Buckwheat, Fava Bean and Hemp Flours Fortified with Anthocyanins and Other Bioactive Phytochemicals as Sustainable Ingredients for Functional Food Development
Abstract
:1. Introduction
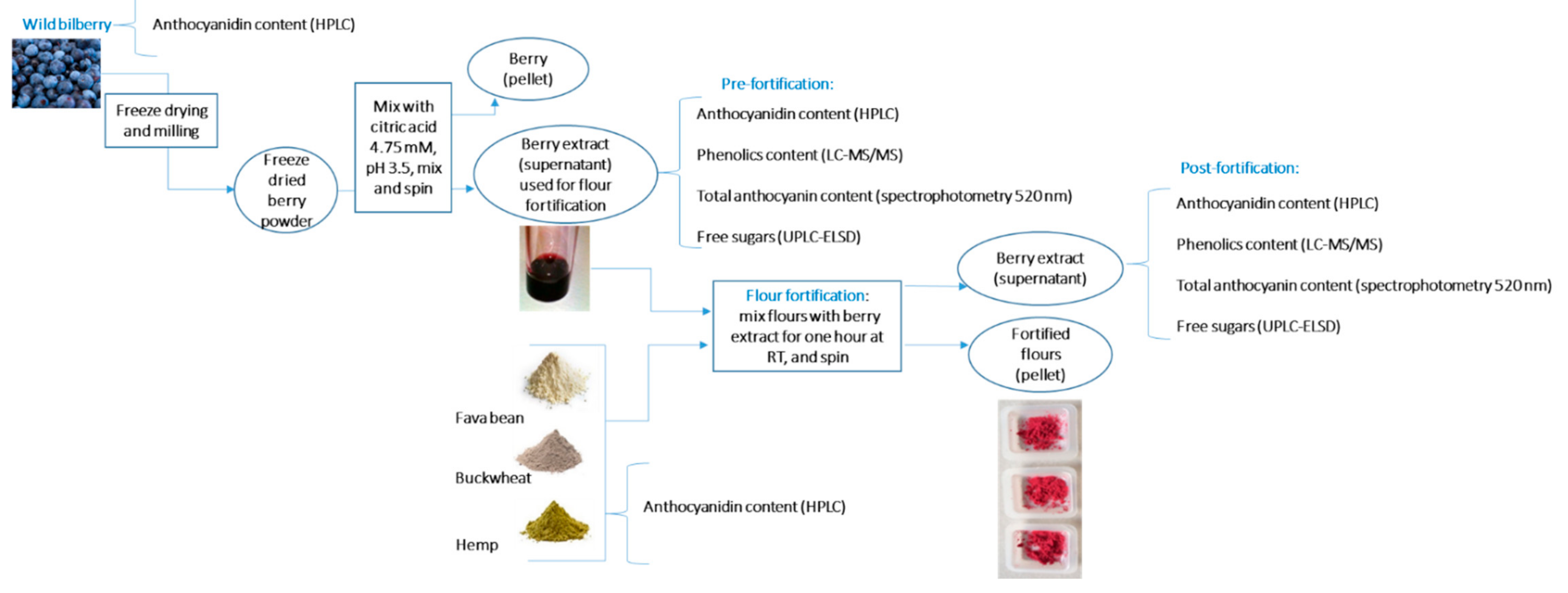
2. Materials and Methods
3. Results
3.1. Anthocyanidin Content of Wild Bilberry, Buckwheat, Fava Bean and Hemp Flours
