The Effect of Smoking Cessation on Body Weight and Other Metabolic Parameters with Focus on People with Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Smoking Cessation and Body Weight in Numbers
2.1. PSCWG in General Population
2.2. The Amount of PSCWG in People with Diabetes
2.3. Factors That Affect PSCWG
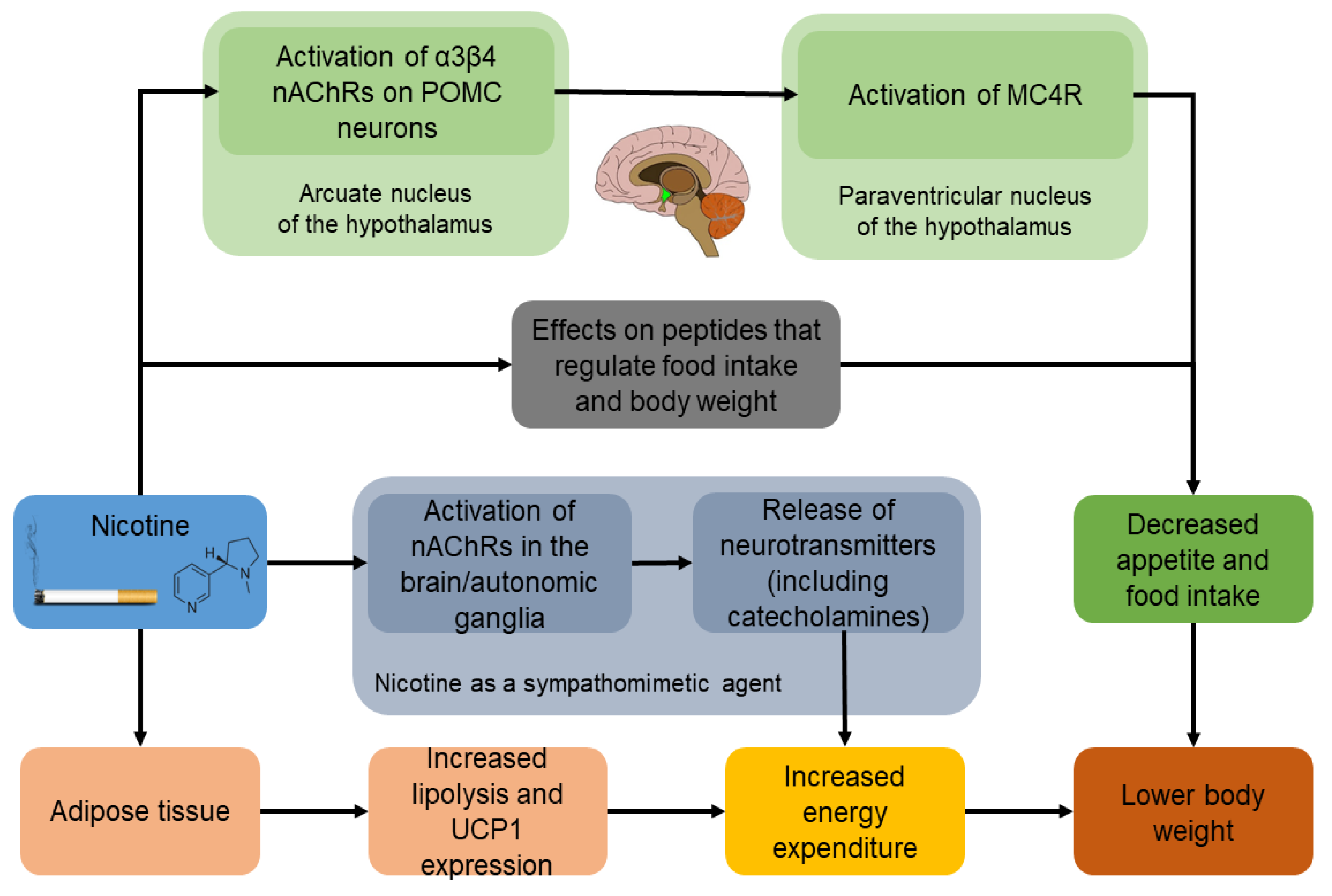
3. Mechanisms of How Smoking Cessation Leads to Weight Gain
3.1. Effect of Nicotine on Appetite and Food Intake
3.2. Effect of Nicotine on Eating Behaviour
3.3. Effect of Nicotine on Energy Expenditure
3.4. Effect of Nicotine on Lipolysis and Fat Oxidation
3.5. Inverse Results of Smoking in the Long-Term
4. Effects of Nicotine and Smoking Cessation on Appetite-Regulating Peptides
4.1. NPY
4.2. Orexins
4.3. Leptin
4.4. Adiponectin
4.5. Ghrelin
4.6. PYY, GLP-1 and CCK
5. Smoking Cessation and Weight Gain on T2DM and Metabolic Profile
5.1. Cigarette Smoking and Risk of Diabetes
5.2. The Effect of Smoking Cessation and PSCWG on Risk of Diabetes
5.3. The Relation of Smoking to Glycemic Control and Complications in Persons with T2DM
5.4. Smoking Cessation and Weight Gain for People with T2DM
5.5. Smoking Cessation and Macro- and Microvascular Diabetic Complications
6. Medications for Smoking Cessation and Weight Gain
6.1. NRT
6.2. Bupropion
6.3. Varenicline
6.4. GLP-1R Agonists
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bush, T.; Hsu, C.; Levine, M.D.; Magnusson, B.; Miles, L. Weight gain and smoking: Perceptions and experiences of obese quitline participants. BMC Public Health 2014, 14, 1229. [Google Scholar] [CrossRef] [PubMed]
- Beebe, L.A.; Bush, T. Post-cessation weight concerns among women calling a state tobacco quitline. Am. J. Prev. Med. 2015, 48, S61–S64. [Google Scholar] [CrossRef] [PubMed]
- Landrau-Cribbs, E.; Cabriales, J.A.; Cooper, T.V. General and smoking cessation weight concern in a Hispanic sample of light and intermittent smokers. Addict. Behav. 2015, 41, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Siahpush, M.; Singh, G.K.; Tibbits, M.; Pinard, C.A.; Shaikh, R.A.; Yaroch, A. It is better to be a fat ex-smoker than a thin smoker: Findings from the 1997–2004 National Health Interview Survey—National Death Index linkage study. Tob. Control 2014, 23, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.F.; Loprinzi, P.D. Association of BMI changes between adolescence and young adulthood with smoking cessation. Am. J. Health Promot. 2019, 33, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Karfopoulou, E.; Mouliou, K.; Koutras, Y.; Yannakoulia, M. Behaviours associated with weight loss maintenance and regaining in a M editerranean population sample. A qualitative study. Clin. Obes. 2013, 3, 141–149. [Google Scholar] [PubMed]
- Yannakoulia, M.; Anastasiou, C.; Zachari, K.; Sidiropoulou, M.; Katsaounou, P.; Tenta, R. Acute effect of smoking and smoking abstinence on energy intake and appetite-related hormones blood concentrations. Physiol. Behav. 2018, 184, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Schauer, G.L.; Bush, T.; Cerutti, B.; Mahoney, L.; Thompson, J.R.; Zbikowski, S.M. Peer Reviewed: Use and Effectiveness of Quitlines for Smokers With Diabetes: Cessation and Weight Outcomes, Washington State Tobacco Quit Line, 2008. Prev. Chronic Dis. 2013, 10, E105. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Audrain-McGovern, J.; Benowitz, N. Cigarette smoking, nicotine, and body weight. Clin. Pharmacol. Ther. 2011, 90, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.F.; Madans, J.; Anda, R.F.; Kleinman, J.C.; Giovino, G.A.; Byers, T. Smoking cessation and severity of weight gain in a national cohort. N. Engl. J. Med. 1991, 324, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Kruger, J.; Ham, S.A.; Prohaska, T.R. Behavioral risk factors associated with overweight and obesity among older adults: The 2005 National Health Interview Survey. Prev. Chronic Dis. 2009, 6, A14. [Google Scholar] [PubMed]
- Bush, T.; Lovejoy, J.C.; Deprey, M.; Carpenter, K.M. The effect of tobacco cessation on weight gain, obesity, and diabetes risk. Obesity 2016, 24, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Mackay, D.F.; Gray, L.; Pell, J.P. Impact of smoking and smoking cessation on overweight and obesity: Scotland-wide, cross-sectional study on 40,036 participants. BMC Public Health 2013, 13, 348. [Google Scholar] [CrossRef] [PubMed]
- Lycett, D.; Munafò, M.; Johnstone, E.; Murphy, M.; Aveyard, P. Associations between weight change over 8 years and baseline body mass index in a cohort of continuing and quitting smokers. Addiction 2011, 106, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Aubin, H.-J.; Farley, A.; Lycett, D.; Lahmek, P.; Aveyard, P. Weight gain in smokers after quitting cigarettes: Meta-analysis. BMJ 2012, 345, e4439. [Google Scholar] [CrossRef]
- Clair, C.; Rigotti, N.A.; Porneala, B.; Fox, C.S.; D’Agostino, R.B.; Pencina, M.J.; Meigs, J.B. Association of smoking cessation and weight change with cardiovascular disease among adults with and without diabetes. JAMA 2013, 309, 1014–1021. [Google Scholar] [CrossRef]
