Effects of Acute Mistletoe (Viscum album L.) Ingestion on Aerobic Exercise Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. VA Supplement and Placebo
2.4. Procedures
2.5. Data Analysis
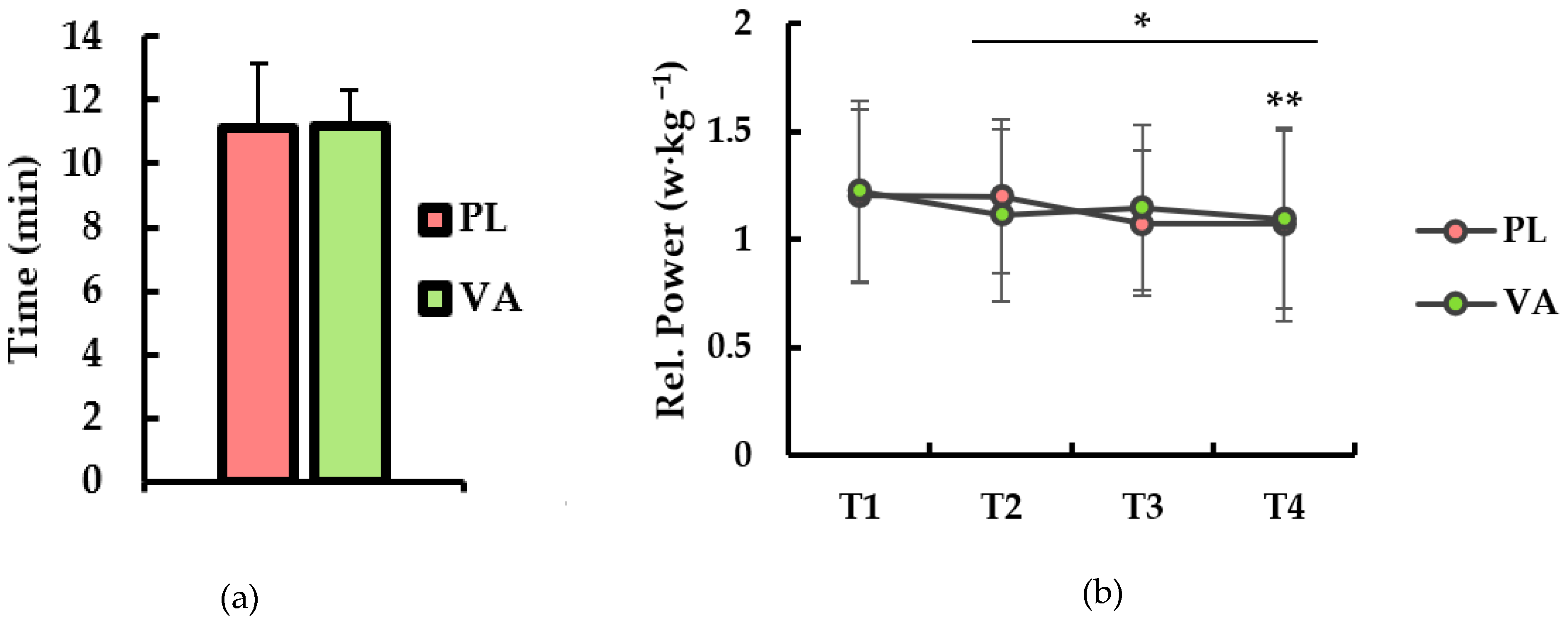
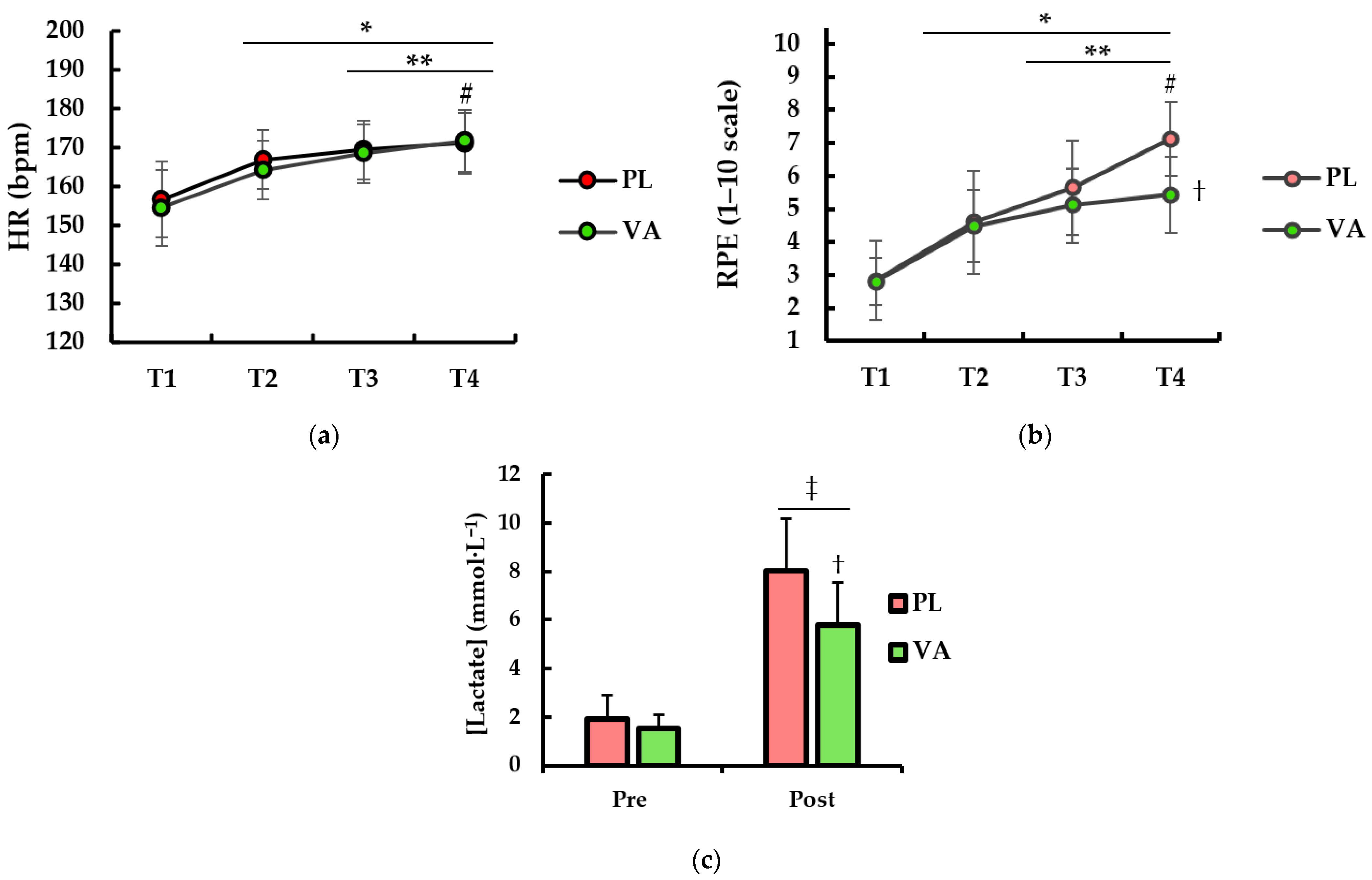
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szurpnicka, A.; Kowalczuk, A.; Szterk, A. Biological activity of mistletoe: In vitro and in vivo studies and mechanisms of action. Arch. Pharmacal Res. 2020, 43, 593–629. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Kim, I.-B.; Kim, J.-B.; Park, D.-H.; Min, K.-J. The effects of Korean mistletoe extract on endurance during exercise in mice. Anim. Cells Syst. 2014, 18, 34–40. [Google Scholar] [CrossRef][Green Version]
- Karagöz, A.; Kesici, S.; Vural, A.; Usta, M.; Tezcan, B.; Semerci, T.; Teker, E. Cardioprotective effects of Viscum album L. ssp. album (Loranthaceae) on isoproterenol-induced heart failure via regulation of the nitric oxide pathway in rats. Anatol. J. Cardiol. 2016, 16, 923. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-Y.; Lee, A.-N.; Song, T.-J.; An, H.-S.; Kim, Y.-H.; Kim, K.-D.; Kim, I.-B.; Kim, K.-S.; Han, B.-S.; Kim, C.-H. Korean mistletoe (Viscum album coloratum) extract improves endurance capacity in mice by stimulating mitochondrial activity. J. Med. Food 2012, 15, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-Y.; Kim, Y.-H.; Kim, I.-B.; Jeong, J.S.; Lee, J.-H.; Do, M.-S.; Jung, S.-P.; Kim, K.-S.; Kim, K.-T.; Kim, J.-B. The Korean mistletoe (Viscum album coloratum) extract has an antiobesity effect and protects against hepatic steatosis in mice with high-fat diet-induced obesity. Evid. Based Complement. Altern. Med. 2013, 2013, 168207. [Google Scholar] [CrossRef][Green Version]
- Singh, B.N.; Saha, C.; Galun, D.; Upreti, D.K.; Bayry, J.; Kaveri, S.V. European Viscum album: A potent phytotherapeutic agent with multifarious phytochemicals, pharmacological properties and clinical evidence. RSC. Adv. 2016, 6, 23837–23857. [Google Scholar] [CrossRef]
- Fukunaga, T.; Kajikawa, I.; Nishiya, K.; Watanabe, Y.; Takeya, K.; Itokawa, H. Studies on the Constituents of the European Mistletoe, Viscum album L. Chem. Pharm. Bull. 1987, 35, 3292–3297. [Google Scholar] [CrossRef][Green Version]
- Krenzelok, E.P.; Jacobsen, T.; Aronis, J. American mistletoe exposures. Am. J. Emerg. Med. 1997, 15, 516–520. [Google Scholar] [CrossRef]
