Green Coffee Extract Microencapsulated: Physicochemical Characteristics, Stability, Bioaccessibility, and Sensory Acceptability through Dairy Beverage Consumption
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Aqueous Green Coffee Extract
2.2. Microencapsulation Procedures
2.3. Characterization of the Encapsulated Extracts
2.3.1. Total Phenolic Compounds
2.3.2. Antioxidant Activity by ABTS Radical Assay
2.3.3. Antioxidant Activity by DPPH Radical Assay
2.3.4. Determination of Caffeine, Chlorogenic Acid, and Trigonelline
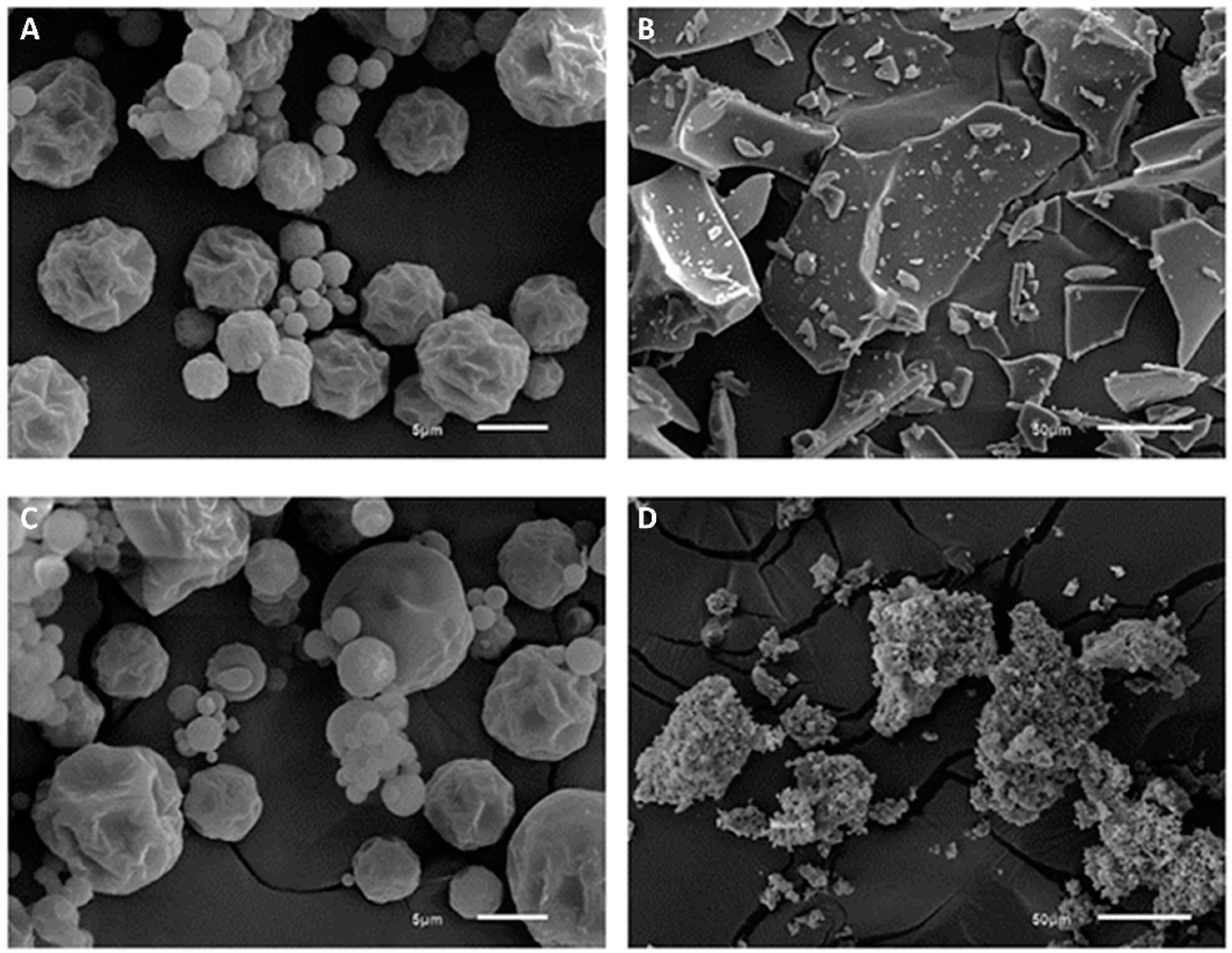
2.3.5. Scanning Electron Microscopy (SEM)
2.4. Incorporation into Dairy Beverages
2.5. Stability of Compounds in the Dairy Beverages during Storage
2.6. Sensory Analysis
2.7. In Vitro Gastrointestinal Digestion
- -
- Bioacessible fraction (%) = (Qt supernatant/Qt digested) × 100
- -
- Residual fraction (%) = (Qt residue/Qt digested) × 100
- -
- Total recovery = bioaccessible fraction (%) + residual fraction (%)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Encapsulated Extracts
3.2. Total Phenolics and Antioxidant Activity of Dairy Beverages during Storage
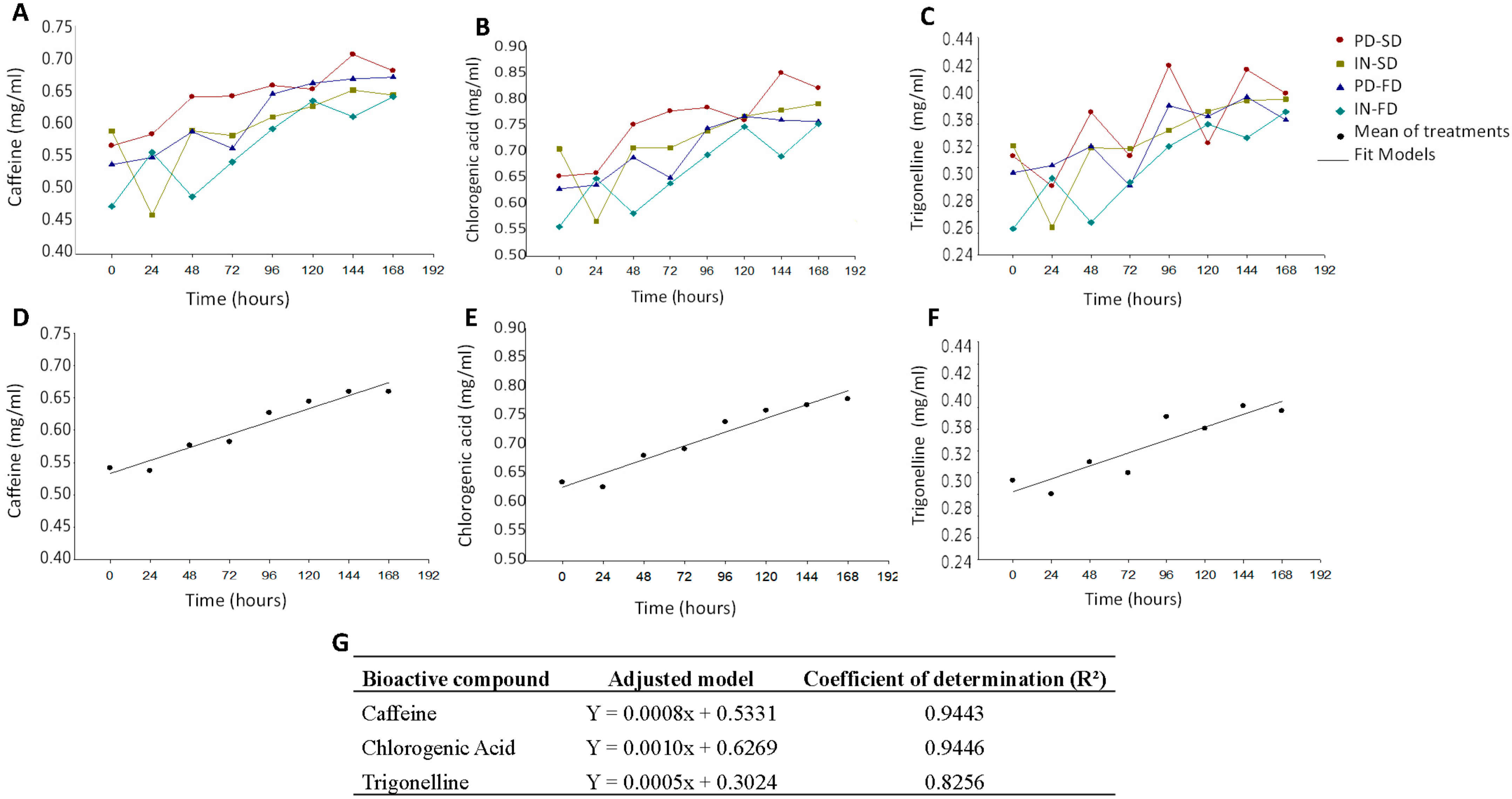
3.3. Bioactive Compounds in Dairy Beverages during Storage
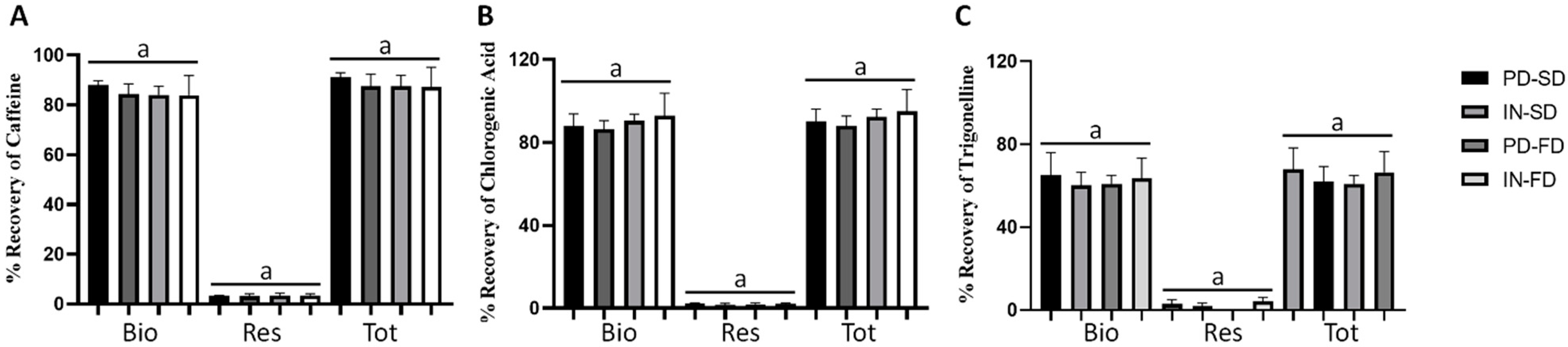
3.4. Percentage of Recovery after In Vitro Gastrointestinal Digestion
3.5. Sensory Analysis of Dairy Beverages
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LIczbiński, P.; Bukowska, B. Tea and coffee polyphenols and their biological properties based on the latest in vitro investigations. Ind. Crops Prod. 2022, 175, 114265. [Google Scholar] [CrossRef]
- Babova, O.; Occhipinti, A.; Maffei, M.E. Chemical partitioning and antioxidant capacity of green coffee (Coffea arabica and Coffea canephora) of different geographical origin. Phytochemistry 2016, 123, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Pimpley, V.; Patil, S.; Srinivasan, K.; Desai, N.; Murthy, P.S. The chemistry of chlorogenic acid from green coffee and its role in attenuation of obesity and diabetes. Prep. Biochem. Biotechnol. 2020, 50, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Zain, M.Z.M.; Baba, A.S.; Shori, A.B. Effect of polyphenols enriched from green coffee bean on antioxidant activity and sensory evaluation of bread. J. King Saud Univ. Sci. 2018, 30, 278–282. [Google Scholar] [CrossRef]