- Tian, J.; Venn, A.; Otahal, P.; Gall, S. The association between quitting smoking and weight gain: A systemic review and meta-analysis of prospective cohort studies. Obes. Rev. 2015, 16, 883–901. [Google Scholar] [CrossRef]
- Veldheer, S.; Yingst, J.; Zhu, J.; Foulds, J. Ten-year weight gain in smokers who quit, smokers who continued smoking and never smokers in the United States, NHANES 2003–2012. Int. J. Obes. 2015, 39, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Danaei, G.; Manson, J.E.; Robins, J.M.; Hernán, M.A. Weight gain after smoking cessation and lifestyle strategies to reduce it. Epidemiology 2020, 31, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Lycett, D.; Nichols, L.; Ryan, R.; Farley, A.; Roalfe, A.; Mohammed, M.A.; Szatkowski, L.; Coleman, T.; Morris, R.; Farmer, A. The association between smoking cessation and glycaemic control in patients with type 2 diabetes: A THIN database cohort study. Lancet Diabetes Endocrinol. 2015, 3, 423–430. [Google Scholar] [CrossRef]
- Liu, G.; Hu, Y.; Zong, G.; Pan, A.; Manson, J.E.; Rexrode, K.M.; Rimm, E.B.; Hu, F.B.; Sun, Q. Smoking cessation and weight change in relation to cardiovascular disease incidence and mortality in people with type 2 diabetes: A population-based cohort study. Lancet Diabetes Endocrinol. 2020, 8, 125–133. [Google Scholar] [CrossRef]
- Tonstad, S.; Lawrence, D. Varenicline in smokers with diabetes: A pooled analysis of 15 randomized, placebo-controlled studies of varenicline. J. Diabetes Investig. 2017, 8, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Rohsenow, D.J.; Johnson, K.C.; Wing, R.R. Smoking and weight loss among smokers with overweight and obesity in Look AHEAD. Health Psychol. 2018, 37, 399. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, P.; Connett, J.E.; Lee, W.W.; Nides, M.; Murray, R.; Wise, R. Early and late weight gain following smoking cessation in the Lung Health Study. Am. J. Epidemiol. 1998, 148, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Kmetova, A.; Kralikova, E.; Stepankova, L.; Zvolska, K.; Blaha, M.; Sticha, M.; Bortlicek, Z.; Schroeder, D.R.; Croghan, I.T. Factors associated with weight changes in successful quitters participating in a smoking cessation program. Addict. Behav. 2014, 39, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, M.; Wada, H.; Ura, S.; Yamakage, H.; Satoh-Asahara, N.; Shimatsu, A.; Koyama, H.; Kono, K.; Takahashi, Y.; Hasegawa, K. Analysis of factors that determine weight gain during smoking cessation therapy. PLoS ONE 2013, 8, e72010. [Google Scholar] [CrossRef] [PubMed]
- Chiolero, A.; Faeh, D.; Paccaud, F.; Cornuz, J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am. J. Clin. Nutr. 2008, 87, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Prod’hom, S.; Locatelli, I.; Giraudon, K.; Marques-Vidal, P.; Clair, C.; Bize, R.; Cornuz, J. Predictors of weight change in sedentary smokers receiving a standard smoking cessation intervention. Nicotine Tobac. Res. 2013, 15, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, I.; Collet, T.-H.; Clair, C.; Rodondi, N.; Cornuz, J. The joint influence of gender and amount of smoking on weight gain one year after smoking cessation. Int. J. Environ. Res. Public Health 2014, 11, 8443–8455. [Google Scholar] [CrossRef] [PubMed]
- Pankova, A.; Kralikova, E.; Zvolska, K.; Stepankova, L.; Blaha, M.; Ovesna, P.; Aveyard, P. Early weight gain after stopping smoking: A predictor of overall large weight gain? A single-site retrospective cohort study. BMJ Open 2018, 8, e023987. [Google Scholar] [CrossRef] [PubMed]
- Filozof, C.; Fernandez Pinilla, M.; Fernández-Cruz, A. Smoking cessation and weight gain. Obes. Rev. 2004, 5, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.K.; Zopey, M.; Friedman, T.C. Metabolic effects of smoking cessation. Nat. Rev. Endocrinol. 2016, 12, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Yang, Z.; Li, M.D. Pharmacological effects and regulatory mechanisms of tobacco smoking effects on food intake and weight control. J. Neuroimmune Pharmacol. 2018, 13, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Calarco, C.A.; Picciotto, M.R. Nicotinic acetylcholine receptor signaling in the hypothalamus: Mechanisms related to nicotine’s effects on food intake. Nicotine Tob. Res. 2020, 22, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Geha, P.Y.; Aschenbrenner, K.; Felsted, J.; O’Malley, S.S.; Small, D.M. Altered hypothalamic response to food in smokers. Am. J. Clin. Nutr. 2013, 97, 15–22. [Google Scholar] [CrossRef]
- Mineur, Y.S.; Abizaid, A.; Rao, Y.; Salas, R.; DiLeone, R.J.; Gündisch, D.; Diano, S.; De Biasi, M.; Horvath, T.L.; Gao, X.-B. Nicotine decreases food intake through activation of POMC neurons. Science 2011, 332, 1330–1332. [Google Scholar] [CrossRef]
- Hughes, J.R. Effects of abstinence from tobacco: Etiology, animal models, epidemiology, and significance: A subjective review. Nicotine Tob. Res. 2007, 9, 329–339. [Google Scholar] [CrossRef]
