Feasibility of a Pilot Randomized Controlled Trial Examining a Multidimensional Intervention in Women with Gynecological Cancer at Risk of Lymphedema
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Sample Size Justification
2.4. Intervention
2.5. Outcome Measures
- Presence and characteristics of lymphedema: The diagnosis of lymphedema was based on clinical examination. Specifically, the presence of soft pitting edema or fibrotic non-pitting edema along the lower limbs and/or the presence of a positive Stemmer sign (thickened skin fold at base of second toe) were evaluated [17,36,37,38]. Lymphedema was staged according to guidelines provided by the International Society of Lymphology 2013 Consensus Document [39].
- Lower limb circumferential measures: Bilateral lower limb circumferential measures were obtained using a tape measure and a measurement board. Participants were in a supine position and the lower limb was placed on the measurement board. Serial circumferential measures to the nearest millimeter were taken with a retractable no-stretch soft tape measure at eight points along each limb: (1) 10 cm from heel around foot; (2) superior to malleoli (about 10 cm from heel); (3) 10 cm above second point; (4) 20 cm above second point; (5) 30 cm above second point; (6) 40 cm above second point; (7) 50 cm above second point; (8) groin. Total and segmental limb volumes for each limb were calculated using the truncated cone method [40].
- Lower limb volume: Total and segmental volume measurements of the bilateral lower limbs were measured with an optoelectronic infra-red volumeter (perometry) (Pero-System Messgeraete GmbH, Wuppertal, Germany, Perometer 350S) [41]. The perometry device was placed on an adjustable table and participants were seated on an adjacent chair with adjustable height. The lower limb was extended with the foot supported so the limb was parallel to the device for measurement. The volume of each limb was measured once, by moving the frame of the device slowly along the limb.
- Body composition: Changes in intracellular and extracellular lower limb fluid were measured by assessing ratios of resistance at infinite (Ri) to resistance at zero (Ro) through bioimpedance spectroscopy (ImpediMed Ltd., Carlsbad, CA, USA, Imp SFB7) [42]. The bioimpedance measurements were obtained using a tetrapolar surface electrode arrangement. Participants were supine and after cleansing the skin surface, four