Formulation of Rosemary Extracts through Spray-Drying Encapsulation or Emulsification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solvents and Reagents
2.2. Plant Material
2.3. Extraction Procedures
2.3.1. Fixed Bed Extraction (FBE)
2.3.2. Ultrasound Assisted Extraction (UAE)
2.4. Spray Drying Encapsulation
- i.
- ii.
- air outlet temperature: 70–85 °C when the inlet temperature was 140 °C, and 85–100 °C when the inlet temperature was 160 °C,
- iii.
- atomization pressure: 5 bar, based on preliminary experiments, and
- iv.
- feed solution temperature: 25 °C and the feed flow rate was adjusted and remained constant at the point where the desired output temperature was reached, according to preliminary experiments.
2.5. Emulsions Preparation and Storage Test
2.6. Analytical Methods
2.6.1. Determination of Total Phenol Content (TPC)
2.6.2. DPPH Free Radical Scavenging Assay
2.6.3. Solid Residue and Selectivity
2.6.4. Encapsulation Yield and Efficiency
2.6.5. Determination of Powder Moisture Content
2.6.6. Emulsion Shelf Life Analysis
2.6.7. HPLC-DAD
2.7. Statistical Analysis
3. Results
3.1. Extraction Yield and Phenolic Compounds Content in the Extracts Obtained by Different Procedures
