Anti-Allergic Effect of Aqueous Extract of Coriander (Coriandrum sativum L.) Leaf in RBL-2H3 Cells and Cedar Pollinosis Model Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sample Preparation
2.3. Cells and Cell Culture
2.4. Mice
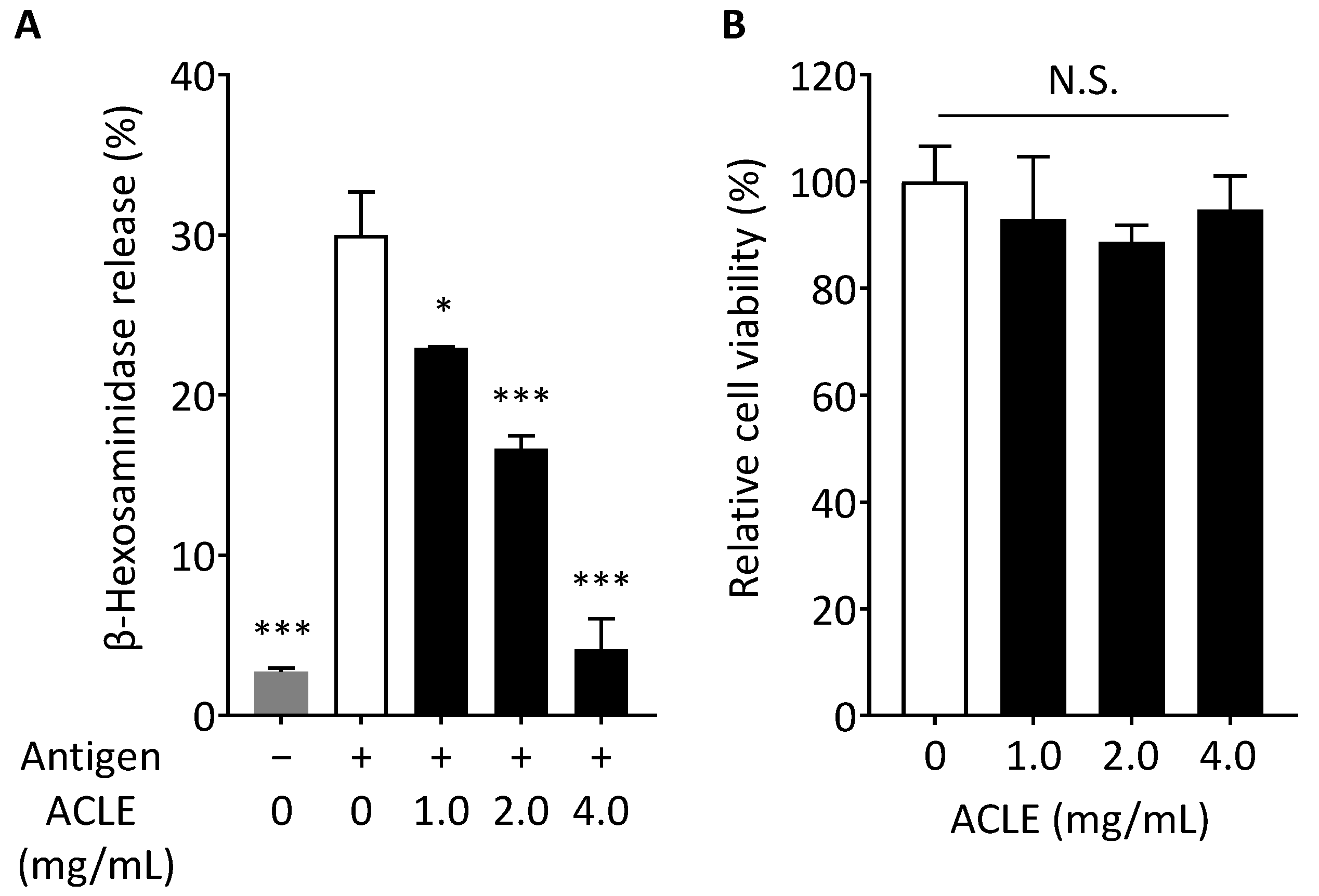
2.5. β-Hexosaminidase Release Assay
2.6. Cell Viability
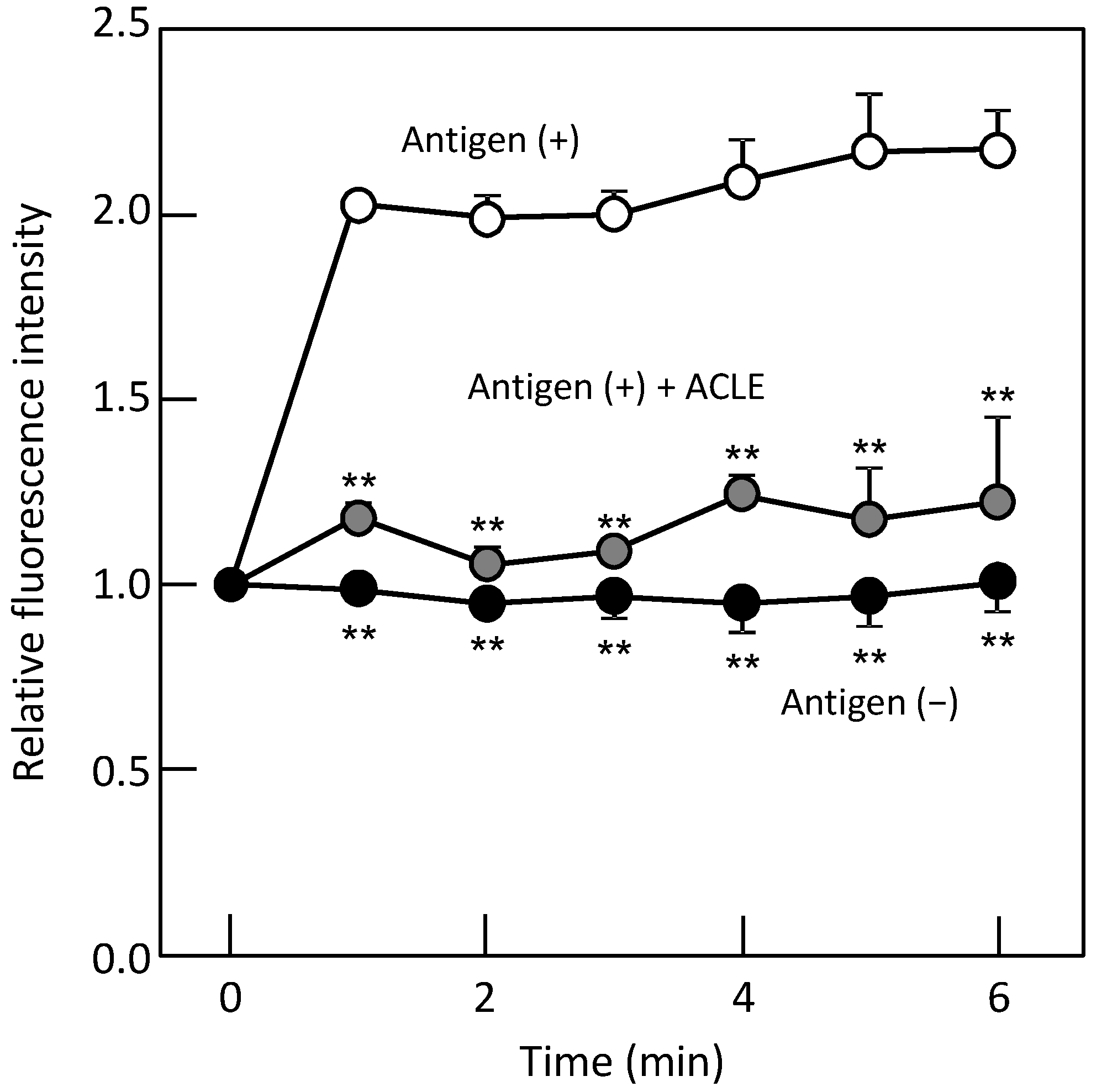
2.7. Monitoring of Intracellular Ca2+ Concentration ([Ca2+]i)
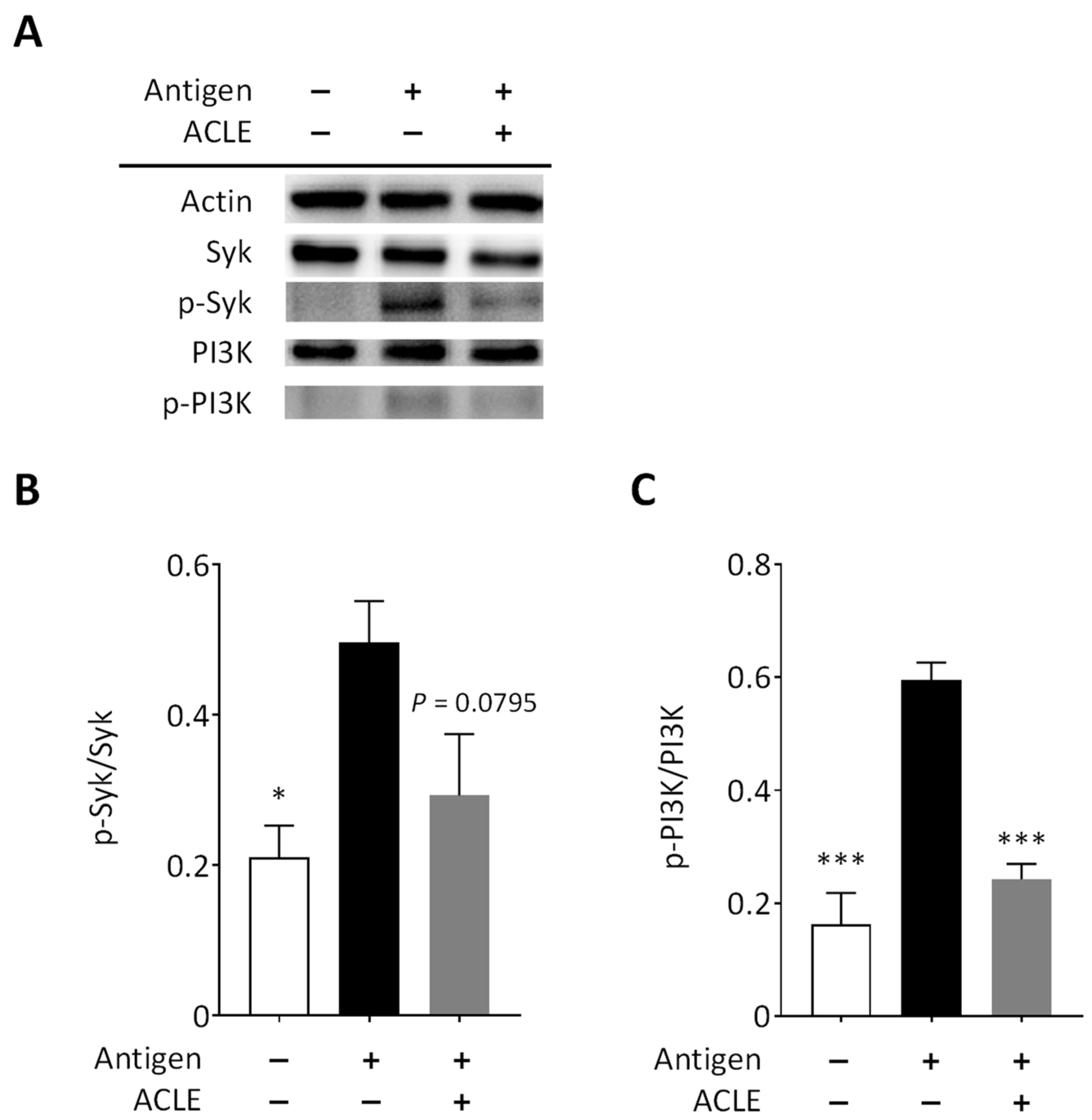
2.8. Immunoblot Analysis
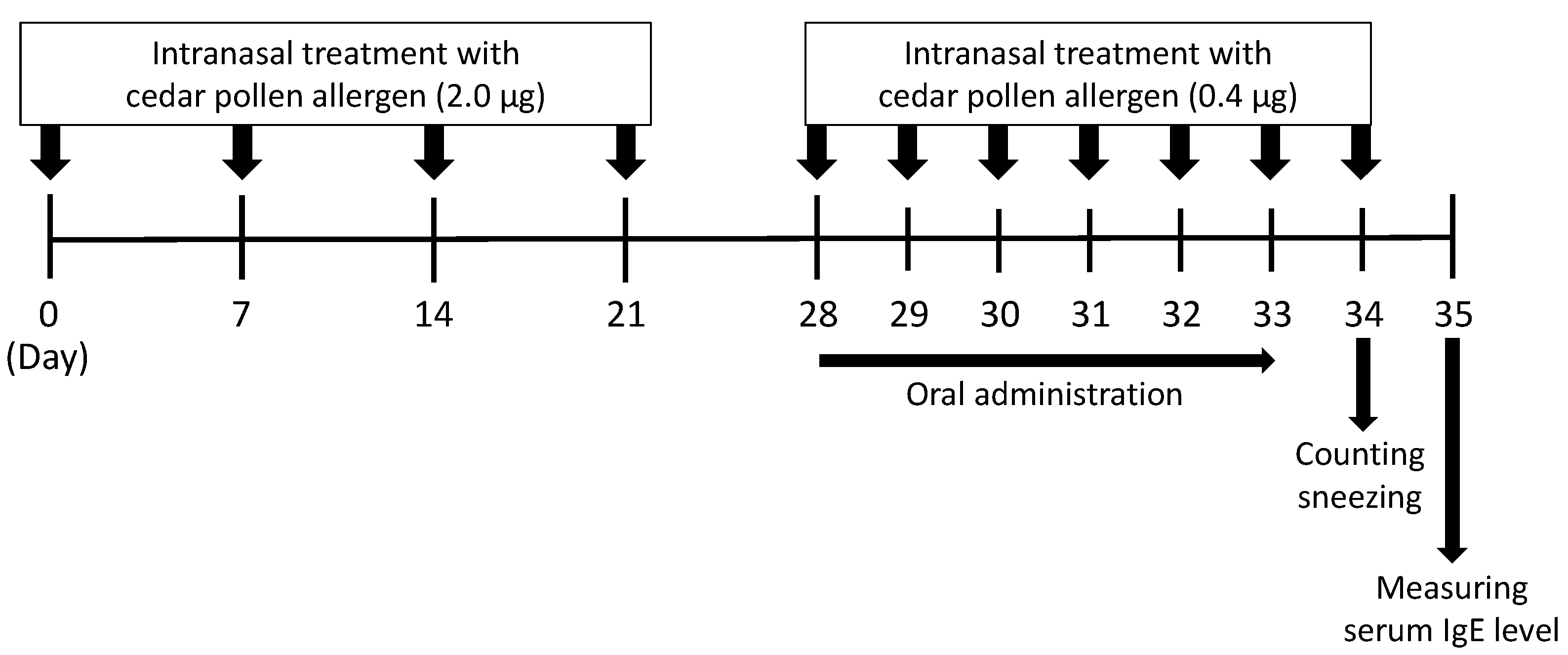
2.9. A Mouse Model of Japanese Cedar Pollinosis
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
2.11. High-Performance Liquid Chromatography (HPLC) Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. Effect of ACLE on Degranulation of RBL-2H3 Cells
3.2. Effect of ACLE on the Elevation of [Ca2+]i Induced by Antigen
3.3. Effect of ACLE on Intracellular Signaling Pathways Leading to Degranulation
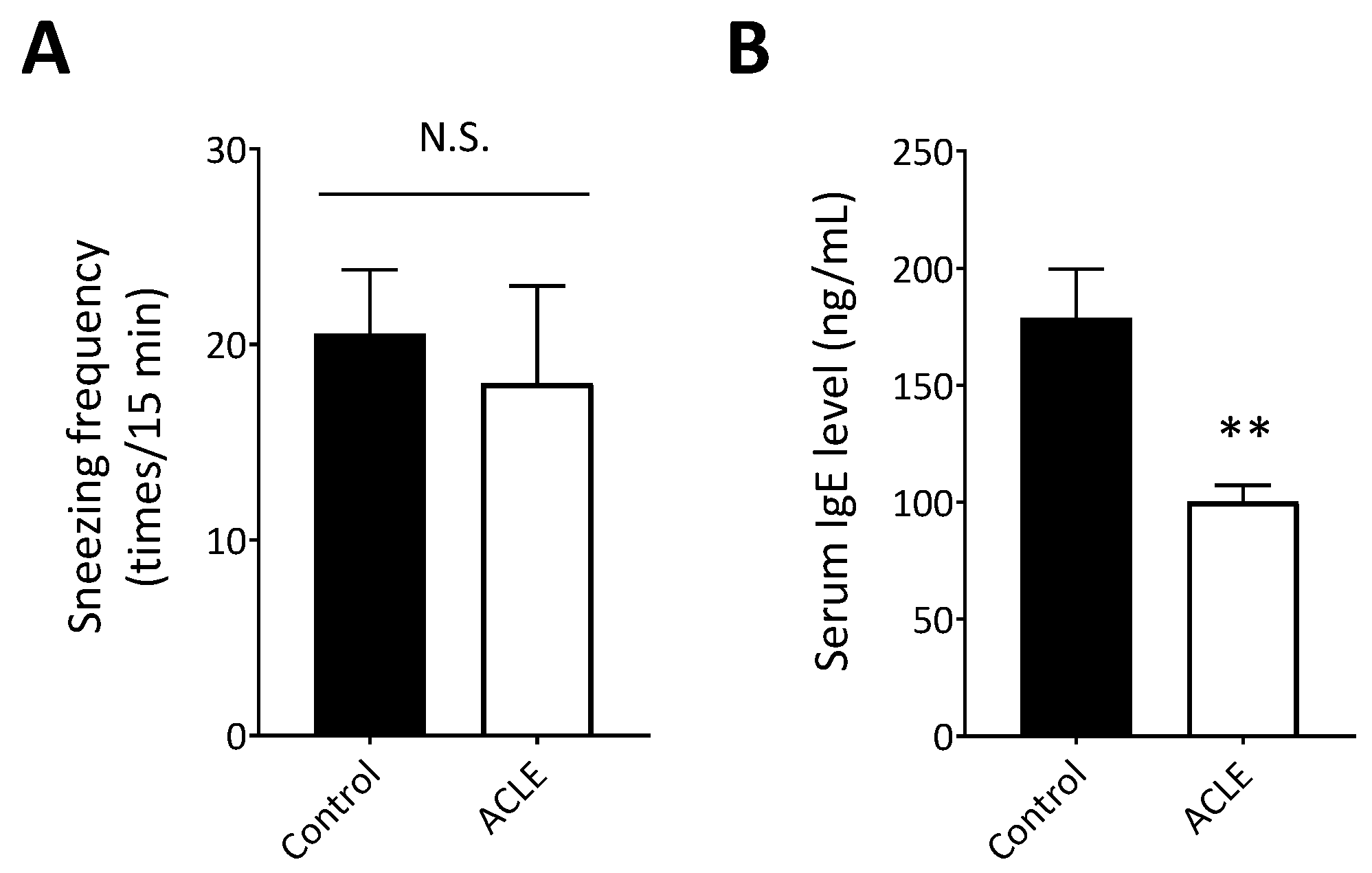
3.4. Effect of ACLE on a Mouse Model of Japanese Cedar Pollinosis
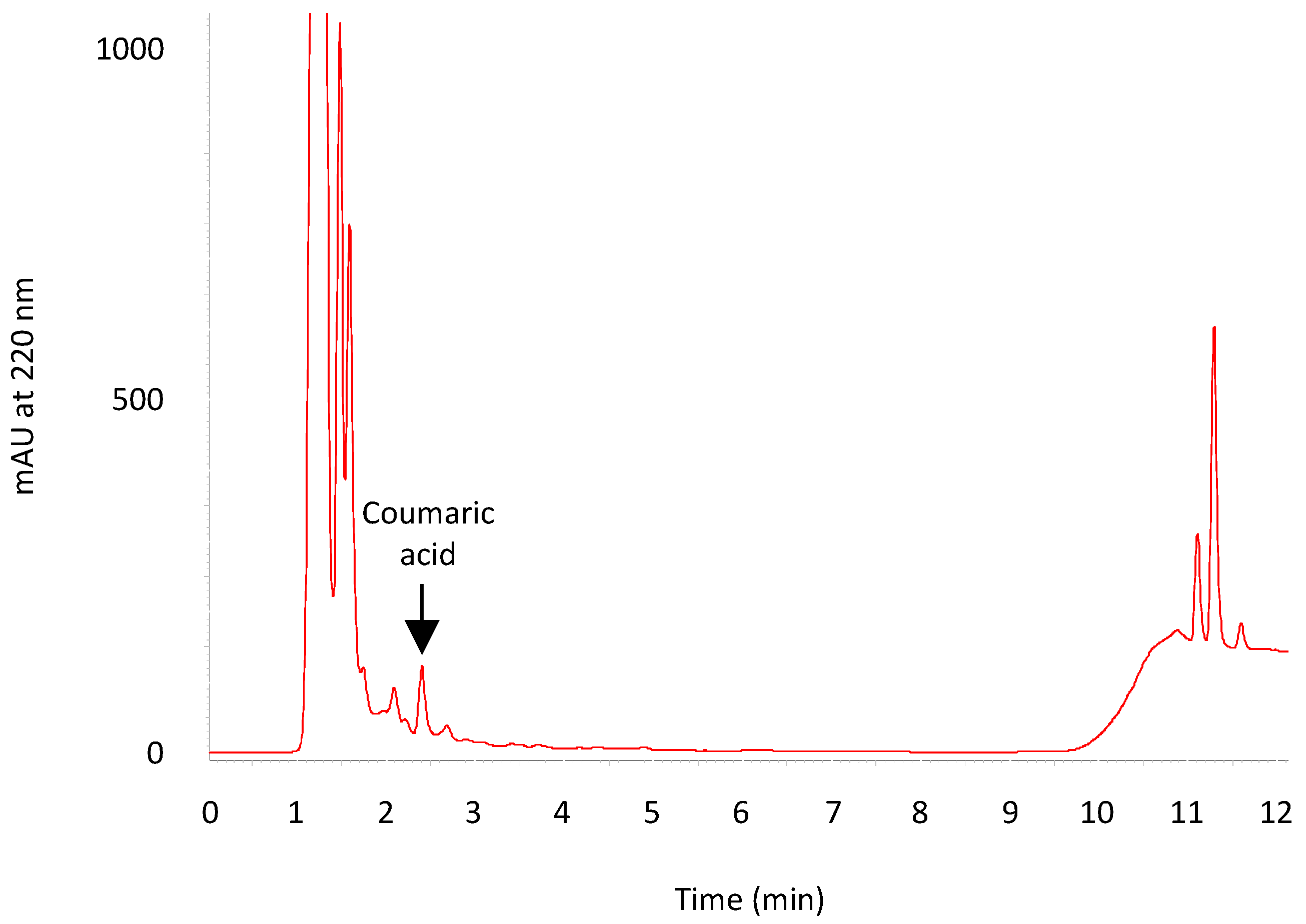
3.5. HPLC Analysis of ACLE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamada, T.; Saito, H.; Fujieda, S. Present state of Japanese cedar pollinosis: The national affliction. J. Allergy Clin. Immunol. 2014, 133, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Nishihata, S.; Murata, T.; Inoue, S.; Okubo, K.; Sahashi, N.; Takahashi, H.; Hirooka, J.; Hoshiyama, Y.; Murayama, K.; Mezawa, A.; et al. Prevalence of Japanese cedar pollinosis in Tokyo: A survey conducted by the Tokyo Metropolitan Government. Clin. Exp. Allergy Rev. 2010, 10, 8–11. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Laribi, B.; Kouki, K.; M’Hamdi, M.; Bettaieb, T. Coriander (Coriandrum sativum L.) and its bioactive constituents. Fitoterapia 2015, 103, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Sahib, N.G.; Anwar, F.; Gilani, A.H.; Hamid, A.A.; Saari, N.; Alkharfy, K.M. Coriander (Coriandrum sativum L.): A potential source of high-value components for functional foods and nutraceuticals—A review. Phytother. Res. 2013, 27, 1439–1456. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.N.; Liu, Z.H.; Zhao, Y.P.; Zhao, L.L.; Xue, T.K.; Lan, Q.K. Phytochemical and bioactive profile of Coriandrum sativum L. Food Chem. 2019, 286, 260–267. [Google Scholar] [CrossRef]
- Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Coriander (Coriandrum sativum): A promising functional food toward the well-being. Food Res. Int. 2018, 105, 305–323. [Google Scholar] [CrossRef]
- Sobhani, Z.; Mohtashami, L.; Amiri, M.S.; Ramezani, M.; Emami, S.A.; Simal-Gandara, J. Ethnobotanical and phytochemical aspects of the edible herb Coriandrum sativum L. J. Food Sci. 2022, 87, 1386–1422. [Google Scholar] [CrossRef]
- Matasyoh, J.C.; Maiyo, Z.C.; Ngure, R.M.; Chepkorir, R. Chemical composition and antimicrobial activity of the essential oil of Coriandrum sativum. Food Chem. 2009, 113, 526–529. [Google Scholar] [CrossRef]
- Begnami, A.F.; Duarte, M.C.T.; Furletti, V.; Rehder, V.L.G. Antimicrobial potential of Coriandrum sativum L. against different Candida species in vitro. Food Chem. 2010, 118, 74–77. [Google Scholar] [CrossRef]