single-use surface electrodes were placed with reference to anatomical markers: two drive electrodes were placed on the dorsal surface of the hand, 1 cm proximal to the metacarpophalangeal joint of the middle finger, and on the mid dorsum of the foot 1 cm proximal to the metatarsophalangeal joint of the second toe; two measurement (voltage-sensing) electrodes were placed on the dorsal surface of each ankle between the medial and lateral malleoli of the ankle. This procedure was repeated on both limbs. Measurements began within five minutes of lying down and were completed within ten minutes. All but two measurements were taken in the morning between 8:00 a.m. and 10:00 a.m.
- Quality of life (QOL): Patient-reported QOL was measured by administering the 30-item European Organization for Research and Treatment of Cancer (EORTC) Core Quality of Life Questionnaire (QLQ-C30) (EORTC Quality of Life Group, version 3.0). This 30-item questionnaire yields five functional subscale scores, nine symptom subscale scores and one global health status score [43].
2.6. Statistical Analysis
3. Results
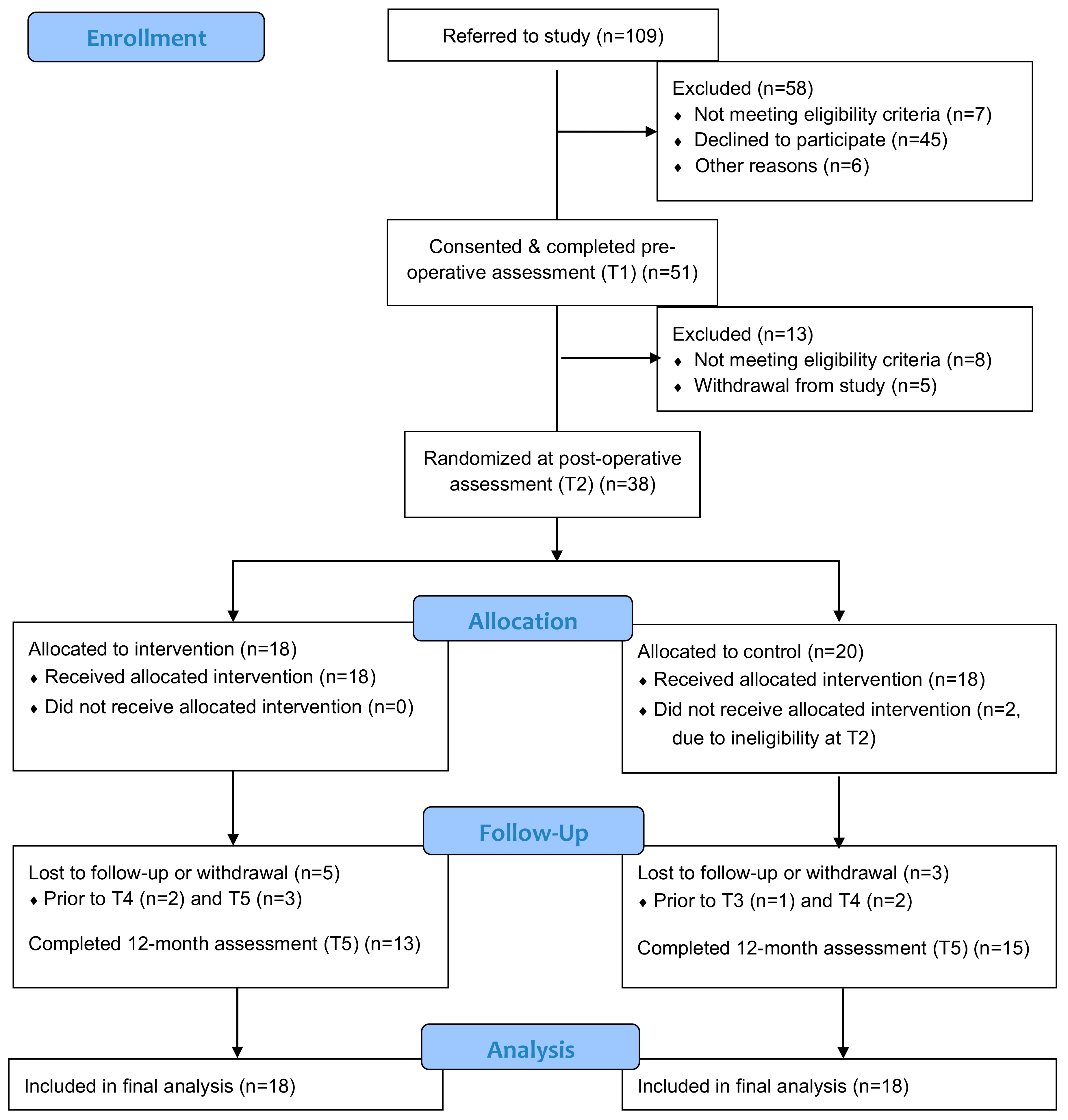
3.1. Recruitment and Retention
3.2. Outcome Assessment Procedures
3.3. Intervention Safety and Feasibility
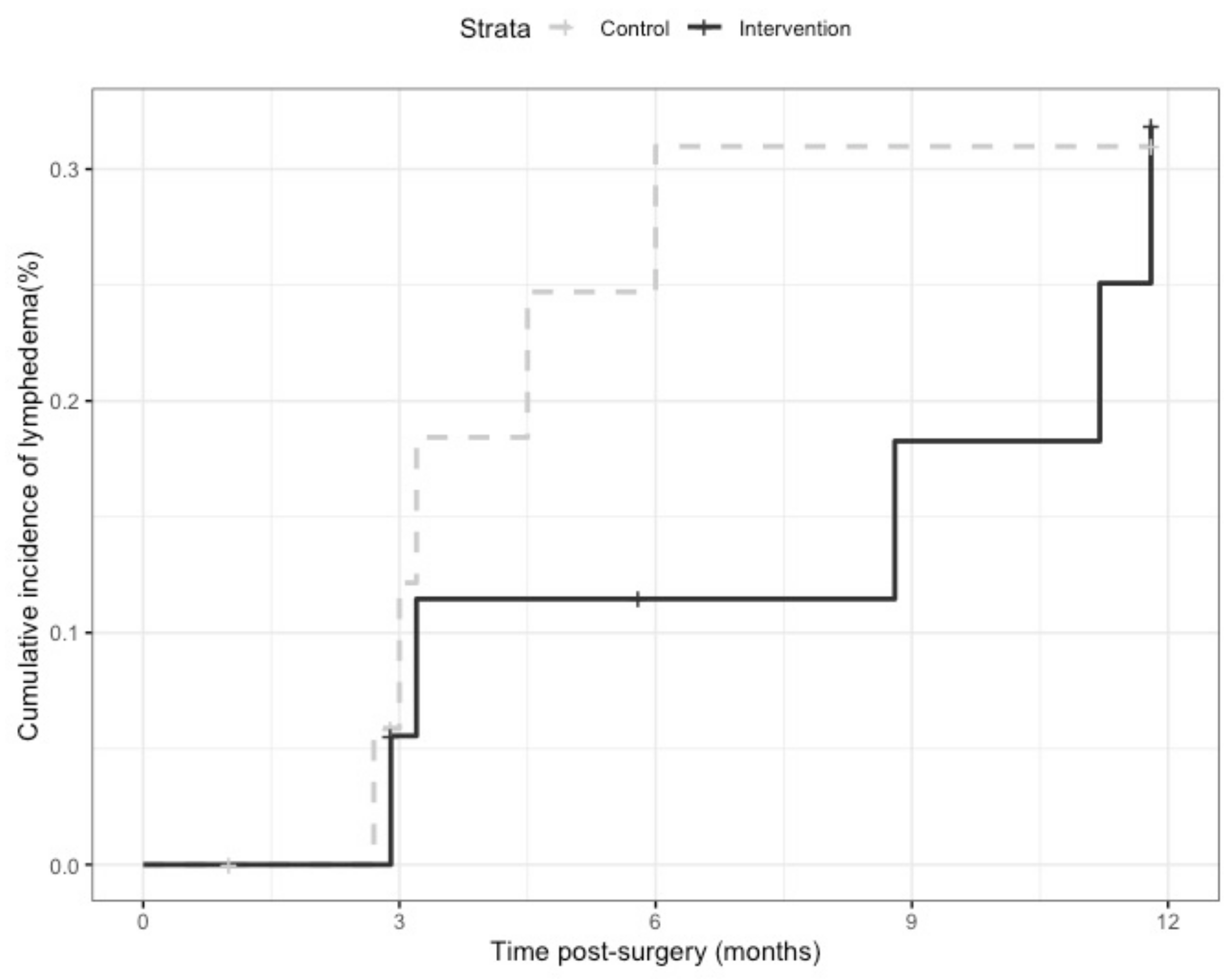
3.4. Preliminary Effectiveness on Lymphedema Incidence
4. Discussion
4.1. Participant Recruitment and Retention
4.2. Outcome Assessment Procedures
4.3. Intervention Safety and Feasibility
4.4. Lymphedema Incidence
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canadian Cancer Society. Canadian Cancer Statistics 2019; Canadian Cancer Society: Toronto, ON, Canada, 2019. [Google Scholar]
- Cormier, J.N.; Askew, R.L.; Mungovan, K.S.; Xing, Y.; Ross, M.I.; Armer, J.M. Lymphedema beyond breast cancer: A systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer 2010, 116, 5138–5149. [Google Scholar] [CrossRef] [PubMed]
- Deura, I.; Shimada, M.; Hirashita, K.; Sugimura, M.; Sato, S.; Sato, S.; Oishi, T.; Itamochi, H.; Harada, T.; Kigawa, J. Incidence and risk factors for lower limb lymphedema after gynecologic cancer surgery with initiation of periodic complex decongestive physiotherapy. Int. J. Clin. Oncol. 2015, 20, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.C.; Janda, M.; Ward, L.C.; Reul-Hirche, H.; Steele, M.L.; Carter, J.; Quinn, M.; Cornish, B.; Obermair, A. Lymphedema following gynecological cancer: Results from a prospective, longitudinal cohort study on prevalence, incidence and risk factors. Gynecol. Oncol. 