Long-Term Sequelae of Frostbite—A Scoping Review
Abstract
1. Background
1.1. Epidemiology
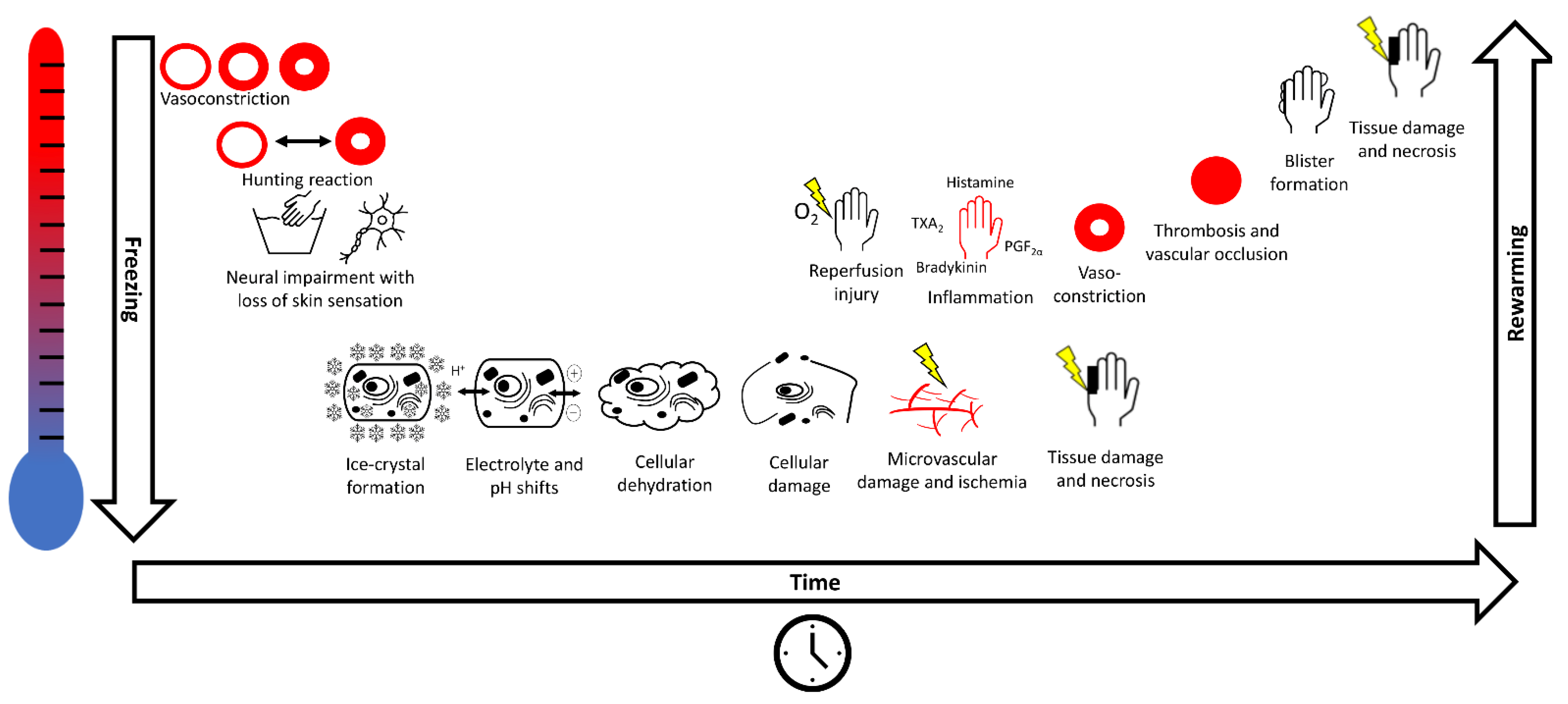
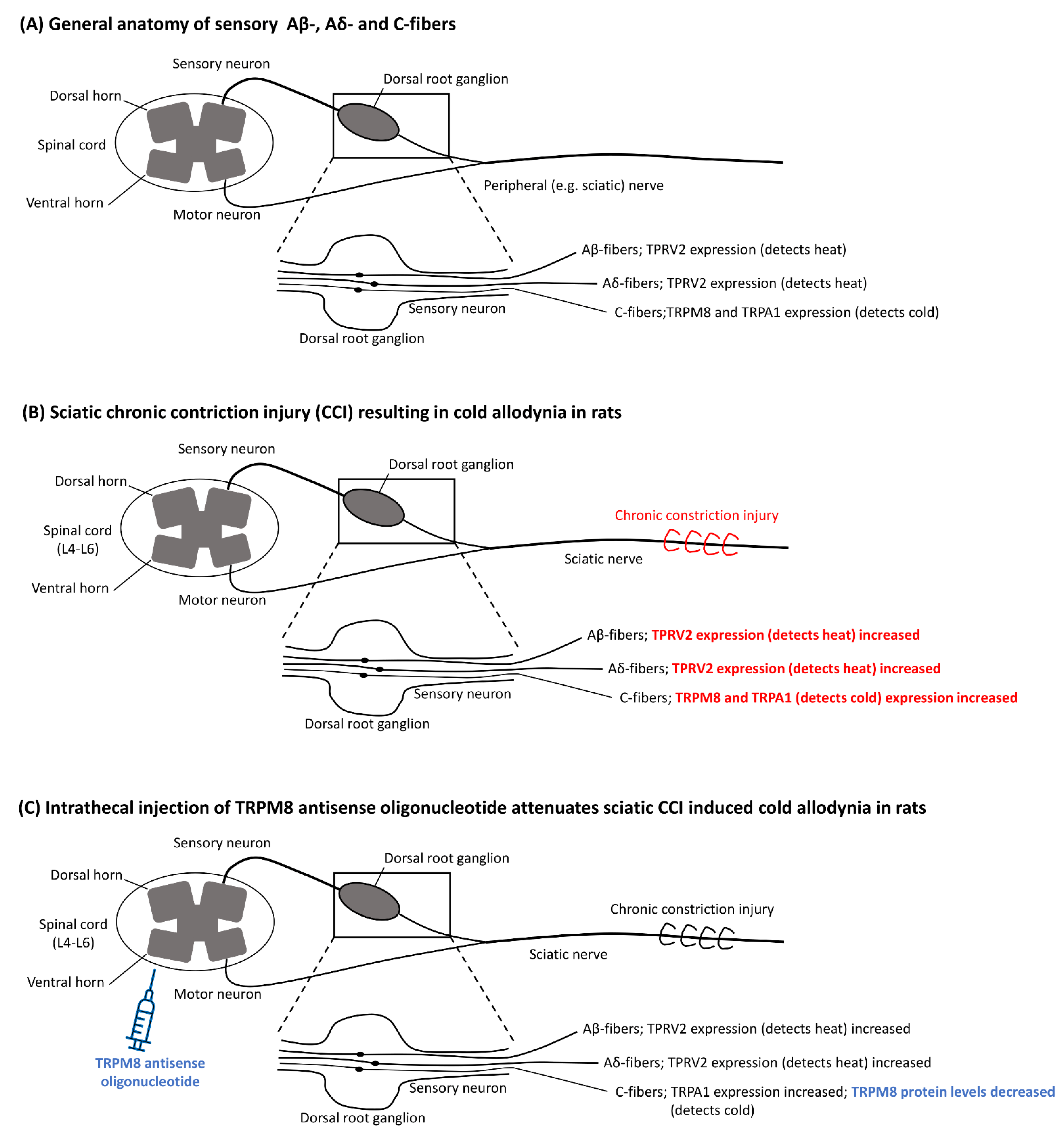
1.2. Pathophysiology
1.3. Classification
1.4. Management
2. Methods
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- McIntosh, S.E.; Freer, L.; Grissom, C.K.; Auerbach, P.S.; Rodway, G.W.; Cochran, A.; Giesbrecht, G.G.; McDevitt, M.; Imray, C.H.; Johnson, E.L.; et al. Wilderness Medical Society Clinical Practice Guidelines for the Prevention and Treatment of Frostbite: 2019 Update. Wilderness Environ. Med. 2019, 30, S19–S32. [Google Scholar] [CrossRef]
- Danielsson, U. Windchill and the risk of tissue freezing. J. Appl. Physiol. 1996, 81, 2666–2673. [Google Scholar] [CrossRef] [PubMed]
- Grieve, A.W.; Davis, P.; Dhillon, S.; Richards, P.; Hillebrandt, D.; Imray, C. A Clinical Review of the Management of Frostbite. J. R. Army Med. Corps 2011, 157, 73–78. [Google Scholar] [CrossRef]
- Imray, C.; Grieve, A.; Dhillon, S.; The Caudwell Xtreme Everest Research Group. Cold damage to the extremities: Frostbite and non-freezing cold injuries. Postgrad. Med. J. 2009, 85, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Gorjanc, J.; Morrison, S.; Blagus, R.; Mekjavić, I.B. Cold Susceptibility of Digit Stumps Resulting from Amputation After Freezing Cold Injury in Elite Alpinists. High Alt. Med. Biol. 2018, 19, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.A.; Dz, D.Z.N.; Gostner, P.; Knapp, R.; Recheis, W.; Seidler, H. The Iceman: Discovery and Imaging. Radiology 2003, 226, 614–629. [Google Scholar] [CrossRef]