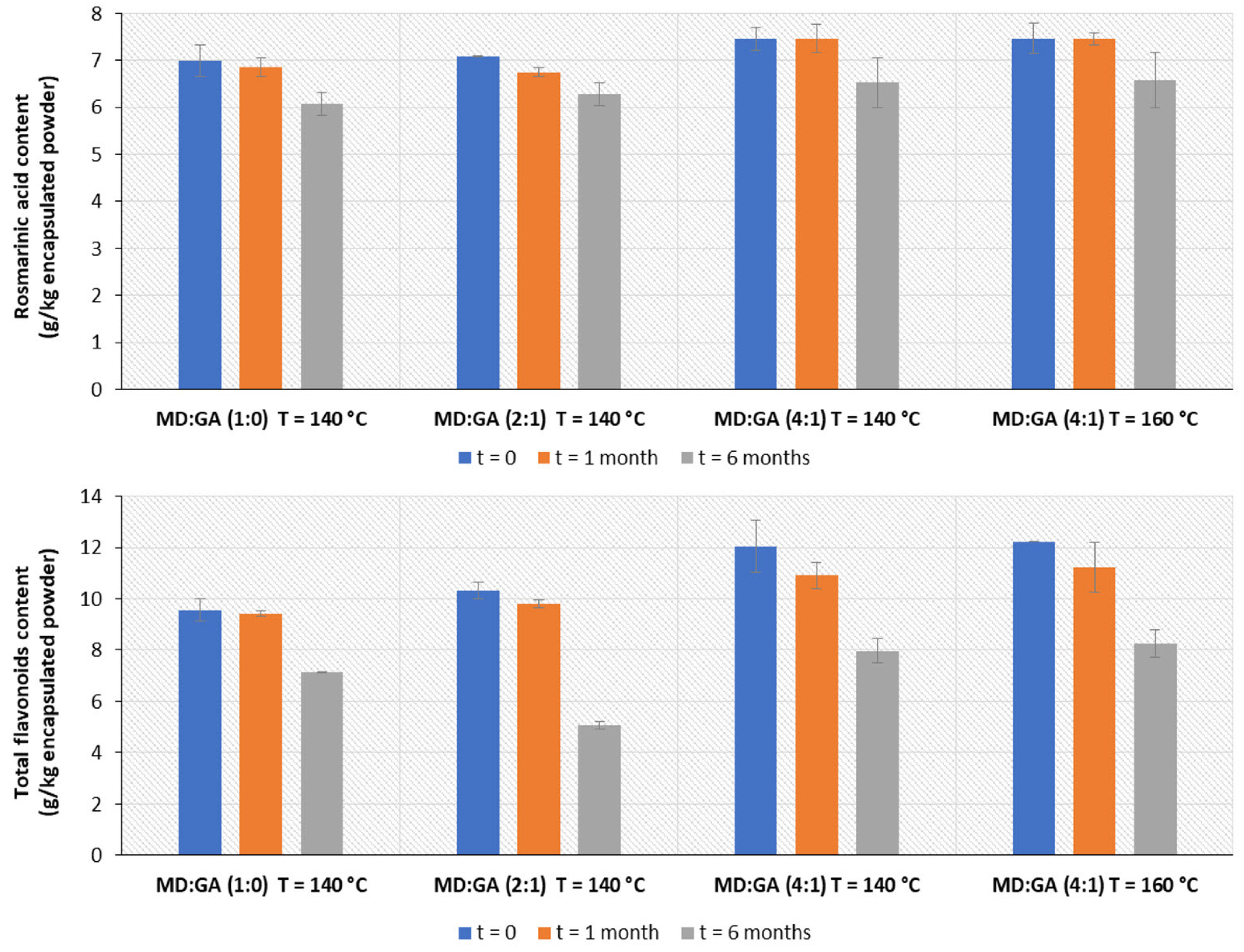
3.2. Encapsulation of the Extracts and Storage Stability of the Encapsulated Products
3.2.1. Encapsulation Yield and Efficiency
3.2.2. Storage Stability of the Encapsulated Products
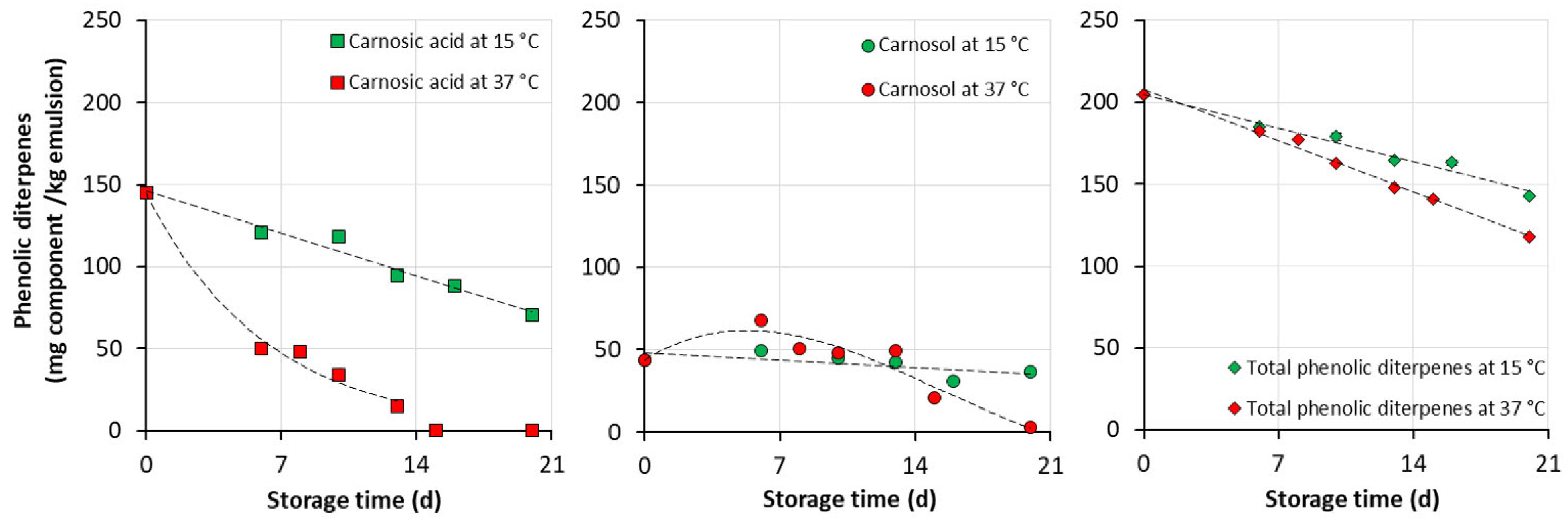
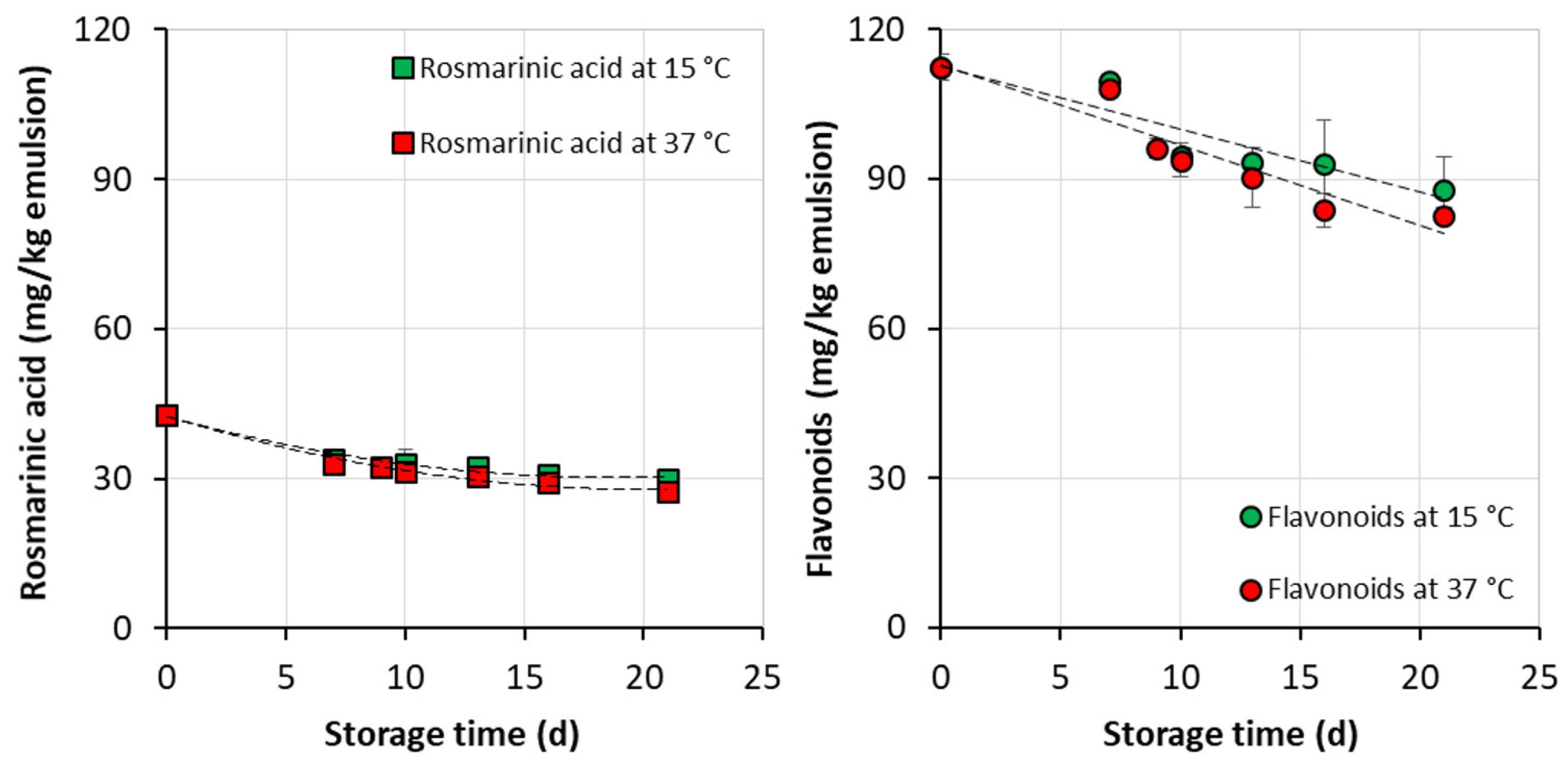
3.3. Formulation of Emulsion Rich in Phenolic Diterpenes and Storage Stability of the Product
4. Discussion
4.1. Extraction Yield and Phenolic Compounds Content in the Extracts Obtained by Different Procedures
4.2. Encapsulation of the Extracts by Spray Drying and Storage Stability of the Products
4.3. Storage Stability of Emulsions Rich in Phenolic Diterpenes
5. Conclusions
- the water rosemary extracts can be very effectively encapsulated in maltodextrin combined with gum arabic with high encapsulation yield (90–100%) and efficiency (97%) for rosmarinic acid and flavonoids;
- the acetone extracts—rich in carnosic acid and carnosol—should be first transferred to an oil solution and then encapsulated as dry powder or emulsion;
- over a period of six months of storage of the encapsulated products, a high retention of rosmarinic acid (88%) and lower of flavonoids (50–75%) were observed; and
- carnosic acid presented lower retention either encapsulated in solid powder (65–70% after one month at ambient temperature) or in emulsion (48% after 20 days of storage at 15 °C), along with its partial conversion to carnosol.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Admassu, S.; Kebede, M. Application of antioxidants in food processing industry: Options to improve the extraction yields and market value of natural products. Adv. Food Technol. Nutr. Sci. Open J. 2019, 5, 38–49. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oreopoulou, V.; Tsironi, T. Plant Antioxidants and Antimicrobials in Edible and Non-edible Active Packaging Films. In Plant Antioxidants and Health; Reference Series in Phytochemistry; Ekiert, H.M., Ramawat, K.G., Arora, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W. Safety assessment of Rosmarinus officinalis (rosemary)-derived ingredients as used in cosmetics. Int. J. Toxicol. 2018, 37 (Suppl. 3), 12S–50S. [Google Scholar] [CrossRef] [Green Version]
- Global Rosemary Extract Market 2019 by Manufacturers, Regions, Type and Application, Forecast to 2024. Available online: https://www.360researchreports.com/global-rosemary-extract-market-14076077 (accessed on 13 January 2020).