- Świeca, M.; Gawlik-Dziki, U.; Dziki, D.; Baraniak, B. Wheat bread enriched with green coffee—In vitro bioaccessibility and bioavailability of phenolics and antioxidant activity. Food Chem. 2017, 221, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Budryn, G.; Nebesny, E.; Żyżelewicz, D.; Oracz, J. Properties of model systems of sunflower oil and green coffee extract after heat treatment and storage. LWT Food Sci. Technol. 2014, 59, 467–478. [Google Scholar] [CrossRef]
- Aguiar, J.; Estevinho, B.N.; Santos, L. Microencapsulation of natural antioxidants for food application—The specific case of coffee antioxidants—A review. Trends Food Sci. Technol. 2016, 58, 21–39. [Google Scholar] [CrossRef]
- Barone, G.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. Interactions between whey proteins and calcium salts and implications for the formulation of dairy protein-based nutritional beverage products: A review. Compr. Rev. Food Sci. Food Saf. 2022, 22, 1254–1274. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 20th ed.; AOAC: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Cai, Y.Z.; Corke, H. Production and properties of spray-dried Amaranthus betacyanin pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- Eastman, J.E.; Moore, C.O. Cold Water Soluble Granular Starch for Gelled Food Compositions. U.S. Patent 4465702-A, 14 August 1984. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Abrahão, S.A.; Pereira, R.G.F.A.; Lima, A.R.; Ferreira, E.; Malta, M.R. Compostos bioativos em café integral e descafeinado e qualidade sensorial da bebida. Pesqui. Agropecu. Bras. 2008, 43, 1799–1804. [Google Scholar] [CrossRef]
- Pinheiro, C.A.; Pereira, L.L.; Fioresi, D.B.; Oliveira, D.D.S.; Osório, V.M.; da Silva, J.A.; Pereira, U.A.; Ferrão, M.A.G.; Souza, E.M.R.; da Fonseca, A.F.A.; et al. Physico-chemical properties and sensory profile of coffea canephora genotypes in high-altitudes. Aust. J. Crop Sci. 2019, 13, 2046–2052. [Google Scholar] [CrossRef]
- Silva, P.I.; Stringheta, P.C.; Teófilo, R.F.; de Oliveira, I.R.N. Parameter optimization for spray-drying microencapsulation of jaboticaba (Myrciaria jaboticaba) peel extracts using simultaneous analysis of responses. J. Food Eng. 2013, 117, 538–544. [Google Scholar] [CrossRef]