2017, 146, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Yamamoto, Y.; Yanagisawa, M.; Kawata, A.; Akiba, N.; Suzuki, K.; Naritaka, K. Risk factors and a prediction model for lower limb lymphedema following lymphadenectomy in gynecologic cancer: A hospital-based retrospective cohort study. BMC Women Health 2017, 17, 50. [Google Scholar] [CrossRef]
- Beesley, V.; Janda, M.; Eakin, E.; Obermair, A.; Battistutta, D. Lymphedema after gynecological cancer treatment: Prevalence, correlates, and supportive care needs. Cancer 2007, 109, 2607–2614. [Google Scholar] [CrossRef]
- Todo, Y.; Yamamoto, R.; Minobe, S.; Suzuki, Y.; Takeshi, U.; Nakatani, M.; Aoyagi, Y.; Ohba, Y.; Okamoto, K.; Kato, H. Risk factors for postoperative lower-extremity lymphedema in endometrial cancer survivors who had treatment including lymphadenectomy. Gynecol. Oncol. 2010, 119, 60–64. [Google Scholar] [CrossRef]
- Ohba, Y.; Todo, Y.; Kobayashi, N.; Kaneuchi, M.; Watari, H.; Takeda, M.; Sudo, S.; Kudo, M.; Kato, H.; Sakuragi, N. Risk factors for lower-limb lymphedema after surgery for cervical cancer. Int. J. Clin. Oncol. 2011, 16, 238–243. [Google Scholar]
- Ryan, M.; Stainton, M.C.; Slaytor, E.K.; Jaconelli, C.; Watts, S.; Mackenzie, P. Aetiology and prevalence of lower limb lymphoedema following treatment for gynaecological cancer. Aust. N. Z. J. Obstet. Gynaecol. 2003, 43, 148–151. [Google Scholar]
- Kim, J.H.; Choi, J.H.; Ki, E.Y.; Lee, S.J.; Yoon, J.H.; Lee, K.H.; Park, T.C.; Park, J.S.; Bae, S.N.; Hur, S.Y. Incidence and risk factors of lower-extremity lymphedema after radical surgery with or without adjuvant radiotherapy in patients with FIGO stage I to stage IIA cervical cancer. Int. J. Gynecol. Cancer 2012, 22, 686–691. [Google Scholar]
- Fuller, J.; Guderian, D.; Kohler, C.; Schneider, A.; Wendt, T.G. Lymph edema of the lower extremities after lymphadenectomy and radiotherapy for cervical cancer. Strahlenther. Onkol. 2008, 184, 206–211. [Google Scholar] [CrossRef]
- Graf, N.; Rufibach, K.; Schmidt, A.M.; Fehr, M.; Fink, D.; Baege, A.C. Frequency and risk factors of lower limb lymphedema following lymphadenectomy in patients with gynecological malignancies. Eur. J. Gynaecol. Oncol. 2013, 34, 23–27. [Google Scholar] [PubMed]