- Oreopoulou, A.; Choulitoudi, E.; Tsimogiannis, D.; Oreopoulou, V. Six Common Herbs with Distinctive Bioactive, Antioxidant Components. A Review of Their Separation Techniques. Molecules 2021, 26, 2920. [Google Scholar] [CrossRef] [PubMed]
- De AR Oliveira, G.; de Oliveira, A.E.; da Conceição, E.C.; Leles, M.I. Multiresponse optimization of an extraction procedure of carnosol and rosmarinic and carnosic acids from rosemary. Food Chem. 2016, 211, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Psarrou, I.; Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction Kinetics of Phenolic Antioxidants from the Hydro Distillation Residues of Rosemary and Effect of Pretreatment and Extraction Parameters. Molecules 2020, 25, 4520. [Google Scholar] [CrossRef]
- Bellumori, M.; Innocenti, M.; Binello, A.; Boffa, L.; Mulinacci, N.; Cravotto, G. Selective recovery of rosmarinic and carnosic acids from rosemary leaves under ultrasound-and microwave-assisted extraction procedures. Comptes Rendus Chim. 2016, 19, 699–706. [Google Scholar] [CrossRef]
- Jacotet-Navarro, M.; Rombaut, N.; Fabiano-Tixier, A.S.; Danguien, M.; Bily, A.; Chemat, F. Ultrasound versus microwave as green processes for extraction of rosmarinic, carnosic and ursolic acids from rosemary. Ultrason. Sonochem. 2015, 27, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer, and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Zabot, G.L.; Moraes, M.N.; Carvalho, P.I.; Meireles, M.A.A. New proposal for extracting rosemary compounds: Process intensification and economic evaluation. Ind. Crops Prod. 2015, 77, 758–771. [Google Scholar] [CrossRef]
- Hossain, M.B.; Barry-Ryan, C.; Martin-Diana, A.B.; Brunton, N.P. Optimisation of accelerated solvent extraction of antioxidant compounds from rosemary (Rosmarinus officinalis L.), marjoram (Origanum marjorana L.) and oregano (Origanum vulgare L.) using response surface methodology. Food Chem. 2011, 126, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Intern. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Labuschagne, B. Impact of wall material physicochemical characteristics on the stability of encapsulated phytochemicals: A review. Food Res. Int. 2018, 107, 227–247. [Google Scholar] [CrossRef] [PubMed]
- Couto, R.O.; Conceição, E.C.; Chaul, L.T.; Oliveira, E.M.; Martins, F.S.; Bara, M.T.F.; Rezende, K.R.; Alves, S.F.; Paula, J.R. Spray-dried rosemary extracts: Physicochemical and antioxidant properties. Food Chem. 2012, 131, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Chaul, L.T.; Conceicão, E.C.; Bara, M.T.F.; Paula, J.R.; Couto, R.O. Engineering spray-dried rosemary extracts with improved physicomechanical properties: A design of experiments issue. Rev. Bras. Farmacogn. 2017, 27, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Tolun, A.; Altintas, Z.; Artik, N. Microencapsulation of grape polyphenols using maltodextrin and gum arabic as two alternative coating materials: Development and characterization. J. Biotechnol. 2016, 239, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Dadi, D.W.; Emire, S.A.; Hagos, A.D.; Eun, J.-B. Development of microencapsulated bioactive product from M. stenopetala leaves extract by optimizing spray drying process parameters. Ann. Food Sci. Technol. 2019, 20, 191–206. [Google Scholar]