- Jo, Y.H.; Talmage, D.A.; Role, L.W. Nicotinic receptor-mediated effects on appetite and food intake. J. Neurobiol. 2002, 53, 618–632. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Fowler, J.S.; Telang, F. Overlapping neuronal circuits in addiction and obesity: Evidence of systems pathology. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 3191–3200. [Google Scholar] [CrossRef]
- Benowitz, N.L. Nicotine addiction. N. Engl. J. Med. 2010, 362, 2295–2303. [Google Scholar] [CrossRef]
- Ioannides-Demos, L.L.; Piccenna, L.; McNeil, J.J. Pharmacotherapies for obesity: Past, current, and future therapies. J. Obes. 2010, 2011, 179674. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L. Cigarette smoking and cardiovascular disease: Pathophysiology and implications for treatment. Prog. Cardiovasc. Dis. 2003, 1, 91–111. [Google Scholar] [CrossRef]
- Hofstetter, A.; Schutz, Y.; Jéquier, E.; Wahren, J. Increased 24-hour energy expenditure in cigarette smokers. N. Engl. J. Med. 1986, 314, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Perkins, K.A. Metabolic effects of cigarette smoking. J. Appl. Physiol. 1992, 72, 401–409. [Google Scholar] [CrossRef]
- Jensen, E.X.; Fusch, C.; Jaeger, P.; Peheim, E.; Horber, F.F. Impact of chronic cigarette smoking on body composition and fuel metabolism. J. Clin. Endocrinol. Metab. 1995, 80, 2181–2185. [Google Scholar]
- Andersson, K.; Arner, P. Systemic nicotine stimulates human adipose tissue lipolysis through local cholinergic and catecholaminergic receptors. Int. J. Obes. 2001, 25, 1225–1232. [Google Scholar] [CrossRef]
- Arai, K.; Kim, K.; Kaneko, K.; Iketani, M.; Otagiri, A.; Yamauchi, N.; Shibasaki, T. Nicotine infusion alters leptin and uncoupling protein 1 mRNA expression in adipose tissues of rats. Am. J. Physiol.-Endocrinol. Metab. 2001, 280, E867–E876. [Google Scholar] [CrossRef]
- Pisinger, C.; Jorgensen, T. Waist circumference and weight following smoking cessation in a general population: The Inter99 study. Prev. Med. 2007, 44, 290–295. [Google Scholar] [CrossRef]
- Stadler, M.; Tomann, L.; Storka, A.; Wolzt, M.; Peric, S.; Bieglmayer, C.; Pacini, G.; Dickson, S.L.; Brath, H.; Bech, P. Effects of smoking cessation on b-cell function, insulin sensitivity, body weight, and appetite. Eur. J. Endocrinol. 2014, 170, 219–227. [Google Scholar] [CrossRef]
- Rom, O.; Reznick, A.Z.; Keidar, Z.; Karkabi, K.; Aizenbud, D. Smoking cessation-related weight gain—Beneficial effects on muscle mass, strength and bone health. Addiction 2015, 110, 326–335. [Google Scholar] [CrossRef]
- Clair, C.; Chiolero, A.; Faeh, D.; Cornuz, J.; Marques-Vidal, P.; Paccaud, F.; Mooser, V.; Waeber, G.; Vollenweider, P. Dose-dependent positive association between cigarette smoking, abdominal obesity and body fat: Cross-sectional data from a population-based survey. BMC Public Health 2011, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.; Bellissimo, N. Nicotine and energy balance: A review examining the effect of nicotine on hormonal appetite regulation and energy expenditure. Appetite 2021, 164, 105260. [Google Scholar] [CrossRef] [PubMed]
- Frankish, H.M.; Dryden, S.; Wang, Q.; Bing, C.; MacFarlane, I.A.; Williams, G. Nicotine administration reduces neuropeptide Y and neuropeptide Y mRNA concentrations in the rat hypothalamus: NPY may mediate nicotine’s effects on energy balance. Brain Res. 1995, 694, 139–146. [Google Scholar] [CrossRef]
- Bishop, C.; Parker, G.C.; Coscina, D.V. Nicotine and its withdrawal alter feeding induced by paraventricular hypothalamic injections of neuropeptide Y in Sprague-Dawley rats. Psychopharmacology 2002, 162, 265–272. [Google Scholar] [CrossRef]
- Chen, H.; Hansen, M.J.; Jones, J.E.; Vlahos, R.; Bozinovski, S.; Anderson, G.P.; Morris, M.J. Cigarette smoke exposure reprograms the hypothalamic neuropeptide Y axis to promote weight loss. Am. J. Respir. Crit. Care Med. 2006, 173, 1248–1254. [Google Scholar] [CrossRef]
- Li, M.D.; Kane, J.K.; Parker, S.L.; McAllen, K.; Matta, S.G.; Sharp, B.M. Nicotine administration enhances NPY expression in the rat hypothalamus. Brain Res. 2000, 867, 157–164. [Google Scholar] [CrossRef]
- Li, M.D.; Parker, S.L.; Kane, J.K. Regulation of feeding-associated peptides and receptors by nicotine. Mol. Neurobiol. 2000, 22, 143–165. [Google Scholar] [CrossRef]
- Kane, J.; Parker, S.; Li, M. Hypothalamic orexin-A binding sites are downregulated by chronic nicotine treatment in the rat. Neurosci. Lett. 2001, 298, 1–4. [Google Scholar] [CrossRef]
- Rudehill, A.; Franco-Cereceda, A.; Hemsén, A.; Stensdotter, M.; Pernow, J.; Lundberg, J. Cigarette smoke-induced elevation of plasma neuropeptide Y levels in man. Clin. Physiol. 1989, 9, 243–248. [Google Scholar] [CrossRef]
- Niedermaier, O.N.; Smith, M.L.; Beightol, L.A.; Zukowska-Grojec, Z.; Goldstein, D.S.; Eckberg, D.L. Influence of cigarette smoking on human autonomic function. Circulation 1993, 88, 562–571. [Google Scholar] [CrossRef]