- Tada, H.; Teramukai, S.; Fukushima, M.; Sasaki, H. Risk factors for lower limb lymphedema after lymph node dissection in patients with ovarian and uterine carcinoma. BMC Cancer 2009, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Coriddi, M.; Salani, R.; Povoski, S.P. Breast and gynecologic cancer-related extremity lymphedema: A review of diagnostic modalities and management options. World J. Surg. Oncol. 2013, 11, 237. [Google Scholar] [CrossRef] [PubMed]
- Hinten, F.; van den Einden, L.C.G.; Hendriks, J.C.M.; van der Zee, A.G.J.; Bulten, J.; Massuger, L.F.A.G.; van de Nieuwenhof, H.P.; de Hullu, J.A. Risk factors for short- and long-term complications after groin surgery in vulvar cancer. Br. J. Cancer 2011, 105, 1279–1287. [Google Scholar] [CrossRef]
- Keast, D.H.; Despatis, M.; Allen, J.O.; Brassard, A. Chronic oedema/lymphoedema: Under-recognised and under-treated. Int. Wound J. 2015, 12, 328–333. [Google Scholar] [CrossRef]
- Biglia, N.; Zanfagnin, V.; Daniele, A.; Robba, E.; Bounous, V.E. Lower body lymphedema in patients with gynecologic cancer. Anticancer. Res. 2017, 37, 4005–4015. [Google Scholar]
- Cohen, S.R.; Payne, D.K.; Tunkel, R.S. Lymphedema: Strategies for management. Cancer 2001, 92 (Suppl. S4), 980–987. [Google Scholar] [CrossRef]
- Ryan, M.; Stainton, M.C.; Jaconelli, C.; Watts, S.; MacKenzie, P.; Mansberg, T. The experience of lower limb lymphedema for women after treatment for gynecologic cancer. Oncol. Nurs. Forum 2003, 30, 417–423. [Google Scholar] [CrossRef]
- Dunberger, G.; Lindquist, H.; Waldenstrom, A.C.; Nyberg, T.; Steineck, G.; Lundqvist, E.A. Lower limb lymphedema in gynecological cancer survivors—Effect on daily life functioning. Support. Care Cancer 2013, 21, 3063–3070. [Google Scholar]
- Brown, J.C.; John, G.M.; Segal, S.; Chu, C.S.; Schmitz, K.H. Physical activity and lower limb lymphedema among uterine cancer survivors. Med. Sci. Sports Exerc. 2013, 45, 2091–2097. [Google Scholar] [CrossRef]
- Brown, J.C.; Chu, C.S.; Cheville, A.L.; Schmitz, K.H. The prevalence of lymphedema symptoms among survivors of long-term cancer with or at risk for lower limb lymphedema. Am. J. Phys. Med. Rehabil. 2013, 92, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, C.J.; Franks, P.J.; Doherty, D.C.; Williams, A.F.; Badger, C.; Jeffs, E.; Bosanquet, N.; Mortimer, P.S. Lymphoedema: An underestimated health problem. QJM Mon. J. Assoc. Physicians 2003, 96, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.F.; Li, S.H.; Huang, H.Y. The efficacy of complex decongestive physiotherapy (CDP) and predictive factors of response to CDP in lower limb lymphedema (LLL) after pelvic cancer treatment. Gynecol. Oncol. 