- Carvalho, I.T.; Estevinho, B.N.; Santos, L. Application of Microencapsulated Essential Oils In Cosmetic And Personal Health Care Products—A Review. Int. J. Cosmet. Sci. 2016, 38, 109–119. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A.; Weiss, J. Emulsion-based delivery systems for lipophilic bioactive components. J. Food Sci. 2007, 72, 109–124. [Google Scholar] [CrossRef]
- Gurpreet, K.; Singh, K. Review of nanoemulsion formulation and characterization techniques. Indian J. Pharmaceut. Sci. 2018, 80, 781–789. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Choulitoudi, E.; Bimpilas, A.; Mitropoulou, G.; Kourkoutas, Y.; Oreopoulou, V. Exploitation of the biological potential of Satureja thymbra essential oil and distillation by-products. J. Appl. Res. Med. Aromat. Plants 2016, 4, 12–20. [Google Scholar] [CrossRef]
- Oreopoulou, A.; Goussias, G.; Tsimogiannis, D.; Oreopoulou, V. Hydro-alcoholic extraction kinetics of phenolics from oregano: Optimization of the extraction parameters. Food Bioprod Process. 2020, 123, 378–389. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (EFSA ANS Panel); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Refined exposure assessment of extracts of rosemary (E 392) from its use as food additive. EFSA J. 2018, 16, e05373. [Google Scholar] [PubMed] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Mahdavi, S.A.; Jafari, S.M.; Assadpoor, E.; Dehnad, D. Microencapsulation optimization of natural anthocyanins with maltodextrin, gum Arabic and gelatin. Int. J. Biol. Macromol. 2016, 85, 379–385. [Google Scholar] [CrossRef]
- Efendy Goon, D.; Sheikh Abdul Kadir, S.H.; Latip, N.A.; Rahim, S.A.; Mazlan, M. Palm Oil in Lipid-Based Formulations and Drug Delivery Systems. Biomolecules 2019, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Amft, J.; Bauer, J.L.; Rostek, J.; Spielvogel, S.; Döring, F.; Schwarz, K. MCT Oil Coating Improves the Oxidative Stability of Surface Lipids in Corn Extrudates. Eur. J. Lipid Sci. Technol. 2020, 122, 1900350. [Google Scholar] [CrossRef]
- Zhang, Y.; Smuts, J.P.; Dodbiba, E.; Rangarajan, R.; Lang, J.C.; Armstrong, D.W. Degradation study of carnosic acid, carnosol, rosmarinic acid, and rosemary extract (Rosmarinus officinalis L.) assessed using HPLC. J. Agric. Food Chem. 2012, 60, 9305–9314. [Google Scholar] [CrossRef]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Phenolic acids of plant origin—A review on their antioxidant activity in vitro (o/w emulsion systems) along with their in vivo health biochemical properties. Foods 2020, 9, 534. [Google Scholar] [CrossRef]
- Kiokias, S.; Oreopoulou, V. A Review of the health protective effects of phenolic acids against a range of severe pathologic conditions (including coronavirus-based infections). Molecules 2021, 26, 5405. [Google Scholar] [CrossRef]
- Mulinacci, N.; Innocenti, M.; Bellumori, M.; Giaccherini, C.; Martini, V.; Michelozzi, M. Storage method, drying processes and extraction procedures strongly affect the phenolic fraction of rosemary leaves: An HPLC/DAD/MS study. Talanta 2011, 85, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Cuvelier, M.E.; Richard, H.; Berset, C. Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. J. Am. Oil Chem. Soc. 1996, 73, 645–652. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.W.; Lee, M.K.; Kim, Y.J.; Asamenew, G.; Cha, Y.S.; Kim, J.B. Phenolic profiling and quantitative determination of common sage (Salvia plebeia R. Br.) by UPLC-DAD-QTOF/MS. Eur. Food Res. Techn. 2018, 244, 1637–1646. [Google Scholar] [CrossRef] [Green Version]