- Silva, F.; Domingues, F.C. Antimicrobial activity of coriander oil and its effectiveness as food preservative. Crit. Rev. Food Sci. Nutr. 2017, 57, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.M.; Flatt, P.R. Insulin-releasing and insulin-like activity of the traditional anti-diabetic plant Coriandrum sativum (coriander). Br. J. Nutr. 1999, 81, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Eidi, M.; Eidi, A.; Saeidi, A.; Molanaei, S.; Sadeghipour, A.; Bahar, M.; Bahar, K. Effect of coriander seed (Coriandrum sativum L.) ethanol extract on insulin release from pancreatic beta cells in streptozotocin-induced diabetic rats. Phytother. Res. 2009, 23, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Chithra, V.; Leelamma, S. Coriandrum sativum—Mechanism of hypoglycemic action. Food Chem. 1999, 67, 229–231. [Google Scholar] [CrossRef]
- Aissaoui, A.; Zizi, S.; Israili, Z.H.; Lyoussi, B. Hypoglycemic and hypolipidemic effects of Coriandrum sativum L. in Meriones shawi rats. J. Ethnopharmacol. 2011, 137, 652–661. [Google Scholar] [CrossRef]
- Emamghoreishi, M.; Khasaki, M.; Aazam, M.F. Coriandrum sativum: Evaluation of its anxiolytic effect in the elevated plus-maze. J. Ethnopharmacol. 2005, 96, 365–370. [Google Scholar] [CrossRef]
- Gastón, M.S.; Cid, M.P.; Vázquez, A.M.; Decarlini, M.F.; Demmel, G.I.; Rossi, L.I.; Aimar, M.L.; Salvatierra, N.A. Sedative effect of central administration of Coriandrum sativum essential oil and its major component linalool in neonatal chicks. Pharm. Biol. 2016, 54, 1954–1961. [Google Scholar] [CrossRef]