2012, 125, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.F.; Huang, M.S.; Li, S.H.; Chen, I.-R.; Wei, T.S.; Kuo, S.J.; Chen, S.T.; Hsu, J.H. Complex decongestive physiotherapy for patients with chronic cancer-associated lymphedema. J. Formos. Med. Assoc. 2004, 103, 344–348. [Google Scholar] [PubMed]
- Franks, P.J.; Moffatt, C.J.; Doherty, D.C.; Williams, A.F.; Jeffs, E.; Mortimer, P.S. Assessment of health-related quality of life in patients with lymphedema of the lower limb. Wound Repair Regen. 2006, 14, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Iwersen, L.F.; Sperandio, F.F.; Toriy, A.M.; Palú, M.; da Luz, C.M. Evidence-based practice in the management of lower limb lymphedema after gynecological cancer. Physiother. Theory Pract. 2017, 33, 1–8. [Google Scholar] [CrossRef]
- Stout, N.L.; Weiss, R.; Feldman, J.L.; Stewart, B.R.; Armer, J.M.; Cormier, J.N.; Shih, Y.-C.T. A systematic review of care delivery models and economic analyses in lymphedema: Health policy impact (2004–2011). Lymphology 2013, 46, 27–41. [Google Scholar]
- Stout, N.L.; Pfalzer, L.A.; Springer, B.; Levy, E.; McGarvey, C.L.; Danoff, J.V.; Gerber, L.H.; Soballe, P.W. Breast cancer-related lymphedema: Comparing direct costs of a prospective surveillance model and a traditional model of care. Phys. Ther. 2012, 92, 152–163. [Google Scholar] [CrossRef]
- McNeely, M.L.; Peddle, C.J.; Yurick, J.L.; Dayes, I.S.; Mackey, J.R. Conservative and dietary interventions for cancer-related lymphedema: A systematic review and meta-analysis. Cancer 2011, 117, 1136–1148. [Google Scholar] [CrossRef]
- Leon, A.C.; Davis, L.L.; Kraemer, H.C. The role and interpretation of pilot studies in clinical research. J. Psychiatr. Res. 2011, 45, 626–629. [Google Scholar] [CrossRef]
- Feeley, N.; Cossette, S.; Côté, J.; Héon, M.; Stremler, R.; Martorella, G.; Purden, M. The importance of piloting an RCT intervention. Can. J. Nurs. Res. 2009, 41, 85–99. [Google Scholar] [PubMed]
- Canadian Society for Exercise Physiology. Canadian Physical Activity Guidelines for Adults (18–64 Years). 2011. Available online: https://csepguidelines.ca/wp-content/uploads/2018/03/CSEP_PAGuidelines_adults_en.pdf (accessed on 25 August 2020).
- Casley-Smith, J.R.; Casley-Smith, J.R. Modern Treatment for Lymphoedema, 5th ed.; Lymphoedema Association of Australia: Adelaide, Australia, 1997.