3.2. Bilberry Anthocyanins Adsorbed by the Buckwheat, Fava Bean and Hemp Flour
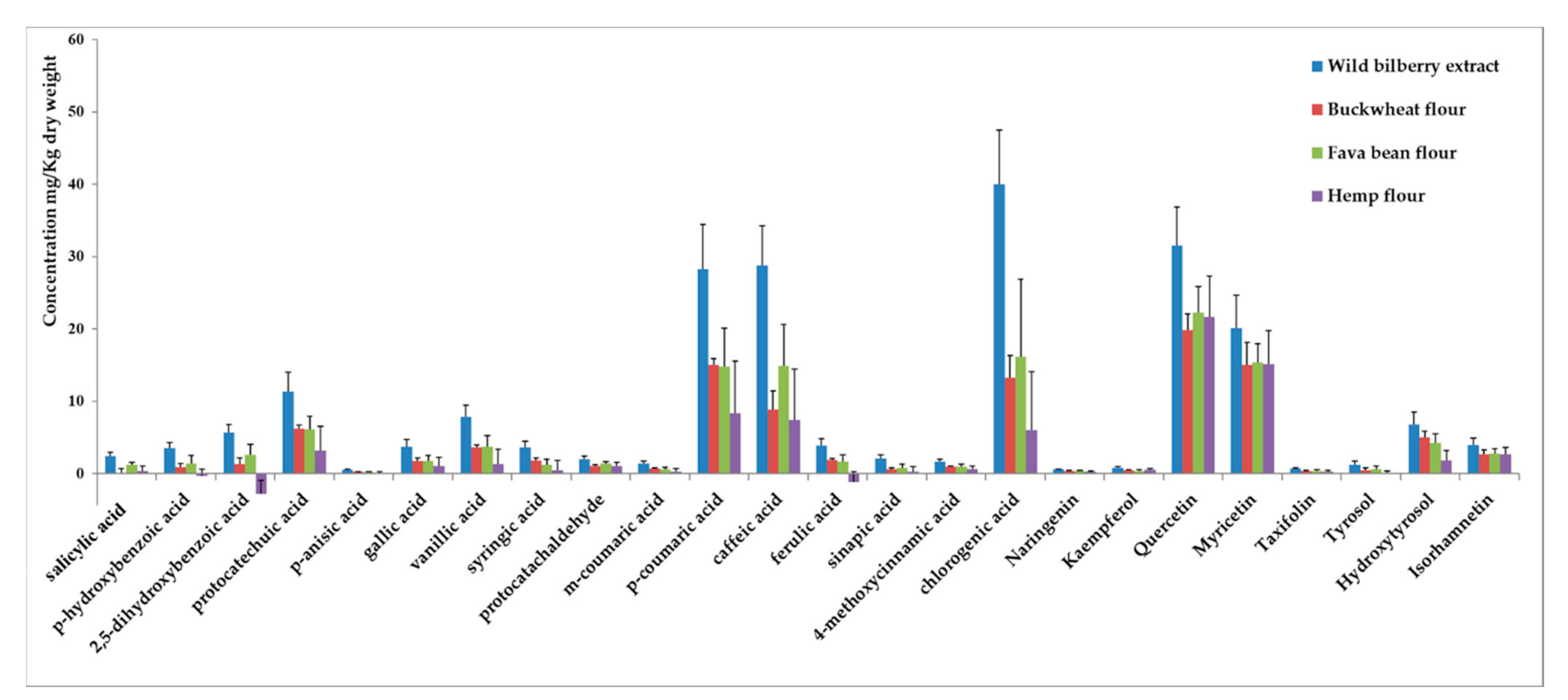
3.3. Other Phenolics from Wild Bilberries Adsorbed on the Flours
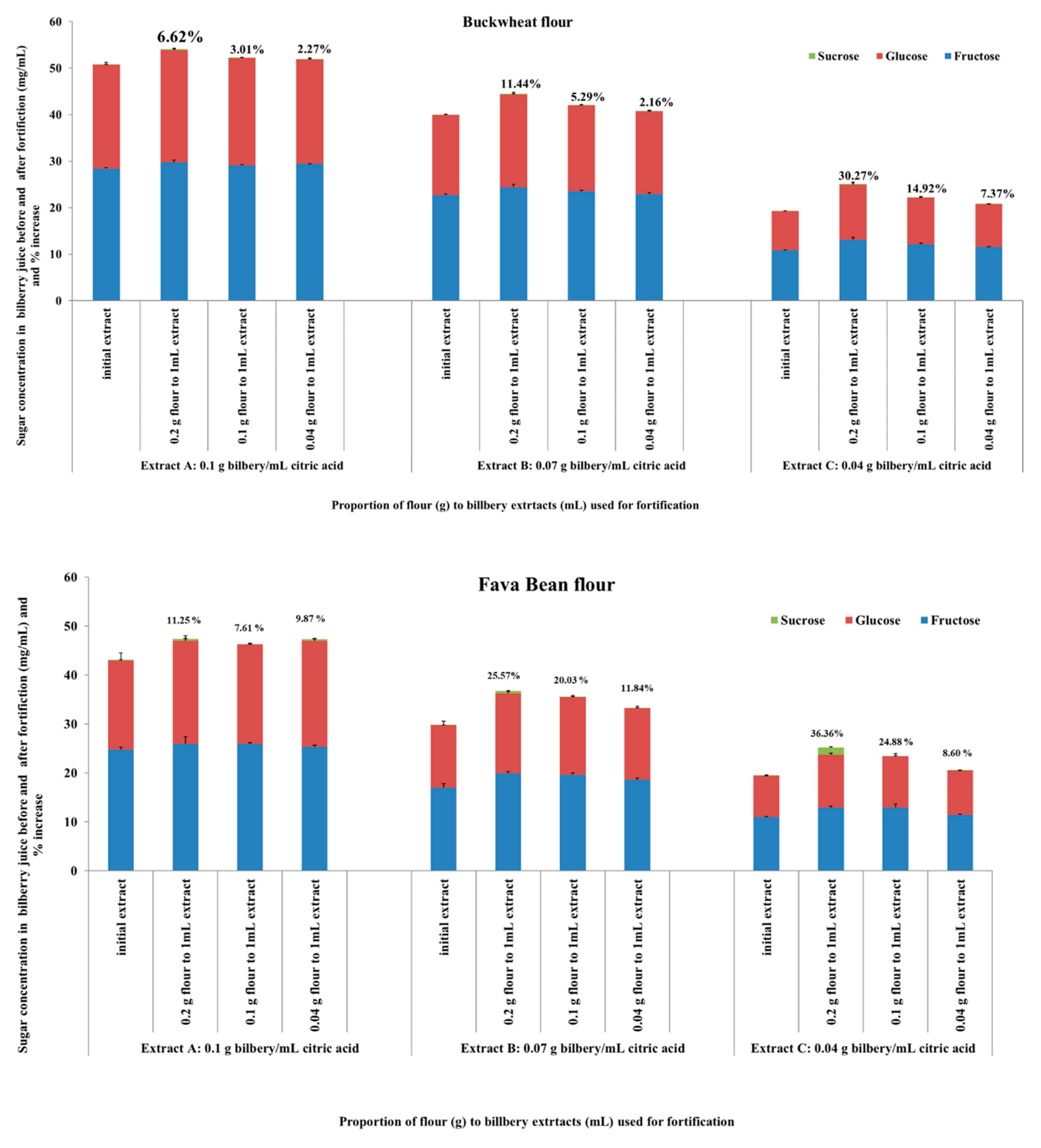
3.4. Sugar Content Following the Flour Fortification with Wild Bilberry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheane, R.; McCosker, C.; Lillywhite, R. Food Waste in Primary Production—A Preliminary Study on Strawberries and Lettuce; Wrap report; Wrap: Banbury, UK, 2017; Available online: https://wrap.org.uk/sites/default/files/2020-10/WRAP-Food_waste_in_primary_production_report.pdf (accessed on 12 May 2022).