- Suhonen, R.; Keskinen, H.; Björkstén, F.; Vaheri, E.; Zitting, A. Allergy to coriander a case report. Allergy 1979, 34, 327–330. [Google Scholar] [CrossRef]
- Jensen-Jarolim, E.; Leitner, A.; Hirschwehr, R.; Kraet, D.; Wüthrich, B.; Scheiner, O.; Graf, J.; Ebner, C. Characterization of allergens in Apiaceae spices: Anise, fennel, coriander and cumin. Clin. Exp. Allergy 1997, 27, 1299–1306. [Google Scholar] [CrossRef]
- Morita, Y.; Siraganian, R.P. Inhibition of IgE-mediated histamine release from rat basophilic leukemia cells and rat mast cells by inhibitors of transmethylation. J. Immunol. 1981, 127, 1339–1344. [Google Scholar]
- Kim, I.H.; Kanayama, Y.; Nishiwaki, H.; Sugahara, T.; Nishi, K. Structure-activity relationships of fish oil derivatives with antiallergic activity in vitro and in vivo. J. Med. Chem. 2019, 62, 9576–9592. [Google Scholar] [CrossRef]
- Nugrahini, A.D.; Ishida, M.; Nakagawa, T.; Nishi, K.; Sugahara, T. Anti-degranulation activity of caffeine: In vitro and in vivo study. J. Funct. Foods 2019, 60, 103422. [Google Scholar] [CrossRef]
- Nugrahini, A.D.; Ishida, M.; Nakagawa, T.; Nishi, K.; Sugahara, T. Trigonelline: An alkaloid with anti-degranulation properties. Mol. Immunol. 2020, 118, 201–209. [Google Scholar] [CrossRef]
- Hada, M.; Nishi, K.; Ishida, M.; Onda, H.; Nishimoto, S.; Sugahara, T. Inhibitory effect of aqueous extract of Cuminum cyminum L. seed on degranulation of RBL-2H3 cells and passive cutaneous anaphylaxis reaction in mice. Cytotechnology 2019, 71, 599–609. [Google Scholar] [CrossRef]