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef] [PubMed]
- Armer, J.M.; Stewart, B.R. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphat. Res. Biol. 2005, 3, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Ward, L. Early Diagnosis in Latent Phase. In Lymphedema; Lee, B.-B., Bergan, J., Rockson., S.G., Eds.; Springer: London, UK, 2011; pp. 105–109. [Google Scholar]
- Stemmer, R. Stemmer’s sign—Possibilities and limits of clinical diagnosis of lymphedema. Wien. Med. Wochenschr. 1999, 149, 85–86. [Google Scholar] [PubMed]
- International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2013 Consensus Document of the International Society of Lymphology. Lymphology 2013, 46, 1–11. [Google Scholar]
- Taylor, R.; Jayasinghe, U.W.; Koelmeyer, L.; Ung, O.; Boyages, J. Reliability and validity of arm volume measurements for assessment of lymphedema. Phys. Ther. 2006, 86, 205–214. [Google Scholar]
- Stanton, A.W.; Northfield, J.W.; Holroyd, B.; Mortimer, P.S.; Levick, J.R. Validation of an optoelectronic limb volumeter (Perometer). Lymphology 1997, 30, 77–97. [Google Scholar]
- Warren, A.G.; Janz, B.A.; Slavin, S.A.; Borud, L.J. The use of bioimpedance analysis to evaluate lymphedema. Ann. Plast. Surg. 2007, 58, 541–543. [Google Scholar]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Keast, D.H.; Towers, A. The rising prevalence of lymphedema in Canada: A continuing dialogue. Pathw. Can. Lymphedema Mag. 2017, Spring, 5–8. [Google Scholar]
- Shallwani, S.M.; Hodgson, P.; Towers, A. Examining obesity in lymphedema: A retrospective study of 178 new patients with suspected lymphedema at a Canadian hospital-based clinic. Physiother. Can. 2020, 72, 18–25. [Google Scholar] [CrossRef]
- Iyer, N.S.; Cartmel, B.; Friedman, L.; Li, F.; Zhou, Y.; Ercolano, E.; Harrigan, M.; Gottlieb, L.; McCorkle, R.; Schwartz, P.E.; et al. Lymphedema in ovarian cancer survivors: Assessing diagnostic methods and the effects of physical activity. Cancer 2018, 124, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Stout, N.; Partsch, H.; Szolnoky, G.; Cordero, I.F.; Mosti, G.; Mortimer, P.; Flour, M.; Damstra, R.; Piller, N.; Geyer, M.J.; et al. Chronic edema of the lower extremities: International consensus recommendations for compression therapy clinical research trials. Int. Angiol. 2012, 31, 316–329. [Google Scholar] [PubMed]
- Dankert, J.; Bouma, J. Recurrent acute leg cellulitis after hysterectomy with pelvic lymphadenectomy. Br. J. Obstet. Gynaecol. 1987, 94, 788–790. [Google Scholar] [PubMed]
- Shallwani, S.M.; Hodgson, P.; Towers, A. Comparisons between cancer-related and noncancer-related lymphedema: An overview of new patients referred to a specialized hospital-based center in Canada. Lymphat. Res. Biol. 2017, 15, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Stuiver, M.M.; de Rooij, J.D.; Lucas, C.; Nieweg, O.E.; Horenblas, S.; van Geel, A.N.; van Beurden, M.; Aaronson, N.K. No evidence of benefit from class-II compression stockings in the prevention of lower-limb lymphedema after inguinal lymph node dissection: Results of a randomized controlled trial. Lymphology 2013, 46, 120–131. [Google Scholar]
- Sawan, S.; Mugnai, R.; Lopes Ade, B.; Hughes, A.; Edmondson, R.J. Lower-limb lymphedema and vulval cancer: Feasibility of prophylactic compression garments and validation of leg volume measurement. Int. J. Gynecol. Cancer 2009, 19, 1649–1654. [Google Scholar] [CrossRef]
- Lam, R.; Wallace, A.; Burbidge, B.; Franks, P.; Moffatt, C. Experiences of patients with lymphoedema. J. Lymphoedema 2006, 1, 16–21. [Google Scholar]
- Ridner, S.H.; Dietrich, M.S.; Kidd, N. Breast cancer treatment-related lymphedema self-care: Education, practices, symptoms, and quality of life. Support. Care Cancer 2011, 19, 631–637. [Google Scholar]
- Al Onazi, M.; Dolgoy, N.; Parkinson, J.; McNeely, M.L. Exploring adherence to daytime compression in women with breast cancer related lymphedema: A multi-methods study. Plast. Aesthetic Res. 2020, 7, 23. [Google Scholar] [CrossRef]