- van Wely, M.; Stoss, M.; Gorter, R.W. Toxicity of a standardized mistletoe extract in immunocompromised and healthy individuals. Am. J. Ther. 1999, 6, 37–43. [Google Scholar] [CrossRef]
- Shahaboddin, M.E.; Pouramir, M.; Moghadamnia, A.-A.; Lakzaei, M.; Mirhashemi, S.M.; Motallebi, M. Antihyperglycemic and antioxidant activity of Viscum album extract. Afr. J. Pharm. Pharmacol. 2011, 5, 433–436. [Google Scholar] [CrossRef]
- Lee, S.-H.; An, H.-S.; Jung, Y.W.; Lee, E.-J.; Lee, H.-Y.; Choi, E.-S.; An, S.W.; Son, H.; Lee, S.-J.; Kim, J.-B. Korean mistletoe (Viscum album coloratum) extract extends the lifespan of nematodes and fruit flies. Biogerontology 2014, 15, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Park, C.-H.; Kim, I.; Kim, Y.-H.; Yoon, J.-M.; Kim, K.-S.; Kim, J.-B. Korean mistletoe (Viscum album coloratum) extract regulates gene expression related to muscle atrophy and muscle hypertrophy. BMC Complement. Altern. Med. 2017, 17, 1–10. [Google Scholar] [CrossRef]
- Lim, N.J.; Shin, J.H.; Kim, H.J.; Lim, Y.; Kim, J.Y.; Lee, W.J.; Han, S.J.; Kwon, O. A combination of Korean mistletoe extract and resistance exercise retarded the decline in muscle mass and strength in the elderly: A randomized controlled trial. Exp. Gerontol. 2017, 87, 48–56. [Google Scholar] [CrossRef]
- Ha, S.-M.; Kim, J.-H.; Kim, J.-W.; Kim, D.-Y.; Ha, M.-S. The Potential Role of Korean Mistletoe Extract as an Anti-Inflammatory Supplementation. J. Immunol. Res. 2021, 2021, 2183427. [Google Scholar] [CrossRef]
- Bentley, M.R.; Patterson, L.B.; Mitchell, N.; Backhouse, S.H. Athlete perspectives on the enablers and barriers to nutritional adherence in high-performance sport. Psychol. Sport Exerc. 2021, 52, 101831. [Google Scholar] [CrossRef]
- Haack, S.A.; Byker, C.J. Recent population adherence to and knowledge of United States federal nutrition guides, 1992–2013: A systematic review. Nutr. Rev. 2014, 72, 613–626. [Google Scholar] [CrossRef]
- Costello, J.T.; Bieuzen, F.; Bleakley, C.M. Where are all the female participants in Sports and Exercise Medicine research? Eur. J. Sport Sci. 2014, 14, 847–851. [Google Scholar] [CrossRef]
- Cowley, E.S.; Olenick, A.A.; McNulty, K.L.; Ross, E.Z. “Invisible sportswomen”: The sex data gap in sport and exercise science research. Women Sport Phys. Act. J. 2021, 29, 146–151. [Google Scholar] [CrossRef]