- Bernardes, A.L.; Moreira, J.A.; Tostes, M.D.G.V.; Costa, N.M.B.; Silva, P.I.; Costa, A.G.V. In vitro bioaccessibility of microencapsulated phenolic compounds of jussara (Euterpe edulis Martius) fruit and application in gelatine model-system. LWT Food Sci. Technol. 2019, 102, 173–180. [Google Scholar] [CrossRef]
- do Carmo, E.L.; Teodoro, R.A.R.; Félix, P.H.C.; de Barros Fernandes, R.V.; de Oliveira, É.R.; Veiga, T.R.L.A.; Borges, S.V.; Botrel, D.A. Stability of spray-dried beetroot extract using oligosaccharides and whey proteins. Food Chem. 2018, 249, 51–59. [Google Scholar] [CrossRef]
- Moayyedi, M.; Eskandari, M.H.; Rad, A.H.E.; Ziaee, E.; Khodaparast, M.H.H.; Golmakani, M.-T. Effect of drying methods (electrospraying, freeze drying and spray drying) on survival and viability of microencapsulated Lactobacillus rhamnosus ATCC 7469. J. Funct. Foods 2018, 40, 391–399. [Google Scholar] [CrossRef]
- Azami, T.; Niakousari, M.; Hashemi, S.M.B.; Torri, L. A three-step sensory-based approach to maximize consumer acceptability for new low-sugar licorice-chocolate-flavored milk drink. LWT Food Sci. Technol. 2018, 91, 375–381. [Google Scholar] [CrossRef]
- Bai, L.; Xie, J.; Liu, J.; Xie, Y. Effect of salt on hygroscopic properties of cement mortar. Constr. Build. Mater. 2021, 305, 124746. [Google Scholar] [CrossRef]
- Tontul, I.; Topuz, A. Spray-drying of fruit and vegetable juices: Effect of drying conditions on the product yield and physical properties. Trends Food Sci. Technol. 2017, 63, 91–102. [Google Scholar] [CrossRef]
- Dias, C.O.; dos Santos Opuski de Almeida, J.; Pinto, S.S.; de Oliveira Santana, F.C.; Verruck, S.; Müller, C.M.O.; Prudêncio, E.S.; de Mello Castanho Amboni, R.D. Development and physico-chemical characterization of microencapsulated bifidobacteria in passion fruit juice: A functional non-dairy product for probiotic delivery. Food Biosci. 2018, 24, 26–36. [Google Scholar] [CrossRef]
- Pinto, S.S.; Fritzen-Freire, C.B.; Benedetti, S.; Murakami, F.S.; Petrus, J.C.C.; Prudêncio, E.S.; Amboni, R.D. Potential use of whey concentrate and prebiotics as carrier agents to protect Bifidobacterium-BB-12 microencapsulated by spray drying. Food Res. Int. 2015, 67, 400–408. [Google Scholar] [CrossRef]
- Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of grape (Vitis labrusca var. Bordo) skin phenolic extract using gum Arabic, polydextrose, and partially hydrolyzed guar gum as encapsulating agents. Food Chem. 2016, 194, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Pasrija, D.; Ezhilarasi, P.; Indrani, D.; Anandharamakrishnan, C. Microencapsulation of green tea polyphenols and its effect on incorporated bread quality. LWT Food Sci. Technol. 2015, 64, 289–296. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze-drying and spray-drying using different coating materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef]
- Chen, C.; Chi, Y.J.; Xu, W. Comparisons on the Functional Properties and Antioxidant Activity of Spray-Dried and Freeze-Dried Egg White Protein Hydrolysate. Food Bioprocess Technol. 2012, 5, 2342–2352. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Świeca, M.; Gawlik-Dziki, U. Soymilk enriched with green coffee phenolics—Antioxidant and nutritional properties in the light of phenolics-food matrix interactions. Food Chem. 2017, 223, 1–7. [Google Scholar] [CrossRef]
- Mareček, V.; Mikyška, A.; Hampel, D.; Čejka, P.; Neuwirthová, J.; Malachová, A.; Cerkal, R. ABTS and DPPH methods as a tool for studying antioxidant capacity of spring barley and malt. J. Cereal Sci. 2017, 73, 40–45. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Martínez-Carballo, E.; Cambeiro-Pérez, N.; Rial-Otero, R.; Figueiredo-González, M.; Cancho-Grande, B. Applicability of an in-vitro digestion model to assess the bioaccessibility of phenolic compounds from olive-related products. Molecules 2021, 26, 6667. [Google Scholar] [CrossRef]
- Kreft, I.; Germ, M.; Golob, A.; Vombergar, B.; Bonafaccia, F.; Luthar, Z. Impact of Rutin and Other Phenolic Substances on the Digestibility of Buckwheat Grain Metabolites. Int. J. Mol. Sci. 2022, 23, 3923. [Google Scholar] [CrossRef]
- Pereira, K.C.P.; Ferreira, D.C.M.; Alvarenga, G.F.; Pereira, M.S.S.; Barcelos, M.C.S.; da Costa, J.M.G. Microencapsulação e liberação controlada por difusão de ingredientes alimentícios produzidos através da secagem por atomização: Revisão. Braz. J. Food Technol. 2018, 21, 1–9. [Google Scholar] [CrossRef]