- Roopchand, D.E.; Kuhn, P.; Rojo, L.E.; Lila, M.A.; Raskin, I. Blueberry polyphenol-enriched soybean flour reduces hyperglycemia; body weight gain and serum cholesterol in mice. Pharmacol. Res. 2013, 69, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akkarachiyasit, S.; Charoenlertkul, P.; Yibchok-anun, S.; Adisakwattana, S. Inhibitor activities of cyanidin and it glycoside and synergistic effect with acarabose against intenstinal α-GLucosidase and pancreatic α-amylase. Int. J. Mol. Sci. 2010, 119, 3387–3396. [Google Scholar]
- Matsui, T.; Ueda, T.; Oki, T.; Sugita, K.; Terahara, N.; Matsumoto, K. α-Glucosidase inhibitory action of natural acylated anthocyanins. 1. Survey of natural pigments with potent inhibitory activity. J. Agric. Food Chem. 2001, 494, 1948–1951. [Google Scholar] [CrossRef]
- Hoggard, N.; Cruickshank, M.; Moar, K.M.; Bestwick, C.; Holst, J.J.; Russell, W.; Horgan, G. A single supplement of a standardised bilberry (Vaccinium myrtillus L.) extract (36% wet weight anthocyanins) modifies glycaemic response in individuals with type 2 diabetes controlled by diet and lifestyle. J. Nutr. Sci. 2013, 2, e22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyssenko, V.; Jonsson, A.; Almgern, P.; Pulizzi, N.; Isomaa, B.; Tuomi, T.; Berglund, G.; Altshuler, D.; Nilsson, P.; Groop, L. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N. Engl. J. Med. 2008, 359, 2220–2232. [Google Scholar] [CrossRef] [Green Version]
- Neacsu, M.; Vaughan, N.J.; Multari, S.; Haljas, E.; Scobbie, L.; Duncan, G.J.; Cantlay, L.; Fyfe, C.; Anderson, S.; Horgan, G.; et al. Hemp and buckwheat are valuable sources of dietary amino acids, beneficially modulating gastrointestinal hormones and promoting satiety in healthy volunteers. Eur. J. Nutr. 2022, 61, 1057–1072. [Google Scholar] [CrossRef]
- Qiu, J.; Liu, Y.; Yue, Y.; Qin, Y.; Li, Z. Dietary tartary buckwheat intake attenuates insulin resistance and improves lipid profiles in patients with type 2 diabetes: A randomized controlled trial. Nutr. Res. 2016, 36, 1392–1401. [Google Scholar] [CrossRef]
- Lee, Y.A.; Cho, E.J.; Tanaka, T.; Yokozawa, T. Inhibitory activities of proanthocyanidins from persimmon against oxidative stress and digestive enzymes related to diabetes. J. Nutr. Sci. Vitaminol. 2007, 53, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Kawa, J.M.; Przybylski, R.; Taylor, C.G. Urinary chiro-inositol and myo-inositol excretion is elevated in the diabetic db/db mouse and streptozotocin diabetic rat. Exp. Biol. Med. 2003, 228, 907–914. [Google Scholar] [CrossRef]
- Yao, Y.; Shan, F.; Bian, J.; Chen, F.; Wang, M.; Ren, G. D-chiro-Inositol-enriched tartary buckwheat bran extract lowers the blood glucose level in KK-Ay mice. J. Agric. Food Chem. 2008, 56, 10027–10031. [Google Scholar] [CrossRef]
- Multari, S.; Neacsu, M.; Scobbie, L.; Cantlay, L.; Duncan, G.; Vaughan, N.; Stewart, D.; Russell, W.R. Nutritional and Phytochemical Content of High-Protein Crops. J. Agric. Food Chem. 2016, 64, 7800–7811. [Google Scholar] [CrossRef] [PubMed]