- Larrey, D.J.; Hall, R.W. Memoirs of Military Surgery, and Campaigns of the French Armies, on the Rhine, in Corsica, Catalonia, Egypt, and Syria; at Boulogne, Ulm, and Austerlitz; in Saxony, Prussia, Poland, Spain, and Austria; Joseph Cushing: Baltimore, MD, USA, 1814. [Google Scholar]
- Joshi, K.; Goyary, D.; Mazumder, B.; Chattopadhyay, P.; Chakraborty, R.; Bhutia, Y.; Karmakar, S.; Dwivedi, S.K. Frostbite: Current status and advancements in therapeutics. J. Therm. Biol. 2020, 93, 102716. [Google Scholar] [CrossRef] [PubMed]
- Handford, C.; Thomas, O.; Imray, C.H.E. Frostbite. Emerg. Med. Clin. N. Am. 2017, 35, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Valnicek, S.M.; Chasmar, L.R.; Clapson, J.B. Frostbite in the prairies: A 12-year review. Plast. Reconstr. Surg. 1993, 92, 633–641. [Google Scholar] [CrossRef]
- Imray, C.H.E.; Oakley, E.H.N. Cold Still Kills: Cold-Related Illnesses in Military Practice Freezing and Non-Freezing Cold Injury. J. R. Army Med. Corps 2005, 151, 218–222. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, S.E.; Campbell, A.; Weber, D.; Dow, J.; Joy, E.; Grissom, C.K. Mountaineering Medical Events and Trauma on Denali, 1992–2011. High Alt. Med. Biol. 2012, 13, 275–280. [Google Scholar] [CrossRef]
- Némethy, M.; Pressman, A.B.; Freer, L.; McIntosh, S.E. Mt Everest Base Camp Medical Clinic “Everest ER”: Epidemiology of Medical Events during the First 10 Years of Operation. Wilderness Environ. Med. 2015, 26, 4–10. [Google Scholar] [CrossRef]
