leonardi, M.C.; et al. Long-term standard sentinel node biopsy after neoadjuvant treatment in breast cancer: A single institution ten-year follow-up. Eur. J. Surg. Oncol. 2021, 47, 804–812. [Google Scholar] [CrossRef]
- Heil, J.; Kuerer, H.M.; Pfob, A.; Rauch, G.; Sinn, H.P.; Golatta, M.; Liefers, G.J.; Vrancken Peeters, M.J. Eliminating the breast cancer surgery paradigm after neoadjuvant systemic therapy: Current evidence and future challenges. Ann. Oncol. 2020, 31, 61–71. [Google Scholar] [CrossRef]
- Kuerer, H.M.; Vrancken Peeters, M.; Rea, D.W.; Basik, M.; De Los Santos, J.; Heil, J. Nonoperative Management for Invasive Breast Cancer after Neoadjuvant Systemic Therapy: Conceptual Basis and Fundamental International Feasibility Clinical Trials. Ann. Surg. Oncol. 2017, 24, 2855–2862. [Google Scholar] [CrossRef]
- Heil, J.; Sinn, P.; Richter, H.; Pfob, A.; Schaefgen, B.; Hennigs, A.; Riedel, F.; Thomas, B.; Thill, M.; Hahn, M.; et al. RESPONDER—Diagnosis of pathological complete response by vacuum-assisted biopsy after neoadjuvant chemotherapy in breast Cancer—A multicenter, confirmative, one-armed, intra-individually-controlled, open, diagnostic trial. BMC Cancer 2018, 18, 851. [Google Scholar] [CrossRef] [PubMed]
- Van der Noordaa, M.; van Duijnhoven, F.H.; Loo, C.E.; van Werkhoven, E.; van de Vijver, K.K.; Wiersma, T.; Winter-Warnars, H.; Sonke, G.S.; Vrancken Peeters, M. Identifying pathologic complete response of the breast after neoadjuvant systemic therapy with ultrasound guided biopsy to eventually omit surgery: Study design and feasibility of the MICRA trial (Minimally Invasive Complete Response Assessment). Breast 2018, 40, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Kuerer, H.M.; Rauch, G.M.; Krishnamurthy, S.; Adrada, B.E.; Caudle, A.S.; DeSnyder, S.M.; Black, D.M.; Santiago, L.; Hobbs, B.P.; Lucci, A.; et al. A Clinical Feasibility Trial for Identification of Exceptional Responders in Whom Breast Cancer Surgery Can Be Eliminated Following Neoadjuvant Systemic Therapy. Ann. Surg. 2018, 267, 946–951. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Eliminating Surgery after Systemic Therapy in Treating Patients with HER2 Positive or Triple Negative Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02945579 (accessed on 14 August 2021).
- Heil, J.; Pfob, A.; Morrow, M. De-escalation of breast and axillary surgery in exceptional responders to neoadjuvant systemic treatment. Lancet Oncol. 2021, 22, 435–436. [Google Scholar] [CrossRef]
- Pfob, A.; Sidey-Gibbons, C.; Lee, H.B.; Tasoulis, M.K.; Koelbel, V.; Golatta, M.; Rauch, G.M.; Smith, B.D.; Valero, V.; Han, W.; et al. Identification of breast cancer patients with pathologic complete response in the breast after neoadjuvant systemic treatment by an intelligent vacuum-assisted biopsy. Eur. J. Cancer 2021, 143, 134–146. [Google Scholar] [CrossRef]
- Veronesi, U.; Stafyla, V.; Luini, A.; Veronesi, P. Breast cancer: From “maximum tolerable” to “minimum effective” treatment. Front. Oncol. 2012, 2, 125. [Google Scholar] [CrossRef]
- Warren Peled, A.; Foster, R.D.; Stover, A.C.; Itakura, K.; Ewing, C.A.; Alvarado, M.; Hwang, E.S.; Esserman, L.J. Outcomes after total skin-sparing mastectomy and immediate reconstruction in 657 breasts. Ann. Surg. Oncol. 2012, 19, 3402–3409. [Google Scholar] [CrossRef]
- Lanitis, S.; Tekkis, P.P.; Sgourakis, G.; Dimopoulos, N.; Al Mufti, R.; Hadjiminas, D.J. Comparison of skin-sparing mastectomy versus non-skin-sparing mastectomy for breast cancer: A meta-analysis of observational studies. Ann. Surg. 2010, 251, 632–639. [Google Scholar] [CrossRef]