- Hussain, T.; Al-Daghri, N.M.; Al-Attas, O.S.; Draz, H.M.; Al-Rahman, S.H.A.; Yakout, S.M. Plasma neuropeptide Y levels relate cigarette smoking and smoking cessation to body weight regulation. Regul. Pept. 2012, 176, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.; Parker, S.; Matta, S.; Fu, Y.; Sharp, B.; Li, M. Nicotine up-regulates expression of orexin and its receptors in rat brain. Endocrinology 2000, 141, 3623–3629. [Google Scholar] [CrossRef] [PubMed]
- al’Absi, M.; Lemieux, A.; Hodges, J.S.; Allen, S. Circulating orexin changes during withdrawal are associated with nicotine craving and risk for smoking relapse. Addict. Biol. 2019, 24, 743–753. [Google Scholar] [CrossRef]
- Perkins, K.A.; Fonte, C. Effects of smoking status and smoking cessation on leptin levels. Nicotine Tob. Res. 2002, 4, 459–466. [Google Scholar] [CrossRef]
- Bergmann, S.; Siekmeier, R. Influence of smoking and body weight on adipokines in middle aged women. Eur. J. Med. Res. 2009, 14, 21. [Google Scholar] [CrossRef][Green Version]
- Hodge, A.; Westerman, R.; De Courten, M.; Collier, G.R.; Zimmet, P.; Alberti, K. Is leptin sensitivity the link between smoking cessation and weight gain? Int. J. Obes. 1997, 21, 50–53. [Google Scholar] [CrossRef]
- Al Mutairi, S.S.; Mojiminiyi, O.A.; Shihab-Eldeen, A.A.; Al Sharafi, A.; Abdella, N. Effect of smoking habit on circulating adipokines in diabetic and non-diabetic subjects. Ann. Nutr. Metab. 2008, 52, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Hilawe, E.H.; Yatsuya, H.; Li, Y.; Uemura, M.; Wang, C.; Chiang, C.; Toyoshima, H.; Tamakoshi, K.; Zhang, Y.; Kawazoe, N. Smoking and diabetes: Is the association mediated by adiponectin, leptin, or C-reactive protein? J. Epidemiol. 2015, 25, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, B.; Smith, U. Leptin levels in smokers and long-term users of nicotine gum. Eur. J. Clin. Investig. 1999, 29, 145–152. [Google Scholar] [CrossRef]
- Nicklas, B.J.; Tomoyasu, N.; Muir, J.; Goldberg, A.P. Effects of cigarette smoking and its cessation on body weight and plasma leptin levels. Metabolism 1999, 48, 804–808. [Google Scholar] [CrossRef]
- Kryfti, M.; Dimakou, K.; Toumbis, M.; Daniil, Z.; Hatzoglou, C.; Gourgoulianis, K.I. Effects of smoking cessation on serum leptin and adiponectin levels. Tob. Induc. Dis. 2015, 13, 30. [Google Scholar] [CrossRef]
- Glahn, A.; Rhein, M.; Frieling, H.; Dette, F.; Bleich, S.; Hillemacher, T.; Muschler, M. Smoking-induced changes in leptin serum levels and c/EBPalpha-related methylation status of the leptin core promotor during smoking cessation. Psychoneuroendocrinology 2019, 100, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Pankova, A.; Kralikova, E.; Kavalkova, P.; Stepankova, L.; Zvolska, K.; Haluzik, M. No change in serum incretins levels but rise of leptin levels after smoking cessation: A pilot study. Physiol. Res. 2016, 65. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Joe, K.-H.; Kim, W.; Park, J.; Lee, D.-H.; Sung, K.-W.; Kim, D.-J. Increased leptin and decreased ghrelin level after smoking cessation. Neurosci. Lett. 2006, 409, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, A.; Nakajima, M.; Hatsukami, D.K.; Allen, S.; al’Absi, M. Changes in circulating leptin levels during the initial stage of cessation are associated with smoking relapse. Psychopharmacology 2015, 232, 3355–3361. [Google Scholar] [CrossRef]
- Gonseth, S.; Locatelli, I.; Bize, R.; Nusslé, S.; Clair, C.; Pralong, F.; Cornuz, J. Leptin and smoking cessation: Secondary analyses of a randomized controlled trial assessing physical activity as an aid for smoking cessation. BMC Public Health 2014, 14, 911. [Google Scholar] [CrossRef][Green Version]
- Komiyama, M.; Wada, H.; Yamakage, H.; Satoh-Asahara, N.; Sunagawa, Y.; Morimoto, T.; Ozaki, Y.; Shimatsu, A.; Takahashi, Y.; Hasegawa, K. Analysis of changes on adiponectin levels and abdominal obesity after smoking cessation. PLoS ONE 2018, 13, e0201244. [Google Scholar] [CrossRef] [PubMed]
- Iwashima, Y.; Katsuya, T.; Ishikawa, K.; Kida, I.; Ohishi, M.; Horio, T.; Ouchi, N.; Ohashi, K.; Kihara, S.; Funahashi, T. Association of hypoadiponectinemia with smoking habit in men. Hypertension 2005, 45, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Arita, Y.; Nishida, M.; Muraguchi, M.; Ouchi, N.; Takahashi, M.; Igura, T.; Inui, Y.; Kihara, S.; Nakamura, T. An adipocyte-derived plasma protein, adiponectin, adheres to injured vascular walls. Horm. Metab. Res. 2000, 32, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Toffolo, M.C.F.; da Silva Gomes, A.; van Keulen, H.V.; Louro, M.B.; Castro, F.M.; Luquetti, S.C.P.D.; Ferreira, A.P.; de Aguiar, A.S. Alteration of inflammatory adipokines after four months of smoking abstinence in multidisciplinary intervention program. Nutr. Hosp. 2018, 35, 434–441. [Google Scholar] [PubMed]
- Miyazaki, T.; Shimada, K.; Mokuno, H.; Daida, H. Adipocyte derived plasma protein, adiponectin, is associated with smoking status in patients with coronary artery disease. Heart 2003, 89, 663. [Google Scholar] [CrossRef][Green Version]