- Nomiya, R.; Okano, M.; Fujiwara, T.; Maeda, M.; Kimura, Y.; Kino, K.; Yokoyama, M.; Hirai, H.; Nagata, K.; Hara, T.; et al. CRTH2 plays an essential role in the pathophysiology of Cry j 1-induced pollinosis in mice. J. Immunol. 2008, 180, 5680–5688. [Google Scholar] [CrossRef]
- Nishi, K.; Kanayama, Y.; Kim, I.H.; Nakata, A.; Nishiwaki, H.; Sugahara, T. Docosahexaenoyl ethanolamide mitigates IgE-mediated allergic reactions by inhibiting mast cell degranulation and regulating allergy-related immune cells. Sci. Rep. 2019, 9, 16213. [Google Scholar] [CrossRef]
- Kondo, M.; Nishi, K.; Sugahara, T. Ishizuchi dark tea suppresses IgE-mediated degranulation of RBL-2H3 cells and nasal rubbing behavior of pollinosis in mice. J. Funct. Foods 2015, 14, 659–669. [Google Scholar] [CrossRef]
- Nishida, K.; Yamasaki, S.; Ito, Y.; Kabu, K.; Hattori, K.; Tezuka, T.; Nishizumi, H.; Kitamura, D.; Goitsuka, R.; Geha, R.S.; et al. FcεRI-mediated mast cell degranulation requires calcium-independent microtubule-dependent translocation of granules to the plasma membrane. J. Cell Biol. 2005, 170, 115–126. [Google Scholar] [CrossRef]
- Kopeć, A.; Panaszek, B.; Fal, A.M. Intracellular signaling pathways in IgE-dependent mast cell activation. Arch. Immunol. Ther. Exp. 2006, 54, 393–401. [Google Scholar] [CrossRef]
- Siraganian, R.P.; Zhang, J.; Suzuki, K.; Sada, K. Protein tyrosine kinase Syk in mast cell signaling. Mol. Immunol. 2002, 38, 1229–1233. [Google Scholar] [CrossRef]