- Kapravelou, G.; Martínez, R.; Andrade, A.M.; Sánchez, C.; Chaves, C.L.; López-Jurado, M.; Aranda, P.; Cantarero, S.; Arrebola, F.; Fernández-Segura, E.; et al. Health promoting effects of Lupin (Lupinus albus var. multolupa) protein hydrolyzate and insoluble fiber in a diet-induced animal experimental model of hypercholesterolemia. Food Res. Int. 2013, 54, 1471–1481. [Google Scholar] [CrossRef]
- Kristensen, M.; Jensen, M.G.; Aarestrup, J.; Petersen, K.E.N.; Sondergaard, L.; Mikkelsen, M.S.; Astrup, A. Flaxseed dietary fibers lower cholesterol and increase fecal fat excretion, but magnitude of effect depend on food type. Nutr. Metab. 2012, 9, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, W.R.; Burkitt, M.J.; Forrester, A.R.; Chesson, A. Oxidative coupling during lignin polymerization is determined by unpaired electron delocalization within parent phenylpropanoid radicals. Arch. Biochem. Biophys. 1996, 332, 357–366. [Google Scholar] [CrossRef]
- Russell, W.R.; Burkitt, M.J.; Scobbie, L.; Chesson, A. Radical formation and coupling of hydroxycinnamic acids containing 1, 2-dihydroxy substituents. Bioorg. Chem. 2003, 31, 206–215. [Google Scholar] [CrossRef]
- Zhang, Z.; Kou, X.; Fugal, K.; Mclaughlin, J. Comparison of HPLC methods for determination of anthocyanins and anthocyanidins in bilberry extracts. J. Agric. Food Chem. 2004, 52, 688–691. [Google Scholar] [CrossRef]
- Lätti, A.K.; Riihinen, K.R.; Kainulainen, P.S. Analysis of anthocyanin variation in wild populations of bilberry (Vaccinium myrtillus L.) in Finland. J. Agric. Food Chem. 2008, 56, 190–503. [Google Scholar] [CrossRef]
- Russell, W.R.; Gratz, S.W.; Duncan, S.H.; Holtrop, G.; Ince, J.; Scobbie, L.; Duncan, G.; Johnstone, A.M.; Lobley, G.E.; Wallace, R.J.; et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 2011, 93, 1062–1072. [Google Scholar] [CrossRef]
- Neacsu, M.; McMonagle, J.; Fletcher, R.J.; Scobbie, L.; Duncan, G.J.; Cantlay, L.; De Roos, B.; Duthie, G.G.; Russell, W.R. Bound phytophenols from ready-to-eat cereals; comparison with other plant-based foods. Food Chem. 2013, 141, 2880–2886. [Google Scholar] [CrossRef]
- Neacsu, M.; Vaughan, N.J.; Raikos, V.; Multari, S.; Duncan, G.J.; Duthie, G.G.; Russell, W.R. Phytochemical profile of food plant powders: Their potential role in healthier food reformulations. Food Chem. 2015, 179, 159–169. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Grace, M.H.; Kuhn, P.; Cheng, D.M.; Plundrich, N.; Poulev, A.; Howell, A.; Fridlender, B.; Lila, M.A.; Raskin, I. Efficient sorption of polyphenols to soybean flour enables natural fortification of foods. Food Chem. 2012, 131, 1193–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouillard, R.; Mazza, G.; Saad, Z.; Albrecht-Gary, A.M.; Cheminat, A. The co-pigmentation reaction of anthocyanins: A microprobe for the structural study of aqueous solutions. J. Am. Chem Soc. 1989, 111, 2604–2610. [Google Scholar] [CrossRef]
- Asen, S.; Stewart, R.N.; Norris, K.H. Copigmentation of anthocyanins in plant tissues and its effect on color. Phytochemistry 1972, 11, 1139–1144. [Google Scholar] [CrossRef]