- Beesley, V.; Eakin, E.; Steginga, S.; Aitken, J.; Dunn, J.; Battistutta, D. Unmet needs of gynaecological cancer survivors: Implications for developing community support services. Psycho Oncol. 2008, 17, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Lin, L.L.; Segal, S.; Chu, C.S.; Haggerty, A.E.; Ko, E.M.; Schmitz, K.H. Physical activity, daily walking, and lower limb lymphedema associate with physical function among uterine cancer survivors. Support. Care Cancer 2014, 22, 3017–3025. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, P.; Greenwood, N. Understanding the Hawthorne effect. BMJ Br. Med. J. 2015, 351, h4672. [Google Scholar] [CrossRef] [PubMed]
- Farrokhzadi, L.; Dhillon, H.M.; Goumas, C.; Young, J.M.; Cust, A.E. Physical activity correlates, barriers, and preferences for women with gynecological cancer. Int. J. Gynecol. Cancer 2016, 26, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.M.; Lowe-Strong, A.; Rankin, J.P.; Campbell, A.; Blaney, J.M.; Gracey, J.H. A focus group study exploring gynecological cancer survivors’ experiences and perceptions of participating in a RCT testing the efficacy of a home-based physical activity intervention. Support. Care Cancer 2013, 21, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Dugan, N.L.; Cohn, J.C.; Chu, C.; Smith, R.G.; Schmitz, K.H. Weight lifting in patients with lower-extremity lymphedema secondary to cancer: A pilot and feasibility study. Arch. Phys. Med. Rehabil. 2010, 91, 1070–1076. [Google Scholar] [CrossRef]
- Fukushima, T.; Tsuji, T.; Sano, Y.; Miyata, C.; Kamisako, M.; Hohri, H.; Yoshimura, C.; Asakura, M.; Okitsu, T.; Muraoka, K.; et al. Immediate effects of active exercise with compression therapy on lower-limb lymphedema. Support. Care Cancer 2017, 25, 2603–2610. [Google Scholar] [CrossRef] [PubMed]
- Do, J.H.; Choi, K.H.; Ahn, J.S.; Jeon, J.Y. Effects of a complex rehabilitation program on edema status, physical function, and quality of life in lower-limb lymphedema after gynecological cancer surgery. Gynecol. Oncol. 2017, 147, 450–455. [Google Scholar] [PubMed]
- Stout Gergich, N.L.; Pfalzer, L.A.; McGarvey, C.; Springer, B.; Gerber, L.H.; Soballe, P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer 2008, 112, 2809–2819. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Ahmed, R.L.; Troxel, A.B.; Cheville, A.; Lewis-Grant, L.; Smith, R.; Bryan, C.J.; Smith, C.T.W.; Chittams, J. Weight lifting for women at risk for breast cancer–related lymphedema: A randomized trial. JAMA 2010, 304, 2699–2705. [Google Scholar]
- Torres Lacomba, M.; Sánchez, M.J.Y.; Goñi, A.Z.; Merino, D.P.; del Moral, O.M.; Téllez, E.C.; Mogollón, E.M. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: Randomised, single blinded, clinical trial. BMJ 2010, 340, b5396. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Hasan, B.; Malandris, K.; Farah, M.H.; Manolopoulos, A.; Ginex, P.K.; Anbari, A.B.; Nayfeh, T.; Rajjoub, M.; Benkhadra, R. Prospective surveillance and risk reduction of cancer treatment-related lymphedema: Systematic review and meta-analysis. Oncol. Nurs. Forum 2020, 47, E161–E170. [Google Scholar] [CrossRef]
- Paskett, E.D.; Rademacher, J.L.; Oliveri, J.M.; Liu, H.; Seisler, D.K.; Sloan, J.A.; Armer, J.M.; Naughton, M.J.; Hock, K.; Schwartz, M.A.; et al. A randomized study to prevent lymphedema in women treated for breast cancer: CALGB 70305 (Alliance). Cancer 2020, 33183. [Google Scholar] [CrossRef] [PubMed]
- Partsch, H.; Stout, N.; Cordero, I.F.; Flour, M.; Moffatt, C.; Szuba, A.; Milic, D.; Szolnoky, G.; Brorson, H.; Abel, M.; et al. Clinical trials needed to evaluate compression therapy in breast cancer related lymphedema (BCRL). Proposals from an expert group. Int. Angiol. 2010, 29, 442–453. [Google Scholar] [PubMed]
- Schmitz, K.H.; Ahmed, R.L.; Troxel, A.; Cheville, A.; Smith, R.; Grant, L.L.; Bryan, C.J.; Smith, C.T.W.; Greene, Q.P. Weight lifting in women with breast-cancer-related lymphedema. N. Engl. J. Med. 2009, 361, 664–673. [Google Scholar] [CrossRef]