- Campelo-Felix, P.H.; Souza, H.J.B.; Figueiredo, J.D.A.; Fernandes, R.V.D.B.; Botrel, D.A.; de Oliveira, C.R.; Yoshida, M.I.; Borges, S.V. Prebiotic carbohydrates: Effect on reconstitution, storage, release, and antioxidant properties of lime essential oil microparticles. J. Agric. Food Chem. 2017, 65, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Narita, Y.; Inouye, K. Chlorogenic Acids from Coffee; Elsevier Inc.: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Grebby, S.; Fisk, I.D. Non-destructive analysis of sucrose, caffeine and trigonelline on single green coffee beans by hyperspectral imaging. Food Res. Int. 2018, 106, 193–203. [Google Scholar] [CrossRef]
- Wang, R.; Xue, J.; Meng, L.; Lee, J.-W.; Zhao, Z.; Sun, P.; Cai, L.; Huang, T.; Wang, Z.; Wang, Z.-K.; et al. Caffeine Improves the Performance and Thermal Stability of Perovskite Solar Cells. Joule 2019, 3, 1464–1477. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Oliveira, A.; Campos, D.; Nunes, J.; Vicente, A.A.; Pintado, M. Simulated digestion of an olive pomace water-soluble ingredient: Relationship between the bioaccessibility of compounds and their potential health benefits. Food Funct. 2020, 11, 2238–2254. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.A.; Bolling, B.W. A review of the efficacy of dietary polyphenols in experimental models of inflammatory bowel diseases. Food Funct. 2015, 6, 1773–1786. [Google Scholar] [CrossRef]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ullah, R.; Ajmal, M.; Jaspal, M.H. Antioxidant properties of Milk and dairy products: A comprehensive review of the current knowledge. Lipids Health Dis. 2019, 18, 41. [Google Scholar] [CrossRef]
- Grancieri, M.; Martino, H.S.D.; de Mejia, E.G. Chia Seed (Salvia hispanica L.) as a Source of Proteins and Bioactive Peptides with Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 480–499. [Google Scholar] [CrossRef] [PubMed]
- Fatkullin, R.; Naumenko, N.; Popova, N.; Ruskina, A.; Kalinina, I.; Potoroko, I. Explaining consumer intentions for foods with antioxidant properties: Predictors of choice and purchase barriers. Int. J. Food Sci. 2021, 2021, 9971425. [Google Scholar] [CrossRef] [PubMed]
| Analysis | Encapsulation Technique | Encapsulating Agent | |
|---|---|---|---|
| PD | IN | ||
| Moisture (g/100 g) | SD | 4.19 ± 0.26 aA | 4.22 ± 0.48 aA |
| FD | 3.30 ± 0.69 aA | 0.35 ± 0.03 bB | |
| Water activity | SD | 0.35 ± 0.03 aA | 0.33 ± 0.01 aA |