- Markovic, J.M.D.; Petranovic, N.A.; Baranac, J.M. A spectrophotometric study of the copigmentation of malvin with caffeic and ferulic acids. J. Agric. Food Chem. 2000, 48, 5530–5536. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Hrazdina, G. Anthocyanins as food colorants: Effect of pH on the formation of anthocyanin-rutin complexes. J. Food Sci. 1979, 44, 66–68. [Google Scholar] [CrossRef]
- Brouillard, R.; Wigand, M.C.; Dangles, O.; Cheminat, A. The pH and solvent effects on the copigmentation reaction of malvin with polyphenols, purine and pyrimidine derivatives. J. Chem. Soc. 1991, 2, 1235–1241. [Google Scholar] [CrossRef]
- Rechner, A.R.; Kroner, C. Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. Thromb. Res. 2005, 116, 327–334. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; Tulipani, S.; Casoli, T.; Di Stefano, G.; González-Paramás, A.M.; Santos-Buelga, C.; Busco, F.; Quiles, J.L.; Cordero, M.D.; et al. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 2014, 25, 289–294. [Google Scholar] [CrossRef]
- Toufektsian, M.C.; De Lorgeril, M.; Nagy, N.; Salen, P.; Donati, M.B.; Giordano, L.; Mock, H.P.; Peterek, S.; Matros, A.; Petroni, K. Chronic dietary intake of plant-derived anthocyanins protects the rat heart against ischemia-reperfusion injury. J. Nutr. 2008, 138, 747–752. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.S.; Hecht, S.S.; Carmella, S.G.; Yu, N.; Larue, B.; Henry, C.; Mcintyre, C.; Rocha, C.; Lechner, J.F.; Stoner, G.D. Anthocyanins in black raspberries prevent esophagul tumours in rats. Cancer Prev. Res. 2009, 2, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Malik, M.; Zhao, C.; Schoene, N.; Guisti, M.M.; Moyer, M.; Magnuson, B.A. Anthocyanin-rich extract from Aronia meloncarpa E. induces a cell cycle block in colon cancer but not normal colonic cells. Nutr. Cancer 2003, 46, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Alnajjar, M.; Barik, S.K.; Bestwick, C.; Campbell, F.; Cruickshank, M.; Farquharson, F.; Holtrop, G.; Horgan, G.; Louis, P.; Moar, K.M.; et al. Anthocyanin-enriched Bilberry extract attenuates glycaemic response in overweight volunteers without changes in insulin. J. Funct. Foods 2020, 64, 103597. [Google Scholar] [CrossRef]
- Barik, S.K.; Dehury, B.; Russell, W.R.; Moar, K.; Cruickshabk, M.; Scobbie, L.; Hoggard, N. Analysis of polyphenolic metabolites from in vitro gastrointestinal digested soft fruit extracts identify malvidin-3-glucoside as an inhibitor of PTP1B. Biochem. Pharmacol. 2020, 178, 114109. [Google Scholar] [CrossRef]
- Echegaray, N.; Munekata, P.E.S.; Gullón, P.; Dzuvor, K.K.O.; Gullón, B.; Kubi, F.; Lorenzo, J.M. Recent advances in food products fortification with anthocyanins. Crit. Rev. Food Sci. Nutr. 2022, 62, 1553–1567. [Google Scholar] [CrossRef]
- Functional Food Ingredients Market by Type & Application—Global Forecast 2026. Functional Food Ingredients Market. Markets and Markets. 2021. Available online: https://www.marketsandmarkets.com/Market-Reports/functional-food-ingredients-market-9242020.html (accessed on 28 April 2022).