- Takefuji, S.; Yatsuya, H.; Tamakoshi, K.; Otsuka, R.; Wada, K.; Matsushita, K.; Sugiura, K.; Hotta, Y.; Mitsuhashi, H.; Oiso, Y. Smoking status and adiponectin in healthy Japanese men and women. Prev. Med. 2007, 45, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Won, W.-Y.; Lee, C.-U.; Chae, J.-H.; Kim, J.-J.; Lee, C.; Kim, D.-J. Changes of plasma adiponectin levels after smoking cessation. Psychiatr. Investig. 2014, 11, 173. [Google Scholar] [CrossRef][Green Version]
- Kotani, K.; Hazama, A.; Hagimoto, A.; Saika, K.; Shigeta, M.; Katanoda, K.; Nakamura, M. Adiponectin and smoking status: A systematic review. J. Atheroscler. Thromb. 2012, 19, 11833. [Google Scholar] [CrossRef] [PubMed]
- Thamer, C.; Stefan, N.; Stumvoll, M.; Häring, H.; Fritsche, A. Reduced adiponectin serum levels in smokers. Atherosclerosis 2005, 179, 421–422. [Google Scholar] [CrossRef]
- Efstathiou, S.P.; Skeva, I.I.; Dimas, C.; Panagiotou, A.; Parisi, K.; Tzanoumis, L.; Kafouri, A.; Bakratsas, K.; Mountokalakis, T.D. Smoking cessation increases serum adiponectin levels in an apparently healthy Greek population. Atherosclerosis 2009, 205, 632–636. [Google Scholar] [CrossRef]
- Otsuka, F.; Kojima, S.; Maruyoshi, H.; Kojima, S.; Matsuzawa, Y.; Funahashi, T.; Kaikita, K.; Sugiyama, S.; Kimura, K.; Umemura, S. Smoking cessation is associated with increased plasma adiponectin levels in men. J. Cardiol. 2009, 53, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Takeshima, F.; Kadota, K.; Yoda, A.; Tatsuta, Y.; Nagaura, Y.; Yoshioka, S.; Nakamichi, S.; Nakao, K.; Ozono, Y. Early effects of smoking cessation and weight gain on plasma adiponectin levels and insulin resistance. Intern. Med. 2011, 50, 707–712. [Google Scholar] [CrossRef]
- Bouros, D.; Tzouvelekis, A.; Anevlavis, S.; Doris, M.; Tryfon, S.; Froudarakis, M.; Zournatzi, V.; Kukuvitis, A. Smoking acutely increases plasma ghrelin concentrations. Clin. Chem. 2006, 52, 777–778. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koopmann, A.; Bez, J.; Lemenager, T.; Hermann, D.; Dinter, C.; Reinhard, I.; Hoffmann, H.; Wiedemann, K.; Winterer, G.; Kiefer, F. Effects of cigarette smoking on plasma concentration of the appetite-regulating peptide ghrelin. Ann. Nutr. Metab. 2015, 66, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Wittekind, D.A.; Kratzsch, J.; Mergl, R.; Enzenbach, C.; Witte, V.; Villringer, A.; Kluge, M. Higher fasting ghrelin serum levels in active smokers than in former and never-smokers. World J. Biol. Psychiatr. 2020, 21, 748–756. [Google Scholar] [CrossRef]
- Kokkinos, A.; Tentolouris, N.; Kyriakaki, E.; Argyrakopoulou, G.; Doupis, J.; Psallas, M.; Kyriaki, D.; Katsilambros, N. Differentiation in the short-and long-term effects of smoking on plasma total ghrelin concentrations between male nonsmokers and habitual smokers. Metabolism 2007, 56, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Kukuvitis, A.; Froudarakis, M.; Tryfon, S.; Tzouvelekis, A.; Saroglou, M.; Karkavitsas, N.; Bouros, D. Acute effect of smoking on plasma Obestatin levels. Tob. Induc. Dis. 2010, 8, 2. [Google Scholar] [CrossRef][Green Version]
- Pilhatsch, M.; Scheuing, H.; Kroemer, N.; Kobiella, A.; Bidlingmaier, M.; Farger, G.; Smolka, M.N.; Zimmermann, U.S. Nicotine administration in healthy non-smokers reduces appetite but does not alter plasma ghrelin. Hum. Psychopharmacol. Clin. Exp. 2014, 29, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, N.B.; Wuttig, F.; Bidlingmaier, M.; Zimmermann, U.S.; Smolka, M.N. Nicotine enhances modulation of food-cue reactivity by leptin and ghrelin in the ventromedial prefrontal cortex. Addict. Biol. 2015, 20, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.E.; Feinle-Bisset, C.; Asarian, L.; Horowitz, M.; Beglinger, C.; Geary, N. Ghrelin, CCK, GLP-1, and PYY (3–36): Secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol. Rev. 2017, 97, 411–463. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Wang, Y.; Talaei, M.; Hu, F.B.; Wu, T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015, 3, 958–967. [Google Scholar] [CrossRef]
- Frati, A.C.; Iniestra, F.; Ariza, C.R. Acute effect of cigarette smoking on glucose tolerance and other cardiovascular risk factors. Diabetes Care 1996, 19, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Willi, C.; Bodenmann, P.; Ghali, W.A.; Faris, P.D.; Cornuz, J. Active smoking and the risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 2007, 298, 2654–2664. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, B. Cigarette smoking and diabetes. Prog. Cardiovasc. Dis. 2003, 45, 405–413. [Google Scholar] [CrossRef]
- Campagna, D.; Alamo, A.; Di Pino, A.; Russo, C.; Calogero, A.; Purrello, F.; Polosa, R. Smoking and diabetes: Dangerous liaisons and confusing relationships. Diabetol. Metab. Syndr. 2019, 11, 85. [Google Scholar] [CrossRef]
- Perry, I.J.; Wannamethee, S.G.; Walker, M.K.; Thomson, A.; Whincup, P.H.; Shaper, A.G. Prospective study of risk factors for development of non-insulin dependent diabetes in middle aged British men. BMJ 1995, 310, 560–564. [Google Scholar] [CrossRef]
- Maddatu, J.; Anderson-Baucum, E.; Evans-Molina, C. Smoking and the risk of type 2 diabetes. Transl. Res. 2017, 184, 101–107. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; Centers for Disease: Atlanta, GA, USA, 2014.