| FD | 0.16 ± 0.04 bA | 0.03 ± 0.001 bB | |
| pH | SD | 5.34 ± 0.07 aA | 5.76 ± 0.09 aB |
| FD | 5.27 ± 0.04 aA | 5.75 ± 0.08 aB | |
| Hygroscopicity (%) | SD | 18.40 ± 0.34 aA | 14.55 ± 0.46 aB |
| FD | 19.91 ± 0.52 bA | 14.92 ± 0.97 aB | |
| Solubility (%) | SD | 97.23 ± 2.23 aA | 98.42 ± 1.66 aA |
| FD | 97.79 ± 1.70 aA | 96.76 ± 3.55 aA | |
| Total Phenolic Compounds (mg GAE/g) | SD | 19.01 ± 5.96 aA | 21.08 ± 2.97 aA |
| FD | 22.43 ± 2.77 aA | 21.17 ± 1.59 aA | |
| Antioxidant activity (ABTS) (µmol Trolox/g) | SD | 153.96 ± 16.93 aA | 143.53 ± 13.71 aA |
| FD | 154.76 ± 28.94 aA | 166.45 ± 30.35 aA | |
| Antioxidant activity (DPPH) (µmol Trolox/g) | SD | 110.85 ± 24.89 aA | 105.03 ± 22.91 aA |
| FD | 88.45 ± 13.77 aA | 97.95 ± 19.07 aA | |
| Caffeine (mg/g) | SD | 9.59 ± 0.91 aA | 9.67 ± 0.51 aA |
| FD | 9.22 ± 0.31 aA | 9.29 ± 0.25 aA | |
| Chlorogenic acid (mg/g) | SD | 18.71 ± 1.79 aA | 18.60 ± 0.98 aA |
| FD | 17.79 ± 0.50 aA | 17.88 ± 0.50 aA | |
| Trigonelline (mg/g) | SD | 5.73 ± 0.45 aA | 4.87 ± 0.41 aB |
| FD | 5.31 ± 0.16 aA | 4.55 ± 0.38 aA | |
| Samples | Storage Time | |||
|---|---|---|---|---|
| T 0 h | T 168 h | % of Remaining | ||
| Total phenolic compounds (mg GAE/mL) | CT | 2.20 ± 0.04 | 2.41 ± 0.07 | 108.79 ± 1.74 |
| PD-SD | 3.42 ± 0.02 * a.A | 3.25 ± 0.10 * a.A | 90.29 ± 8.09 * a | |
| IN-SD | 3.27 ± 0.10 * ab.A | 3.20 ± 0.14 * a.A | 94.24 ± 2.31 * a | |
| PD-FD | 3.30± 0.12 * ab.A | 3.15 ± 0.04 * a.A | 92.34 ± 3.12 * a | |
| IN-FD | 3.00± 0.08 * b.A | 3.05 ± 0.07 * a.A | 96.71 ± 6.38 a | |
| Antioxidant activity (ABTS) (µmol Trolox/mL) | CT | 84.59 ± 5.07 | 85.35 ± 2.31 | 98.91 ± 2.02 |
| PD-SD | 102.90 ± 4.03 * a.A | 101.25 ± 1.35 * a.A | 93.47 ± 2.29 a | |
| IN-SD | 103.78 ± 6.00 * a.A | 104.43 ± 1.30 * a.A | 97.09 ± 5.58 a | |
| PD-FD | 105.41 ± 2.41 * a.A | 109.30 ± 3.90 * a.A | 100.24 ± 2.59 a | |
| IN-FD | 105.33 ± 4.79 * a.A | 107.62 ± 0.95 * a.A | 99.77 ± 6.93 a | |
| Antioxidant activity (DPPH) (µmol Trolox/mL) | CT | 11.31 ± 0.66 | 2.82 ± 1.51 | 24.72 ± 10.72 |
| PD-SD | 12.89 ± 0.35 a.A | 9.01 ± 1.12 * a.B | 66.23 ± 3.43* a | |
| IN-SD | 14.56 ± 0.58 * a.A | 6.7 ± 0.37 * a.B | 44.92 ± 3.74 a | |
| PD-FD | 14.02 ± 0.83 a.A | 7.77 ± 1.22 * a.B | 53.87 ± 11.42 a | |
| IN-FD | 13.53 ± 0.80 a.A | 8.13 ± 2.94 * a.B | 57.87 ± 149.59 a | |