- Kroll, S.S.; Khoo, A.; Singletary, S.E.; Ames, F.C.; Wang, B.G.; Reece, G.P.; Miller, M.J.; Evans, G.R.; Robb, G.L. Local recurrence risk after skin-sparing and conventional mastectomy: A 6-year follow-up. Plast. Reconstr. Surg. 1999, 104, 421–425. [Google Scholar] [CrossRef]
- Newman, L.A.; Kuerer, H.M.; Hunt, K.K.; Kroll, S.S.; Ames, F.C.; Ross, M.I.; Feig, B.W.; Singletary, S.E. Presentation, treatment, and outcome of local recurrence after skin-sparing mastectomy and immediate breast reconstruction. Ann. Surg. Oncol. 1998, 5, 620–626. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz, L.; Moody, A.M.; Tappy, E.E.; Blankenship, S.A.; Hecht, E.M. Overall Survival, Disease-Free Survival, Local Recurrence, and Nipple-Areolar Recurrence in the Setting of Nipple-Sparing Mastectomy: A Meta-Analysis and Systematic Review. Ann. Surg. Oncol. 2015, 22, 3241–3249. [Google Scholar] [CrossRef]
- Valero, M.G.; Muhsen, S.; Moo, T.A.; Zabor, E.C.; Stempel, M.; Pusic, A.; Gemignani, M.L.; Morrow, M.; Sacchini, V.S. Increase in Utilization of Nipple-Sparing Mastectomy for Breast Cancer: Indications, Complications, and Oncologic Outcomes. Ann. Surg. Oncol. 2020, 27, 344–351. [Google Scholar] [CrossRef]
- Galimberti, V.; Morigi, C.; Bagnardi, V.; Corso, G.; Vicini, E.; Fontana, S.; Naninato, P.; Ratini, S.; Magnoni, F.; Toesca, A.; et al. Oncological Outcomes of Nipple-Sparing Mastectomy: A Single-Center Experience of 1989 Patients. Ann. Surg. Oncol. 2018, 25, 3849–3857. [Google Scholar] [CrossRef]
- Headon, H.L.; Kasem, A.; Mokbel, K. The Oncological Safety of Nipple-Sparing Mastectomy: A Systematic Review of the Literature with a Pooled Analysis of 12,358 Procedures. Arch. Plast. Surg. 2016, 43, 328–338. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Kim, H.J.; Lee, J.W.; Chung, I.Y.; Kim, J.S.; Lee, S.B.; Son, B.H.; Eom, J.S.; Kim, S.B.; Gong, G.Y.; et al. Oncologic Outcomes of Nipple-sparing Mastectomy and Immediate Reconstruction after Neoadjuvant Chemotherapy for Breast Cancer. Ann. Surg. 2020. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Bae, Y.; Lee, S. Nipple-sparing mastectomy for breast cancer close to the nipple: A single institution’s 11-year experience. Breast Cancer 2020, 27, 999–1006. [Google Scholar] [CrossRef]
- Corso, G.; De Lorenzi, F.; Vicini, E.; Pagani, G.; Veronesi, P.; Sargenti, M.; Magnoni, F.; Naninato, P.; Maisonneuve, P.; Sangalli, C.; et al. Nipple-sparing mastectomy with different approaches: Surgical incisions, complications, and cosmetic results. Preliminary results of 100 consecutive patients at a single center. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 1751–1760. [Google Scholar] [CrossRef]
- Vicini, E.; De Lorenzi, F.; Invento, A.; Corso, G.; Radice, D.; Bozzo, S.; Kahler Ribeiro Fontana, S.; Caldarella, P.; Veronesi, P.; Galimberti, V. Is Nipple-Sparing Mastectomy Indicated after Previous Breast Surgery? A Series of 387 Institutional Cases. Plast. Reconstr. Surg. 2021, 148, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Sigalove, S.; Maxwell, G.P.; Sigalove, N.M.; Storm-Dickerson, T.L.; Pope, N.; Rice, J.; Gabriel, A. Prepectoral Implant-Based Breast Reconstruction: Rationale, Indications, and Preliminary Results. Plast. Reconstr. Surg. 2017, 139, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Houvenaeghel, G.; Bannier, M.; Rua, S.; Barrou, J.; Heinemann, M.; Knight, S.; Lambaudie, E.; Cohen, M. Robotic breast and reconstructive surgery: 100 procedures in 2-years for 80 patients. Surg. Oncol. 2019, 31, 38–45. [Google Scholar] [CrossRef]
- Lai, H.W.; Wang, C.C.; Lai, Y.C.; Chen, C.J.; Lin, S.L.; Chen, S.T.; Lin, Y.J.; Chen, D.R.; Kuo, S.J. The learning curve of robotic nipple sparing mastectomy for breast cancer: An analysis of consecutive 39 procedures with cumulative sum plot. Eur. J. Surg. Oncol. 2019, 45, 125–133. [Google Scholar] [CrossRef]