- Holloway, A.; Lim, G.; Petrik, J.; Foster, W.; Morrison, K.; Gerstein, H. Fetal and neonatal exposure to nicotine in Wistar rats results in increased beta cell apoptosis at birth and postnatal endocrine and metabolic changes associated with type 2 diabetes. Diabetologia 2005, 48, 2661–2666. [Google Scholar] [CrossRef]
- Li, S.; Shin, H.J.; Ding, E.L.; van Dam, R.M. Adiponectin levels and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 2009, 302, 179–188. [Google Scholar] [CrossRef]
- Bergman, B.C.; Perreault, L.; Hunerdosse, D.; Kerege, A.; Playdon, M.; Samek, A.M.; Eckel, R.H. Novel and reversible mechanisms of smoking-induced insulin resistance in humans. Diabetes 2012, 61, 3156–3166. [Google Scholar] [CrossRef]
- Yeh, H.-C.; Duncan, B.B.; Schmidt, M.I.; Wang, N.-Y.; Brancati, F.L. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: A cohort study. Ann. Intern. Med. 2010, 152, 10–17. [Google Scholar] [CrossRef]
- Morimoto, A.; Ohno, Y.; Tatsumi, Y.; Nishigaki, Y.; Maejima, F.; Mizuno, S.; Watanabe, S. Impact of smoking cessation on incidence of diabetes mellitus among overweight or normal-weight Japanese men. Diabetes Res. Clin. Pract. 2012, 96, 407–413. [Google Scholar] [CrossRef]
- Kamaura, M.; Fujii, H.; Mizushima, S.; Tochikubo, O. Weight gain and risk of impaired fasting glucose after smoking cessation. J. Epidemiol. 2011, 21, 431. [Google Scholar] [CrossRef]
- Hu, Y.; Zong, G.; Liu, G.; Wang, M.; Rosner, B.; Pan, A.; Willett, W.C.; Manson, J.E.; Hu, F.B.; Sun, Q. Smoking cessation, weight change, type 2 diabetes, and mortality. N. Engl. J. Med. 2018, 379, 623–632. [Google Scholar] [CrossRef]
- Tamura, U.; Tanaka, T.; Okamura, T.; Kadowaki, T.; Yamato, H.; Tanaka, H.; Nakamura, M.; Okayama, A.; Ueshima, H.; Yamagata, Z. Changes in weight, cardiovascular risk factors and estimated risk of coronary heart disease following smoking cessation in Japanese male workers: HIPOP-OHP study. J. Atheroscler. Thromb. 2010, 17, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.H.; Asthana, A.; Smith, S.S.; Piper, M.E.; Loh, W.-Y.; Fiore, M.C.; Baker, T.B. Smoking cessation and the risk of diabetes mellitus and impaired fasting glucose: Three-year outcomes after a quit attempt. PLoS ONE 2014, 9, e98278. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Kim, T.H.; Han, E. Smoking Cessation, Weight Change, Diabetes, and Hypertension in Korean Adults. Am. J. Prev. Med. 2021, 60, 205–212. [Google Scholar] [CrossRef]
- Oba, S.; Noda, M.; Waki, K.; Nanri, A.; Kato, M.; Takahashi, Y.; Poudel-Tandukar, K.; Matsushita, Y.; Inoue, M.; Mizoue, T. Smoking cessation increases short-term risk of type 2 diabetes irrespective of weight gain: The Japan Public Health Center-Based Prospective Study. PLoS ONE 2012, 7, e17061. [Google Scholar] [CrossRef]
- Sung, Y.-T.; Hsiao, C.-T.; Chang, I.-J.; Lin, Y.-C.; Yueh, C.-Y. Smoking cessation carries a short-term rising risk for newly diagnosed diabetes mellitus independently of weight gain: A 6-year retrospective cohort study. J. Diabetes Res. 2016, 2016, 3961756. [Google Scholar] [CrossRef]
- Nilsson, P.; Gudbjörnsdottir, S.; Eliasson, B.; Cederholm, J.; Register, S.C.o.t.S.N.D. Smoking is associated with increased HbA1c values and microalbuminuria in patients with diabetes—Data from the National Diabetes Register in Sweden. Diabetes Metab. 2004, 30, 261–268. [Google Scholar] [CrossRef]
- Kaizu, S.; Kishimoto, H.; Iwase, M.; Fujii, H.; Ohkuma, T.; Ide, H.; Jodai, T.; Kikuchi, Y.; Idewaki, Y.; Hirakawa, Y. Impact of leisure-time physical activity on glycemic control and cardiovascular risk factors in Japanese patients with type 2 diabetes mellitus: The Fukuoka Diabetes Registry. PLoS ONE 2014, 9, e98768. [Google Scholar] [CrossRef]
- Peng, K.; Chen, G.; Liu, C.; Mu, Y.; Ye, Z.; Shi, L.; Zhao, J.; Chen, L.; Li, Q.; Yang, T. Association between smoking and glycemic control in diabetic patients: R esults from the R isk E valuation of c A ncers in C hinese diabe T ic I ndividuals: A l ON gitudinal (REACTION) study. J. Diabetes 2018, 10, 408–418. [Google Scholar] [CrossRef]
- Cacciola, R.R.; Guarino, F.; Polosa, R. Relevance of endothelial-haemostatic dysfunction in cigarette smoking. Curr. Med. Chem. 2007, 14, 1887–1892. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, N.; Chen, G.; Wan, Q.; Yan, L.; Wang, G.; Qin, Y.; Luo, Z.; Tang, X.; Huo, Y. Interaction between smoking and diabetes in relation to subsequent risk of cardiovascular events. Cardiovasc. Diabetol. 2022, 21, 14. [Google Scholar] [CrossRef]
- Fagard, R.H.; Nilsson, P.M. Smoking and diabetes—The double health hazard! Prim. Care Diabetes 2009, 3, 205–209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martín-Timón, I.; Sevillano-Collantes, C.; Segura-Galindo, A.; del Cañizo-Gómez, F.J. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J. Diabetes 2014, 5, 444. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Chen, T.; Lou, Q.; Yu, D. Excess risk of mortality and cardiovascular events associated with smoking among patients with diabetes: Meta-analysis of observational prospective studies. Int. J. Cardiol. 2013, 167, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Ma, L.; Liu, J.; Fu, P. Cigarette smoking as a risk factor for diabetic nephropathy: A systematic review and meta-analysis of prospective cohort studies. PLoS ONE 2019, 14, e0210213. [Google Scholar] [CrossRef]
- Kar, D.; Gillies, C.; Nath, M.; Khunti, K.; Davies, M.J.; Seidu, S. Association of smoking and cardiometabolic parameters with albuminuria in people with type 2 diabetes mellitus: A systematic review and meta-analysis. Acta Diabetol. 2019, 56, 839–850. [Google Scholar] [CrossRef]
- Clair, C.; Cohen, M.J.; Eichler, F.; Selby, K.J.; Rigotti, N.A. The effect of cigarette smoking on diabetic peripheral neuropathy: A systematic review and meta-analysis. J. Gen. Intern. Med. 2015, 30, 1193–1203. [Google Scholar] [CrossRef]