- Singh, B.; Disipio, T.; Peake, J.; Hayes, S.C. Systematic review and meta-analysis of the effects of exercise for those with cancer-related lymphedema. Arch. Phys. Med. Rehabil. 2016, 97, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Buchan, J.; Janda, M.; Box, R.; Schmitz, K.; Hayes, S. A randomized trial on the effect of exercise mode on breast cancer-related lymphedema. Med. Sci. Sports Exerc. 2016, 48, 1866–1874. [Google Scholar] [CrossRef]
| Characteristics | Overall | IG | CG |
|---|---|---|---|
| Sample size | 36 | 18 | 18 |
| Age (years), mean (sd) | 57.6 (9.6) | 56.3 (10.1) | 58.9 (9.1) |
| Employment status | |||
| Full time | 22 (61) | 11 (61) | 11 (61) |
| Part time | 5 (14) | 3 (17) | 2 (11) |
| Retired | 8 (22) | 3 (17) | 5 (28) |
| Unemployed | 1 (3) | 1 (6) | 0 (0) |
| Body mass index b (kg/m2) | |||
| Mean (sd) | 27.5 (5.9) | 27.2 (6.2) | 27.9 (5.8) |
| Range | 17.9–40.9 | 18.5–40.9 | 17.9–38.5 |
| Body mass index category b | |||
| Underweight | 1 (3) | 0 (0) | 1 (6) |
| Normal weight | 14 (39) | 9 (50) | 5 (28) |
| Overweight | 9 (25) | 5 (28) | 4 (22) |
| Obese | 12 (33) | 4 (22) | 8 (44) |
| Treatment status | |||
| Radiotherapy | 19 (53) | 9 (50) | 10 (56) |
| No radiotherapy | 17 (47) | 9 (50) | 8 (44) |
| Cancer diagnosis | |||
| Endometrial | 26 (73) | 13 (72) | 13 (72) |
| Cervical | 7 (19) | 4 (22) | 3 (17) |
| Vulvar | 3 (8) | 1 (6) | 2 (11) |
| Comorbidities c | |||
| None | 12 (33) | 7 (39) | 5 (28) |
| One | 10 (28) | 7 (39) | 3 (17) |
| Two or more | 14 (39) | 4 (22) | 10 (56) |
| Marital status d | |||
| Married | 23 (64) | 11 (61) | 12 (67) |
| Other | 13 (36) | 7 (39) | 6 (33) |
| Study Time Point | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|
| Number of participants | 51 | 38 | 35 | 31 | 28 |
| Clinical examination | 51 (100%) | 38 (100%) | 35 (100%) | 31 (100%) | 28 (100%) |
| Circumferential measures | 50 (98%) | 38 (100%) | 35 (100%) | 31 (100%) | 28 (100%) |
| Perometry | 43 (84.3%) | 31 (81.6%) | 29 (82.9%) | 24 (77.4%) | 24 (85.7%) |
| BIS | 48 (94.1%) | 33 (86.8%) | 35 (100%) | 26 (83.9%) | 22 (78.6%) |
| EORTC-QLQ-C30 | 51 (100%) | 38 (100%) | 33 (94.3%) | 29 (93.5%) | 26 (92.9%) |
| T1 | T2 | T3 | T4 | T5 | |
|---|---|---|---|---|---|
| CG | 62% (n = 13/21) | 56% (n = 10/18) | 53% (n = 9/17) | 60% (n = 9/15) | 53% (n = 8/15) |
| IG | 61% (n = 11/18) | 28% (n = 5/18) | 56% (n = 10/18) | 56% (n = 9/16) | 69% (n = 9/13) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shallwani, S.M.; Towers, A.; Newman, A.; Salvador, S.; Yung, A.; Gilbert, L.; Gotlieb, W.H.; Zeng, X.; Thomas, D. Feasibility of a Pilot Randomized Controlled Trial Examining a Multidimensional Intervention in Women with Gynecological Cancer at Risk of Lymphedema. Curr. Oncol. 2021, 28, 455-470. https://doi.org/10.3390/curroncol28010048
Shallwani SM, Towers A, Newman A, Salvador S, Yung A, Gilbert L, Gotlieb WH, Zeng X, Thomas D. Feasibility of a Pilot Randomized Controlled Trial Examining a Multidimensional Intervention in Women with Gynecological Cancer at Risk of Lymphedema. Current Oncology. 2021; 28(1):455-470. https://doi.org/10.3390/curroncol28010048
Chicago/Turabian StyleShallwani, Shirin M., Anna Towers, Anne Newman, Shannon Salvador, Angela Yung, Lucy Gilbert, Walter H. Gotlieb, Xing Zeng, and Doneal Thomas. 2021. "Feasibility of a Pilot Randomized Controlled Trial Examining a Multidimensional Intervention in Women with Gynecological Cancer at Risk of Lymphedema" Current Oncology 28, no. 1: 455-470. https://doi.org/10.3390/curroncol28010048
APA StyleShallwani, S. M., Towers, A., Newman, A., Salvador, S., Yung, A., Gilbert, L., Gotlieb, W. H., Zeng, X., & Thomas, D. (2021). Feasibility of a Pilot Randomized Controlled Trial Examining a Multidimensional Intervention in Women with Gynecological Cancer at Risk of Lymphedema. Current Oncology, 28(1), 455-470. https://doi.org/10.3390/curroncol28010048