- Nayak, A.; Bhushan, B. An overview of the recent trends on the waste valorization techniques for food wastes. J. Environ. Manag. 2019, 233, 352–370. [Google Scholar] [CrossRef]
- Różańska, D.; Regulska-Ilow, B. The significance of anthocyanins in the prevention and treatment of type 2 diabetes. Adv. Clin. Exp. Med. 2018, 27, 135–142. [Google Scholar] [CrossRef] [Green Version]


| Flour | Aqueous Wild Bilberry Extracts | ||
|---|---|---|---|
| A * | B * | C * | |
| Buckwheat | |||
| 0.2 g/mL extract | 24.93 ± 0.72 (97.02 ± 0.69) | 10.07 ± 0.55 (94.56 ± 1.98) | 5.26 ± 0.87 (92.91 ± 1.53) |
| 0.1 g/mL extract | 48.11 ± 2.19 (92.93 ± 1.6) | 19.5 ± 1.08 (92 ± 1.76) | 10.31 ± 1.67 (91 ± 1.38) |
| 0.04 g/mL extract | 95.69 ± 12.47 (78.64 ± 3.15) | 26.23 ± 4.39 (48.64 ± 8.69) | 14.27 ± 4.6 (8.56 ± 5.14) |
| Fava bean | |||
| 0.2 g/mL extract | 24.43 ± 3.46 (91.26 ± 0.34) | 10.76 ± 0.43 (85.04 ± 0.18) | 12.91 ± 0.99 (90.85 ± 0.4) |
| 0.1 g/mL extract | 41.68 ± 6.51 (76.69 ± 2.9) | 19.32 ± 0.66 (75.97 ± 1.05) | 25.4 ± 1.99 (88.67 ± 0.66) |
| 0.04 g/mL extract | 88.86 ± 8.44 (50.46 ± 2.94) | 24 ± 6.73 (37.02 ± 8.93) | 31.58 ± 4.54 (52.55 ± 2.02) |
| Hemp | |||
| 0.2 g/mL extract | 23.05 ± 0.95 (94.33 ± 0.4) | 8.66 ± 0.89 (90.72 ± 1.01) | 7.04 ± 0.4 (92.1 ± 0.93) |
| 0.1 g/mL extract | 44.92 ± 2.1 (91.83 ± 0.28) | 16.48 ± 1.7 (86.67 ± 1.07) | 14.07 ± 0.89 (91.6 ± 1.25) |
| 0.04 g/mL extract | 101.64 ± 1.57 (88.45 ± 0.88) | 36.29 ± 6.28 (78.95 ± 3.61) | 20.2 ± 0.62 (60.75 ± 0.65) |
| Flour | Flours Anthocyanins Fortification (%) | ||||
|---|---|---|---|---|---|
| Delphinidin-3-Glucoside | Cyanidin-3-Glucoside | Cyanidin-3-Galactoside | Peonidin-3-Glucoside | Malvidin-3-Glucoside | |
| Buckwheat | 64.52 ± 1.57 | 65.55 ± 0.74 | 52.65 ± 0.2 | 51.53 ± 0.51 | 46.94 ± 0.44 |
| Fava bean | 55.25 ± 0.33 | 50.25 ± 0.35 | 44.53 ± 0.3 | 37.46 ± 0.51 | 33.93 ± 0.42 |
| Hemp | 68.29 ± 2.31 | 71.06 ± 0.79 | 57.68 ± 0.78 | 58.62 ± 1.51 | 53.83 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neacsu, M.; Christie, J.S.; Duncan, G.J.; Vaughan, N.J.; Russell, W.R. Buckwheat, Fava Bean and Hemp Flours Fortified with Anthocyanins and Other Bioactive Phytochemicals as Sustainable Ingredients for Functional Food Development. Nutraceuticals 2022, 2, 150-161. https://doi.org/10.3390/nutraceuticals2030011
Neacsu M, Christie JS, Duncan GJ, Vaughan NJ, Russell WR. Buckwheat, Fava Bean and Hemp Flours Fortified with Anthocyanins and Other Bioactive Phytochemicals as Sustainable Ingredients for Functional Food Development. Nutraceuticals. 2022; 2(3):150-161. https://doi.org/10.3390/nutraceuticals2030011
Chicago/Turabian StyleNeacsu, Madalina, James S. Christie, Gary J. Duncan, Nicholas J. Vaughan, and Wendy R. Russell. 2022. "Buckwheat, Fava Bean and Hemp Flours Fortified with Anthocyanins and Other Bioactive Phytochemicals as Sustainable Ingredients for Functional Food Development" Nutraceuticals 2, no. 3: 150-161. https://doi.org/10.3390/nutraceuticals2030011
APA StyleNeacsu, M., Christie, J. S., Duncan, G. J., Vaughan, N. J., & Russell, W. R. (2022). Buckwheat, Fava Bean and Hemp Flours Fortified with Anthocyanins and Other Bioactive Phytochemicals as Sustainable Ingredients for Functional Food Development. Nutraceuticals, 2(3), 150-161. https://doi.org/10.3390/nutraceuticals2030011