- Liguori, G.; Medicine, A.C.o.S. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2020. [Google Scholar]
- Peters, B.; Ballmann, C.; Quindry, T.; Zehner, E.G.; McCroskey, J.; Ferguson, M.; Ward, T.; Dumke, C.; Quindry, J. Experimental woodsmoke exposure during exercise and blood oxidative stress. J. Occup. Environ. Med. 2018, 60, 1073. [Google Scholar] [CrossRef] [PubMed]
- Karow, M.C.; Rogers, R.R.; Pederson, J.A.; Williams, T.D.; Marshall, M.R.; Ballmann, C.G. Effects of Preferred and Nonpreferred Warm-Up Music on Exercise Performance. Percept. Mot. Skills 2020, 127, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.R.; Langley, H.N.; Rogers, R.R.; Benjamin, C.L.; Williams, T.D.; Ballmann, C.G. Effects of Zembrin®(Sceletium tortuosum) Supplementation on Mood, Soreness, and Performance Following Unaccustomed Resistance Exercise: A Pilot Study. Nutraceuticals 2021, 1, 2–11. [Google Scholar] [CrossRef]
- Barnes, M.E.; Cowan, C.R.; Boag, L.E.; Hill, J.G.; Jones, M.L.; Nixon, K.M.; Parker, M.G.; Parker, S.K.; Raymond, M.V.; Sternenberg, L.H.; et al. Effects of Acute Yohimbine Hydrochloride Supplementation on Repeated Supramaximal Sprint Performance. Int. J. Environ. Res. Public Health 2022, 19, 1316. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.D.; Langley, H.N.; Roberson, C.C.; Rogers, R.R.; Ballmann, C.G. Effects of Short-Term Golden Root Extract (Rhodiola rosea) Supplementation on Resistance Exercise Performance. Int. J. Environ. Res. Public Health 2021, 18, 6953. [Google Scholar] [CrossRef]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: New York, NY, USA, 1988. [Google Scholar]
- Bhattaram, V.A.; Graefe, U.; Kohlert, C.; Veit, M.; Derendorf, H. Pharmacokinetics and bioavailability of herbal medicinal products. Phytomedicine 2002, 9, 1–33. [Google Scholar] [CrossRef]
- Kienle, G.; Berrino, F.; Bussing, A.; Portalupi, E.; Rosenzweig, S.; Kiene, H. Mistletoe in cancer: A systematic review on controlled clinical trials. In Database of Abstracts of Reviews of Effects (DARE), Quality-Assessed Reviews [Internet]; 2003. Available online: https://www.ncbi.nlm.nih.gov/books/NBK69731/ (accessed on 5 July 2022).