- Herrero, M.; Plaza, M.; Cifuentes, A.; Ibáñez, E. Green processes for the extraction of bioactives from Rosemary: Chemical and functional characterization via ultra-performance liquid chromatography-tandem mass spectrometry and in-vitro assays. J. Chromatogr. A 2010, 1217, 2512–2520. [Google Scholar] [CrossRef] [PubMed]
- Sansone, F.; Mencherini, T.; Picerno, P.; d’Amore, M.; Aquino, R.P.; Lauro, M.R. Maltodextrin/pectin microparticles by spray drying as carrier for nutraceutical extracts. J. Food Eng. 2011, 105, 468–476. [Google Scholar] [CrossRef]
- Moradi, S.; Navideh, A. Preparation and characterization of α-tocopherol nanocapsules based on gum Arabic-stabilized nanoemulsions. Food Sci. Biotechnol. 2018, 28, 413–421. [Google Scholar] [CrossRef]
- Garofulić, I.E.; Zorić, Z.; Pedisić, S.; Dragović-Uzelac, V. Retention of polyphenols in encapsulated sour cherry juice in dependence of drying temperature and wall material. LWT-Food Sci. Technol. 2017, 83, 110–117. [Google Scholar] [CrossRef]
- Bušić, A.; Komes, D.; Belščak-Cvitanović, A.; Vojvodić Cebin, A.; Špoljarić, I.; Mršić, G.; Miao, S. The potential of combined emulsification and spray drying techniques for encapsulation of polyphenols from rosemary (Rosmarinus officinalis L.) leaves. Food Technol. Biotechnol. 2018, 56, 494–505. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2003, 17, 25–39. [Google Scholar] [CrossRef]
- Mahdavi, S.A.; Jafari, S.M.; Assadpour, E.; Ghorbani, M. Storage stability of encapsulated barberry’s anthocyanin and its application in jelly formulation. J. Food Eng. 2016, 181, 59–66. [Google Scholar] [CrossRef]
- Da Silva, F.C.; da Fonseca, C.R.; de Alencar, S.M.; Thomazini, M.; Balieiro, J.C.d.C.; Pittia, P.; Favaro-Trindade, C.S. Assessment of production efficiency, physicochemical properties and storage stability of spray-dried propolis, a natural food additive, using gum Arabic and OSA starch-based carrier systems. Food Bioprod. Process. 2013, 91, 28–36. [Google Scholar] [CrossRef]
- Tan, S.P.; Kha, T.C.; Parks, S.; Stathopoulos, C.; Roach, P.D. Optimising the Encapsulation of an Aqueous Bitter Melon Extract by Spray-Drying. Foods 2015, 4, 400–419. [Google Scholar] [CrossRef] [Green Version]
- Koç, M.; Koç, B.; Susyal, G.; Sakin Yilmazer, M.; Kaymak Ertekin, F.; Bağdatlıoğlu, N. Functional and physicochemical properties of whole egg powder: Effect of spray drying conditions. J. Food Sci. Technol. 2011, 48, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Moser, P.; Telis, V.R.N.; de Andrade Neves, N.; García-Romero, E.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Storage stability of phenolic compounds in powdered BRS Violeta grape juice microencapsulated with protein and maltodextrin blends. Food Chem. 2017, 214, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Birtić, S.; Dussort, P.; Pierre, F.X.; Bily, A.C.; Roller, M. Carnosic acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.W.; Frankel, E.N.; Schwarz, K.; Aeschbach, R.; German, J.B. Antioxidant activity of carnosic acid and methyl carnosate in bulk oils and oil-in-water emulsions. J. Agric. Food Chem. 1996, 44, 2951–2956. [Google Scholar] [CrossRef]
- Lu, W.; Kelly, A.L.; Miao, S. Emulsion-based encapsulation and delivery systems for polyphenols. Trends Food Sci. Technol. 2016, 47, 1–9. [Google Scholar] [CrossRef]