- Sarfati, B.; Struk, S.; Leymarie, N.; Honart, J.F.; Alkhashnam, H.; Tran de Fremicourt, K.; Conversano, A.; Rimareix, F.; Simon, M.; Michiels, S.; et al. Robotic Prophylactic Nipple-Sparing Mastectomy with Immediate Prosthetic Breast Reconstruction: A Prospective Study. Ann. Surg. Oncol. 2018, 25, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Toesca, A.; Invento, A.; Massari, G.; Girardi, A.; Peradze, N.; Lissidini, G.; Sangalli, C.; Maisonneuve, P.; Manconi, A.; Gottardi, A.; et al. Update on the Feasibility and Progress on Robotic Breast Surgery. Ann. Surg. Oncol. 2019, 26, 3046–3051. [Google Scholar] [CrossRef]
- Filipe, M.D.; de Bock, E.; Postma, E.L.; Bastian, O.W.; Schellekens, P.; Vriens, M.R.; Witkamp, A.J.; Richir, M.C. Robotic nipple-sparing mastectomy complication rate compared to traditional nipple-sparing mastectomy: A systematic review and meta-analysis. J. Robot. Surg. 2021, 1–8. [Google Scholar] [CrossRef]
- Toesca, A.; Sangalli, C.; Maisonneuve, P.; Massari, G.; Girardi, A.; Baker, J.L.; Lissidini, G.; Invento, A.; Farante, G.; Corso, G.; et al. A Randomized Trial of Robotic Mastectomy versus Open Surgery in Women with Breast Cancer or BRCA Mutation. Ann. Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Beeraka, N.M.; Zhang, J.; Reshetov, I.V.; Nikolenko, V.N.; Sinelnikov, M.Y.; Mikhaleva, L.M. Efficacy of da Vinci robot-assisted lymph node surgery than conventional axillary lymph node dissection in breast cancer—A comparative study. Int. J. Med. Robot. 2021, e2307. [Google Scholar] [CrossRef]
- Yip, C.H.; Newman, L.A. American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Guideline for Management of Hereditary Breast Cancer. JAMA Surg. 2021, 156, 284–285. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.M.; Boughey, J.C.; Pierce, L.J.; Robson, M.E.; Bedrosian, I.; Dietz, J.R.; Dragun, A.; Gelpi, J.B.; Hofstatter, E.W.; Isaacs, C.J.; et al. Management of Hereditary Breast Cancer: American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Guideline. J. Clin. Oncol. 2020, 38, 2080–2106. [Google Scholar] [CrossRef]
- Corso, G.; Magnoni, F. Hereditary breast cancer: Translation into clinical practice of recent American Society of Clinical Oncology, American Society of Radiation Oncology, and Society of Surgical Oncology recommendations. Eur. J. Cancer Prev. 2021, 30, 311–314. [Google Scholar] [CrossRef]
- Corso, G.; Figueiredo, J.; La Vecchia, C.; Veronesi, P.; Pravettoni, G.; Macis, D.; Karam, R.; Lo Gullo, R.; Provenzano, E.; Toesca, A.; et al. Hereditary lobular breast cancer with an emphasis on E-cadherin genetic defect. J. Med. Genet. 2018, 55, 431–441. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnoni, F.; Alessandrini, S.; Alberti, L.; Polizzi, A.; Rotili, A.; Veronesi, P.; Corso, G. Breast Cancer Surgery: New Issues. Curr. Oncol. 2021, 28, 4053-4066. https://doi.org/10.3390/curroncol28050344
Magnoni F, Alessandrini S, Alberti L, Polizzi A, Rotili A, Veronesi P, Corso G. Breast Cancer Surgery: New Issues. Current Oncology. 2021; 28(5):4053-4066. https://doi.org/10.3390/curroncol28050344
Chicago/Turabian StyleMagnoni, Francesca, Sofia Alessandrini, Luca Alberti, Andrea Polizzi, Anna Rotili, Paolo Veronesi, and Giovanni Corso. 2021. "Breast Cancer Surgery: New Issues" Current Oncology 28, no. 5: 4053-4066. https://doi.org/10.3390/curroncol28050344
APA StyleMagnoni, F., Alessandrini, S., Alberti, L., Polizzi, A., Rotili, A., Veronesi, P., & Corso, G. (2021). Breast Cancer Surgery: New Issues. Current Oncology, 28(5), 4053-4066. https://doi.org/10.3390/curroncol28050344