- Khan, T.; Ali, S.; Qayyum, R.; Hussain, I.; Wahid, F.; Shah, A.J. Intestinal and vascular smooth muscle relaxant effect of Viscum album explains its medicinal use in hyperactive gut disorders and hypertension. BMC Complement. Altern. Med. 2016, 16, 1–8. [Google Scholar] [CrossRef]
- Irving, B.A.; Rutkowski, J.; Brock, D.W.; Davis, C.K.; Barrett, E.J.; Gaesser, G.A.; Weltman, A. Comparison of Borg-and OMNI-RPE as markers of the blood lactate response to exercise. Med. Sci. Sports Exerc. 2006, 38, 1348–1352. [Google Scholar] [CrossRef]
- Steed, J.; Gaesser, G.A.; Weltman, A. Rating of perceived exertion and blood lactate concentration during submaximal running. Med. Sci. Sports Exerc. 1994, 26, 797. [Google Scholar] [CrossRef]
- Kilpatrick, M.W.; Robertson, R.J.; Powers, J.M.; Mears, J.L.; Ferrer, N.F. Comparisons of RPE before, during, and after self-regulated aerobic exercise. Med. Sci. Sports Exerc. 2009, 41, 682–687. [Google Scholar] [CrossRef]
- Szmedra, L.; Bacharach, D. Effect of music on perceived exertion, plasma lactate, norepinephrine and cardiovascular hemodynamics during treadmill running. Int. J. Sports Med. 1998, 19, 32–37. [Google Scholar] [CrossRef]
- Arda, N.; Onay, E.; Koz, O.; Kirmizigul, S. Monosaccharides and polyols from mistletoes (Viscum album L.) growing on two different host species. Biol. Bratisl. 2003, 58, 1037–1042. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goode, E.C.; Van Duser, S.E.; Rogers, R.R.; Williams, T.D.; Ballmann, C.G. Effects of Acute Mistletoe (Viscum album L.) Ingestion on Aerobic Exercise Performance. Nutraceuticals 2022, 2, 162-169. https://doi.org/10.3390/nutraceuticals2030012
Goode EC, Van Duser SE, Rogers RR, Williams TD, Ballmann CG. Effects of Acute Mistletoe (Viscum album L.) Ingestion on Aerobic Exercise Performance. Nutraceuticals. 2022; 2(3):162-169. https://doi.org/10.3390/nutraceuticals2030012
Chicago/Turabian StyleGoode, Emily C., Sarah E. Van Duser, Rebecca R. Rogers, Tyler D. Williams, and Christopher G. Ballmann. 2022. "Effects of Acute Mistletoe (Viscum album L.) Ingestion on Aerobic Exercise Performance" Nutraceuticals 2, no. 3: 162-169. https://doi.org/10.3390/nutraceuticals2030012
APA StyleGoode, E. C., Van Duser, S. E., Rogers, R. R., Williams, T. D., & Ballmann, C. G. (2022). Effects of Acute Mistletoe (Viscum album L.) Ingestion on Aerobic Exercise Performance. Nutraceuticals, 2(3), 162-169. https://doi.org/10.3390/nutraceuticals2030012