- Ahmed, K.; Li, Y.; McClements, D.J.; Xiao, H. Nanoemulsion-and emulsion-based delivery systems for curcumin: Encapsulation and release properties. Food Chem. 2012, 132, 799–807. [Google Scholar] [CrossRef]
- Costa, M.; Losada-Barreiro, S.; Bravo-Díaz, C.; Vicente, A.A.; Monteiro, L.S.; Paiva-Martins, F. Influence of AO chain length, droplet size and oil to water ratio on the distribution and on the activity of gallates in fish oil-in-water emulsified systems: Emulsion and nanoemulsion comparison. Food Chem. 2020, 310, 125716. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Wu, C.; Chen, Y.; Xie, M. Antioxidant effects of different polar gallic acid and its alkyl esters in oil-in-water emulsions. J. Chin. Instit. Food Sci. Technol. 2019, 19, 13–22. [Google Scholar]
- Lei, C.; Tang, X.; Chen, M.; Chen, H.; Yu, S. Alpha-tocopherol-based microemulsion improving the stability of carnosic acid and its electrochemical analysis of antioxidant activity. Colloids Surf. Physicochem. Eng. Asp. 2019, 580, 123708. [Google Scholar] [CrossRef]
- Zheng, H.; Wijaya, W.; Zhang, H.; Feng, K.; Liu, Q.; Zheng, T.; Yin, Z.; Cao, y.; Huang, Q. Improving the bioaccessibility and bioavailability of carnosic acid using a lecithin-based nanoemulsion: Complementary in vitro and in vivo studies. Food Funct. 2020, 11, 8141–8149. [Google Scholar] [CrossRef] [PubMed]
- Choulitoudi, E.; Xristou, M.; Tsimogiannis, D.; Oreopoulou, V. The effect of temperature on the phenolic content and oxidative stability of o/w emulsions enriched with natural extracts from Satureja thymbra. Food Chem. 2021, 349, 129206. [Google Scholar] [CrossRef] [PubMed]


| Extraction | TPC Yield (g GAE/kg dw) 1 | Antiradical Activity (g Trolox/kg dw) 1 | Selectivity (%) |
|---|---|---|---|
| Acetone 100% (FBE 2) | 17.5 ± 1.4 a | 27.2 ± 2.9 a | 14.8 ± 1.2 a |
| Water 100% (FBE) | 44.5 ± 3.8 b | 76.3 ± 6.1 bc | 19.6 ± 1.7 b |
| Acetone:water 80:20 (FBE) | 60.0 ± 2.6 c | 127.3 ± 4.5 d | 24.4 ± 0.6 c |
| Acetone:water 80:20 (UAE 3) | 64.5 ± 6.0 c | 91.6 ± 7.7 c | 26.9 ± 2.5 c |
| Εthanol:water 60:40 (UAE) | 62.5 ± 4.2 c | 63.2 ± 5.5 b | 26.3 ± 1.8 c |
| Identified Flavonoids | Acetone Extract (FBE 1) C (g/kg dw 3) | Water Extract (FBE) C (g/kg dw) | Acetone:Water 80:20 (FBE) C (g/kg dw) | Acetone:Water 80:(UAE 2) C (g/kg dw) | Ethanol:Water 60:40 (UAE) C (g/kg dw) |
|---|---|---|---|---|---|
| Phenolic diterpenes | |||||
| carnosic acid | 9.5 ± 0.5 | − | 13.9 ± 0.5 | 12.7 ± 0.5 | 7.0 ± 0.3 |
| carnosol | 2.1 ± 0.2 | − | 2.4 ± 0.1 | 2.8 ± 0.1 | 5.0 ± 0.3 |
| other phenolic diterpenes | 1.5 ± 0.0 | − | 1.9 ± 0.2 | 1.9 ± 0.1 | 1.7 ± 0.1 |
| Total phenolic diterpenes | 13.1 ± 0.7 | − | 18.2 ± 0.3 | 17.4 ± 0.7 | 13.7 ± 0.7 |
| Phenolic acids | |||||
| caffeic acid | − | 1.2 ± 0.1 | 1.3 ± 0.1 | 1.14 ± 0.1 | 1.04 ± 0.1 |
| rosmarinic acid | 3.2 ± 0.3 | 16.3 ± 1.7 | 21.3 ± 1.2 | 20.7 ± 1.4 | 18.0 ± 0.2 |
| Flavonoids | |||||
| Nepitrin 4 | 1.6 ± 0.1 | 9.5 ± 0.9 | 13.4 ± 1.4 | 13.7 ± 1.8 | 12.2 ± 0.2 |
| Homoplantaginin 4 | 0.8 ± 0.1 | 5.0 ± 0.6 | 4.5 ± 0.1 | 5.1 ± 0.1 | 4.9 ± 0.4 |
| Isoscutellarein 4 | 1.0 ± 0.1 | 6.1 ± 1.0 | 5.8 ± 0.7 | 8.4 ± 1.0 | 7.3 ± 0.9 |
| Hispidoulin 4 | 0.4 ± 0.0 | 0.2 ± 0.0 | 1.1 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 |
| Ladanein 4 | 0.7 ± 0.0 | 0.1 ± 0.0 | 1.4 ± 0.1 | 1.1 ± 0.0 | 1.1 ± 0.0 |