- Parravicini, V.; Gadina, M.; Kovarova, M.; Odom, S.; Gonzalez-Espinosa, C.; Furumoto, Y.; Saitoh, S.; Samelson, L.E.; O’Shea, J.J.; Rivera, J. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat. Immunol. 2002, 3, 741–748. [Google Scholar] [CrossRef]
- Ménasché, G.; Longé, C.; Bratti, M.; Blank, U. Cytoskeletal transport, reorganization, and fusion regulation in mast cell-stimulus secretion coupling. Front. Cell Dev. Biol. 2021, 9, 652077. [Google Scholar] [CrossRef]
- Furumoto, Y.; Gonzalez-Espinosa, C.; Gomez, G.; Kovarova, M.; Odom, S.; Parravicini, V.; Ryan, J.J.; Rivera, J. Rethinking the role of Src family protein tyrosine kinases in the allergic response. Immunol. Res. 2004, 30, 241–253. [Google Scholar] [CrossRef]
- Goncalves, M.D.; Hopkins, B.D.; Cantley, L.C. Phosphatidylinositol 3-kinase, growth disorders, and Cancer. N. Engl. J. Med. 2018, 379, 2052–2062. [Google Scholar] [CrossRef]
- Alzahrani, A.S. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin. Cancer Biol. 2019, 59, 125–132. [Google Scholar] [CrossRef]
- Cortés-Eslava, J.; Gómez-Arroyo, S.; Villalobos-Pietrini, R.; Espinosa-Aguirre, J.J. Antimutagenicity of coriander (Coriandrum sativum) juice on the mutagenesis produced by plant metabolites of aromatic amines. Toxicol. Lett. 2004, 153, 283–292. [Google Scholar] [CrossRef]
- Huang, H.; Nakamura, T.; Yasuzawa, T.; Ueshima, S. Effects of Coriandrum sativum on migration and invasion abilities of cancer cells. J. Nutr. Sci. Vitaminol. 2020, 66, 468–477. [Google Scholar] [CrossRef]