- Pan, A.; Wang, Y.; Talaei, M.; Hu, F.B. Relation of smoking with total mortality and cardiovascular events among patients with diabetes mellitus: A meta-analysis and systematic review. Circulation 2015, 132, 1795–1804. [Google Scholar] [CrossRef]
- Association, A.D. 4. Lifestyle management: Standards of medical care in diabetes—2018. Diabetes Care 2018, 41, S38–S50. [Google Scholar] [CrossRef]
- Iino, K.; Iwase, M.; Tsutsu, N.; Iida, M. Smoking cessation and glycaemic control in type 2 diabetic patients. Diabetes Obes. Metab. 2004, 6, 181–186. [Google Scholar] [CrossRef]
- Feldstein, A.C.; Nichols, G.A.; Smith, D.H.; Stevens, V.J.; Bachman, K.; Rosales, A.G.; Perrin, N. Weight change in diabetes and glycemic and blood pressure control. Diabetes Care 2008, 31, 1960–1965. [Google Scholar] [CrossRef]
- Tonstad, S. Cigarette smoking, smoking cessation, and diabetes. Diabetes Res. Clin. Pract. 2009, 85, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Rossouw, J.; Margolis, K.L. Smoking cessation, weight change, and coronary heart disease among postmenopausal women with and without diabetes. JAMA 2013, 310, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Luque-Ramírez, M.; de Burgoa, V.S. Impact of smoking cessation on estimated cardiovascular risk in Spanish type 2 diabetes mellitus patients: The DIABETES study. Rev. Clín. Esp. Engl. Ed. 2018, 218, 391–398. [Google Scholar] [CrossRef]
- Blomster, J.I.; Woodward, M.; Zoungas, S.; Hillis, G.S.; Harrap, S.; Neal, B.; Poulter, N.; Mancia, G.; Chalmers, J.; Huxley, R. The harms of smoking and benefits of smoking cessation in women compared with men with type 2 diabetes: An observational analysis of the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron modified release Controlled Evaluation) trial. BMJ Open 2016, 6, e009668. [Google Scholar] [CrossRef] [PubMed]
- Chuahirun, T.; Hudson, C.; Seipel, T.; Khanna, A.; Simoni, J.; Harrist, R.B.; Wesson, D.E. Cigarette smoking exacerbates and its cessation ameliorates renal injury in type 2 diabetes. Am. J. Med. Sci. 2004, 327, 57–67. [Google Scholar] [CrossRef]
- Phisitkul, K.; Hegazy, K.; Chuahirun, T.; Hudson, C.; Wesson, D.E.; Simoni, J.; Rajab, H. Continued smoking exacerbates but cessation ameliorates progression of early type 2 diabetic nephropathy. Am. J. Med. Sci. 2008, 335, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Voulgari, C.; Katsilambros, N.; Tentolouris, N. Smoking cessation predicts amelioration of microalbuminuria in newly diagnosed type 2 diabetes mellitus: A 1-year prospective study. Metabolism 2011, 60, 1456–1464. [Google Scholar] [CrossRef]
- Stead, L.F.; Koilpillai, P.; Fanshawe, T.R.; Lancaster, T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst. Rev. 2016, 10, CD008286. [Google Scholar] [CrossRef]
- Nagrebetsky, A.; Brettell, R.; Roberts, N.; Farmer, A. Smoking cessation in adults with diabetes: A systematic review and meta-analysis of data from randomised controlled trials. BMJ Open 2014, 4, e004107. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur. Heart J. 2020, 41, 255–323. [Google Scholar]
- Tobacco, T.C.P.G.T. A clinical practice guideline for treating tobacco use and dependence: 2008 update: A US public health service report. Am. J. Prev. Med. 2008, 35, 158–176. [Google Scholar]
- Hartmann-Boyce, J.; Chepkin, S.C.; Ye, W.; Bullen, C.; Lancaster, T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst. Rev. 2018, 5, CD000146. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.C.; Jaén, C.R.; Baker, T.B.; Bailey, W.C.; Benowitz, N.L.; Curry, S.J.; Dorfman, S.F.; Froelicher, E.S.; Goldstein, M.G.; Healton, C.G. Treating Tobacco Use and Dependence: 2008 Update; US Department of Health and Human Services: Rockville, MD, USA, 2008.
- Dale, L.C.; Schroeder, D.R.; Wolter, T.D.; Croghan, I.T.; Hurt, R.D.; Offord, K.P. Weight change after smoking cessation using variable doses of transdermal nicotine replacement. J. Gen. Intern. Med. 1998, 13, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Schnoll, R.A.; Wileyto, E.P.; Lerman, C. Extended duration therapy with transdermal nicotine may attenuate weight gain following smoking cessation. Addict. Behav. 2012, 37, 565–568. [Google Scholar] [CrossRef]
- Jorenby, D.E.; Leischow, S.J.; Nides, M.A.; Rennard, S.I.; Johnston, J.A.; Hughes, A.R.; Smith, S.S.; Muramoto, M.L.; Daughton, D.M.; Doan, K. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N. Engl. J. Med. 1999, 340, 685–691. [Google Scholar] [CrossRef]
- Farley, A.C.; Hajek, P.; Lycett, D.; Aveyard, P. Interventions for preventing weight gain after smoking cessation. Cochrane Database Syst. Rev. 2012, 1, CD006219. [Google Scholar] [CrossRef]
- Hartmann-Boyce, J.; Theodoulou, A.; Farley, A.; Hajek, P.; Lycett, D.; Jones, L.L.; Kudlek, L.; Heath, L.; Hajizadeh, A.; Schenkels, M. Interventions for preventing weight gain after smoking cessation. Cochrane Database Syst. Rev. 2021, 10, CD006219. [Google Scholar]
- Hsieh, M.T.; Tseng, P.T.; Wu, Y.C.; Tu, Y.K.; Wu, H.C.; Hsu, C.W.; Lei, W.T.; Stubbs, B.; Carvalho, A.F.; Liang, C.S. Effects of different pharmacologic smoking cessation treatments on body weight changes and success rates in patients with nicotine dependence: A network meta-analysis. Obes. Rev. 2019, 20, 895–905. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Gourlay, S.G. Cardiovascular toxicity of nicotine: Implications for nicotine replacement therapy. J. Am. Coll. Cardiol. 1997, 29, 1422–1431. [Google Scholar] [CrossRef]
- Eliasson, B.r.; Taskinen, M.-R.; Smith, U. Long-term use of nicotine gum is associated with hyperinsulinemia and insulin resistance. Circulation 1996, 94, 878–881. [Google Scholar] [CrossRef]
- Mills, E.J.; Thorlund, K.; Eapen, S.; Wu, P.; Prochaska, J.J. Cardiovascular events associated with smoking cessation pharmacotherapies: A network meta-analysis. Circulation 2014, 129, 28–41. [Google Scholar] [CrossRef]