| Samples | Attributes | |||||
|---|---|---|---|---|---|---|
| Appearance | Aroma | Body | Flavor | Overall Impression | Purchase Intent | |
| CT | 7.97 | 7.30 | 7.26 | 7.68 | 7.58 | 4.04 |
| PD-SD | 7.20 *a | 7.24 a | 7.11 a | 7.32 *a | 7.28 *a | 3.74 *a |
| IN-SD | 7.31 *a | 7.05 a | 7.26 a | 7.30 *a | 7.31 *a | 3.74 *a |
| PD-FD | 7.16 *a | 7.18 a | 7.26 a | 7.76 b | 7.43 ab | 3.93 ab |
| IN-FD | 7.31 *a | 7.26 a | 7.23 a | 7.81 b | 7.64 b | 3.97 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Carmo, L.B.; Benincá, D.B.; Grancieri, M.; Pereira, L.V.; Lima Filho, T.; Saraiva, S.H.; Silva, P.I.; Oliveira, D.d.S.; Costa, A.G.V. Green Coffee Extract Microencapsulated: Physicochemical Characteristics, Stability, Bioaccessibility, and Sensory Acceptability through Dairy Beverage Consumption. Int. J. Environ. Res. Public Health 2022, 19, 13221. https://doi.org/10.3390/ijerph192013221
do Carmo LB, Benincá DB, Grancieri M, Pereira LV, Lima Filho T, Saraiva SH, Silva PI, Oliveira DdS, Costa AGV. Green Coffee Extract Microencapsulated: Physicochemical Characteristics, Stability, Bioaccessibility, and Sensory Acceptability through Dairy Beverage Consumption. International Journal of Environmental Research and Public Health. 2022; 19(20):13221. https://doi.org/10.3390/ijerph192013221
Chicago/Turabian Styledo Carmo, Laísa Bernabé, Daiane Bonizioli Benincá, Mariana Grancieri, Lucélia Vieira Pereira, Tarcísio Lima Filho, Sérgio Henriques Saraiva, Pollyanna Ibrahim Silva, Daniela da Silva Oliveira, and André Gustavo Vasconcelos Costa. 2022. "Green Coffee Extract Microencapsulated: Physicochemical Characteristics, Stability, Bioaccessibility, and Sensory Acceptability through Dairy Beverage Consumption" International Journal of Environmental Research and Public Health 19, no. 20: 13221. https://doi.org/10.3390/ijerph192013221
APA Styledo Carmo, L. B., Benincá, D. B., Grancieri, M., Pereira, L. V., Lima Filho, T., Saraiva, S. H., Silva, P. I., Oliveira, D. d. S., & Costa, A. G. V. (2022). Green Coffee Extract Microencapsulated: Physicochemical Characteristics, Stability, Bioaccessibility, and Sensory Acceptability through Dairy Beverage Consumption. International Journal of Environmental Research and Public Health, 19(20), 13221. https://doi.org/10.3390/ijerph192013221