- Qu, C.; Zheng, D.; Li, S.; Liu, Y.; Lidofsky, A.; Holmes, J.A.; Chen, J.; He, L.; Wei, L.; Liao, Y.; et al. Tyrosine kinase SYK is a potential therapeutic target for liver fibrosis. Hepatology 2018, 68, 1125–1139. [Google Scholar] [CrossRef]
- Bartaula-Brevik, S.; Lindstad Brattås, M.K.; Tvedt, T.H.A.; Reikvam, H.; Bruserud, Ø. Splenic tyrosine kinase (SYK) inhibitors and their possible use in acute myeloid leukemia. Expert Opin. Investig. Drugs 2018, 27, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Yu, Q.; Ding, C. Investigational spleen tyrosine kinase (SYK) inhibitors for the treatment of autoimmune diseases. Expert Opin. Investig. Drugs 2022, 31, 291–303. [Google Scholar] [CrossRef]
- Denyer, J.; Patel, V. Syk kinase inhibitors in allergic diseases. Drug News Perspect. 2009, 22, 146–150. [Google Scholar] [CrossRef]
- Burdock, G.A.; Carabin, I.G. Safety assessment of coriander (Coriandrum sativum L.) essential oil as a food ingredient. Food Chem. Toxicol. 2009, 47, 22–34. [Google Scholar] [CrossRef]
- Shoko, T.; Manhivi, V.E.; Mtlhako, M.; Sivakumar, D. Changes in functional compounds, volatiles, and antioxidant properties of culinary herb coriander leaves (Coriandrum sativum) stored under red and blue LED light for different storage times. Front. Nutr. 2022, 9, 856484. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kondo, K.; Kitajima, J. Water-soluble constituents of coriander. Chem. Pharm. Bull. 2003, 51, 32–39. [Google Scholar] [CrossRef][Green Version]
- Zeković, Z.; Kaplan, M.; Pavlić, B.; Olgun, E.O.; Vladić, J.; Canlı, O.; Vidović, S. Chemical characterization of polyphenols and volatile fraction of coriander (Coriandrum sativum L.) extracts obtained by subcritical water extraction. Ind. Crops Prod. 2016, 87, 54–63. [Google Scholar] [CrossRef]