| Genkwanin 4 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.6 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| Salvigenin 4 | 2.3 ± 0.2 | 0.1 ± 0.0 | 3.4 ± 0.1 | 2.8 ± 0.0 | 2.6 ± 0.1 |
| 4’-methoxytectochrysin 4 | 0.6 ± 0.0 | − | 0.8 ± 0.0 | 0.6 ± 0.0 | 0.4 ± 0.0 |
| Total flavonoids 4 | 9.4 ± 0.6 | 27.4 ± 2.6 | 35.6 ± 3.1 | 37.4 ± 1.6 | 33.3 ± 1.8 |
| Wall (MD:GA) | Inlet Air Temperature (°C) | Powder Moisture (%) | EY (%) | EE (%) | ||
|---|---|---|---|---|---|---|
| Rosmarinic Acid | Total Flavonoids | Rosmarinic Acid | Total Flavonoids | |||
| 1:0 | 140 | 3.3 ± 0.0 a | 95.4 ± 4.5 a | 84.2 ± 4.5 a | 96.9 ± 0.2 a | 96.5 ± 0.2 a |
| 4:1 | 140 | 4.2 ± 0.3 b | 100.0 ± 3.3 a | 95.3 ± 5.4 ab | 96.8 ± 1.5 a | 97.2 ± 0.5 ab |
| 4:1 | 160 | 3.1 ± 0.0 a | 100.0 ± 4.4 a | 96.9 ± 0.0 b | 97.9 ± 0.1 a | 97.4 ± 0.2 b |
| 2:1 | 140 | 4.1 ± 0.1b | 98.9 ± 0.1a | 90.9 ± 3.2 ab | 97.4 ± 0.3 a | 97.6 ± 0.2 b |
| Extract | Wall (MD:GA) | Core (%) | Powder Moisture (%) | Rosmarinic Acid | Total Flavonoids | Carnosic Acid | Carnosol | Other Phenolic Diterpenes |
|---|---|---|---|---|---|---|---|---|
| EY (%) | ||||||||
| Acetone:Water 80:20 | 1:0 | 10 | 3.8 ± 0.5 a | 98.7 ± 0.4 a | 86.3 ± 0.1 a | 57.8 ± 1.1 a | 93.2 ± 6.8 a | 67.6 ± 4.8 a |
| Acetone:Water 80:20 | 4:1 | 10 | 3.9 ± 0.0 a | 100.0 ± 0.2 a | 91.3 ± 0.0 b | 62.3 ± 0.6 b | 98.0 ± 1.5 ab | 67.2 ± 0.8 a |
| Ethanol:Water 60:40 | 4:1 | 10 | 4.2 ± 0.1 a | 99.1 ± 1.2 a | 95.5 ± 5.0 b | 65.7 ± 1.2 c | 100.0 ± 0.8 b | 94.9 ± 3.8 b |
| EE (%) | ||||||||
| Acetone:Water 80:20 | 1:0 | 10 | 3.8 ± 0.5 a | 92.4 ± 0.0 a | 90.4 ± 0.0 a | 26.4 ± 2.8 a | 64.5 ± 3.9 a | 35.2 ± 4.6 a |
| Acetone: Water 80:20 | 4:1 | 10 | 3.9 ± 0.0 a | 95.1 ± 0.5 b | 91.8 ± 0.2 b | 31.8 ± 0.1 b | 43.5 ± 2.1 b | 37.7 ± 0.8 a |
| Ethanol: Water 60:40 | 4:1 | 10 | 4.2 ± 0.1 a | 97.2 ± 0.7 c | 91.5 ± 0.9 b | 34.3 ± 0.7 b | 66.7 ± 1.1 a | 53.0 ± 1.9 b |
| Core (%) | Powder Moisture (%) | EY% | EE% | ||||
|---|---|---|---|---|---|---|---|
| Carnosic Acid | Carnosol | Other Phenolic Diterpenes | Carnosic Acid | Carnosol | Other Phenolic Diterpenes | ||
| 5 | 2.3 ± 0.1 a | 72.8 ± 0.9 a | 100.0 ± 0.0 a | 100.0 ± 0.1 a | 75.2 ± 0.5 a | 70.8 ± 2.0 a | 88.7 ± 0.2 a |
| 10 | 3.3 ± 0.1 b | 44.4 ± 1.4 b | 49.4 ± 3.3 b | 30.5 ± 1.7 b | 73.0 ± 0.8 b | 60.7 ± 3.6 b | 65.8 ± 1.4 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanakidi, L.-D.; Tsimogiannis, D.; Kiokias, S.; Oreopoulou, V. Formulation of Rosemary Extracts through Spray-Drying Encapsulation or Emulsification. Nutraceuticals 2022, 2, 1-21. https://doi.org/10.3390/nutraceuticals2010001
Kanakidi L-D, Tsimogiannis D, Kiokias S, Oreopoulou V. Formulation of Rosemary Extracts through Spray-Drying Encapsulation or Emulsification. Nutraceuticals. 2022; 2(1):1-21. https://doi.org/10.3390/nutraceuticals2010001
Chicago/Turabian StyleKanakidi, Lamprini-Danai, Dimitrios Tsimogiannis, Sotirios Kiokias, and Vassiliki Oreopoulou. 2022. "Formulation of Rosemary Extracts through Spray-Drying Encapsulation or Emulsification" Nutraceuticals 2, no. 1: 1-21. https://doi.org/10.3390/nutraceuticals2010001
APA StyleKanakidi, L.-D., Tsimogiannis, D., Kiokias, S., & Oreopoulou, V. (2022). Formulation of Rosemary Extracts through Spray-Drying Encapsulation or Emulsification. Nutraceuticals, 2(1), 1-21. https://doi.org/10.3390/nutraceuticals2010001