- Hughes, J.R.; Stead, L.F.; Hartmann-Boyce, J.; Cahill, K.; Lancaster, T. Antidepressants for smoking cessation. Cochrane Database Syst. Rev. 2014, 4, CD000031. [Google Scholar] [CrossRef] [PubMed]
- Hurt, R.D.; Sachs, D.P.; Glover, E.D.; Offord, K.P.; Johnston, J.A.; Dale, L.C.; Khayrallah, M.A.; Schroeder, D.R.; Glover, P.N.; Sullivan, C.R. A comparison of sustained-release bupropion and placebo for smoking cessation. N. Engl. J. Med. 1997, 337, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Hays, J.T.; Hurt, R.D.; Rigotti, N.A.; Niaura, R.; Gonzales, D.; Durcan, M.J.; Sachs, D.P.; Wolter, T.D.; Buist, A.S.; Johnston, J.A. Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation: A randomized, controlled trial. Ann. Intern. Med. 2001, 135, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.D.; Perkins, K.A.; Kalarchian, M.A.; Cheng, Y.; Houck, P.R.; Slane, J.D.; Marcus, M.D. Bupropion and cognitive behavioral therapy for weight-concerned women smokers. Arch. Intern. Med. 2010, 170, 543–550. [Google Scholar] [CrossRef]
- Lustman, P.J.; Williams, M.M.; Sayuk, G.S.; Nix, B.D.; Clouse, R.E. Factors influencing glycemic control in type 2 diabetes during acute-and maintenance-phase treatment of major depressive disorder with bupropion. Diabetes Care 2007, 30, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Cahill, K.; Stevens, S.; Perera, R.; Lancaster, T. Pharmacological interventions for smoking cessation: An overview and network meta-analysis. Cochrane Database Syst. Rev. 2013, 2013, CD009329. [Google Scholar] [CrossRef]
- Niaura, R.; Hays, J.T.; Jorenby, D.E.; Leone, F.T.; Pappas, J.E.; Reeves, K.R.; Williams, K.E.; Billing Jr, C.B. The efficacy and safety of varenicline for smoking cessation using a flexible dosing strategy in adult smokers: A randomized controlled trial. Curr. Med. Res. Opin. 2008, 24, 1931–1941. [Google Scholar] [CrossRef]
- Tashkin, D.P.; Rennard, S.; Hays, J.T.; Ma, W.; Lawrence, D.; Lee, T.C. Effects of varenicline on smoking cessation in patients with mild to moderate COPD: A randomized controlled trial. Chest 2011, 139, 591–599. [Google Scholar] [CrossRef]
- Ponciano-Rodriguez, G.; Paez-Martinez, N.; Villa-Romero, A.; Gutierrez-Grobe, Y.; Mendez-Sanchez, N. Early changes in the components of the metabolic syndrome in a group of smokers after tobacco cessation. Metab. Syndr. Relat. Disord. 2014, 12, 242–250. [Google Scholar] [CrossRef]
- Taniguchi, C.; Tanaka, H.; Nakamura, N.; Saka, H.; Oze, I.; Ito, H.; Tachibana, K.; Tokoro, A.; Nozaki, Y.; Nakamichi, N. Varenicline is more effective in attenuating weight gain than nicotine patch 12 months after the end of smoking cessation therapy: An observational study in Japan. Nicotine Tob. Res. 2014, 16, 1026–1029. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Duan, W.; Meng, X.; Li, H.; Jia, C. Varenicline is associated with a modest limitation in weight gain in smokers after smoking cessation: A meta-analysis. J. Public Health 2018, 40, e126–e132. [Google Scholar] [CrossRef]
- Yammine, L.; Green, C.E.; Kosten, T.R.; de Dios, C.; Suchting, R.; Lane, S.D.; Verrico, C.D.; Schmitz, J.M. Exenatide Adjunct to Nicotine Patch Facilitates Smoking Cessation and May Reduce Post-Cessation Weight Gain: A Pilot Randomized Controlled Trial. Nicotine Tob. Res. 2021, 23, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
| Appetite-Regulating Peptides | Smoking | Smoking Cessation |
|---|---|---|
| NPY | ↓ levels of NPY | ↑ levels of NPY correlated with body weight |
| Orexins | dose-dependent ↑ prepro-orexin mRNA production upon chronic nicotine administration | ↓ orexin levels during the initial withdrawal period (24 h of abstinence) |
| Leptin | ↓ or ↑ plasma leptin concentration | ↑ serum leptin levels positively correlated with ↑ body weight, BMI and body fat mass |
| Adiponectin | ↓ plasma concentrations of adiponectin | ↑ serum adiponectin levels in individuals with less abdominal obesity |
| Ghrelin | ↑ or ↓ plasma levels of ghrelin, non-effect result in smokers | No data |
| PYY, GLP-1 and CCK | Not affected | Not affected |
| Smoking-Cessation Treatment | Weight Change | Diabetes Effect |
|---|---|---|
| NRT | weight-gain suppression | can have a negative impact on glucose metabolism |
| Bupropion | maybe the largest effect on weight-gain suppression | safe for people with DM, treatment of choice in obese patients and patients with depression and DM |
| Varenicline | modest limitation of weight gain | effective and well-tolerated treatment for smokers with DM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Driva, S.; Korkontzelou, A.; Tonstad, S.; Tentolouris, N.; Katsaounou, P. The Effect of Smoking Cessation on Body Weight and Other Metabolic Parameters with Focus on People with Type 2 Diabetes Mellitus. Int. J. Environ. Res. Public Health 2022, 19, 13222. https://doi.org/10.3390/ijerph192013222
Driva S, Korkontzelou A, Tonstad S, Tentolouris N, Katsaounou P. The Effect of Smoking Cessation on Body Weight and Other Metabolic Parameters with Focus on People with Type 2 Diabetes Mellitus. International Journal of Environmental Research and Public Health. 2022; 19(20):13222. https://doi.org/10.3390/ijerph192013222
Chicago/Turabian StyleDriva, Stamatina, Aliki Korkontzelou, Serena Tonstad, Nikolaos Tentolouris, and Paraskevi Katsaounou. 2022. "The Effect of Smoking Cessation on Body Weight and Other Metabolic Parameters with Focus on People with Type 2 Diabetes Mellitus" International Journal of Environmental Research and Public Health 19, no. 20: 13222. https://doi.org/10.3390/ijerph192013222
APA StyleDriva, S., Korkontzelou, A., Tonstad, S., Tentolouris, N., & Katsaounou, P. (2022). The Effect of Smoking Cessation on Body Weight and Other Metabolic Parameters with Focus on People with Type 2 Diabetes Mellitus. International Journal of Environmental Research and Public Health, 19(20), 13222. https://doi.org/10.3390/ijerph192013222