- Chen, H.J.; Shih, C.K.; Hsu, H.Y.; Chiang, W. Mast cell-dependent allergic responses are inhibited by ethanolic extract of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) testa. J. Agric. Food Chem. 2010, 58, 2596–2601. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitamura, Y.; Nishi, K.; Ishida, M.; Nishimoto, S.; Sugahara, T. Anti-Allergic Effect of Aqueous Extract of Coriander (Coriandrum sativum L.) Leaf in RBL-2H3 Cells and Cedar Pollinosis Model Mice. Nutraceuticals 2022, 2, 170-180. https://doi.org/10.3390/nutraceuticals2030013
Kitamura Y, Nishi K, Ishida M, Nishimoto S, Sugahara T. Anti-Allergic Effect of Aqueous Extract of Coriander (Coriandrum sativum L.) Leaf in RBL-2H3 Cells and Cedar Pollinosis Model Mice. Nutraceuticals. 2022; 2(3):170-180. https://doi.org/10.3390/nutraceuticals2030013
Chicago/Turabian StyleKitamura, Yurika, Kosuke Nishi, Momoko Ishida, Sogo Nishimoto, and Takuya Sugahara. 2022. "Anti-Allergic Effect of Aqueous Extract of Coriander (Coriandrum sativum L.) Leaf in RBL-2H3 Cells and Cedar Pollinosis Model Mice" Nutraceuticals 2, no. 3: 170-180. https://doi.org/10.3390/nutraceuticals2030013
APA StyleKitamura, Y., Nishi, K., Ishida, M., Nishimoto, S., & Sugahara, T. (2022). Anti-Allergic Effect of Aqueous Extract of Coriander (Coriandrum sativum L.) Leaf in RBL-2H3 Cells and Cedar Pollinosis Model Mice. Nutraceuticals, 2(3), 170-180. https://doi.org/10.3390/nutraceuticals2030013