- Harirchi, I.; Arvin, A.; Vash, J.H.; Zafarmand, V. Frostbite: Incidence and predisposing factors in mountaineers. Br. J. Sports Med. 2005, 39, 898–901. [Google Scholar] [CrossRef] [PubMed]
- Norheim, A.J.; Borud, E.K. Frostbite in the Norwegian Armed Forces. Tidsskr. Nor. Legeforening 2018, 138. [Google Scholar] [CrossRef]
- Carlsson, D.; Burström, L.; Lilliesköld, V.H.; Nilsson, T.; Nordh, E.; Wahlström, J. Neurosensory sequelae assessed by thermal and vibrotactile perception thresholds after local cold injury. Int. J. Circumpolar Health 2014, 73, 384. [Google Scholar] [CrossRef] [PubMed]
- Koljonen, V.; Andersson, K.; Mikkonen, K.; Vuola, J. Frostbite Injuries Treated in the Helsinki Area from 1995 to 2002. J. Trauma Inj. Infect. Crit. Care 2004, 57, 1315–1320. [Google Scholar] [CrossRef]
- Ervasti, O.; Hassi, J.; Rintamäki, H.; Virokannas, H.; Kettunen, P.; Pramila, S.; Linna, T.; Tolonen, U.; Manelius, J. Sequelae of moderate finger frostbite as assessed by subjective sensations, clinical signs, and thermophysiological responses. Int. J. Circumpolar Health 2000, 59, 137–145. [Google Scholar]
- Arvesen, A.; Wilson, J.; Rosén, L. Nerve conduction velocity in human limbs with late sequelae after local cold injury. Eur. J. Clin. Investig. 1996, 26, 443–450. [Google Scholar] [CrossRef]
- Rosén, L.; Eltvik, L.; Arvesen, A.; Stranden, E. Local cold injuries sustained during military service in the Norwegian Army. Arct. Med. Res. 1991, 50, 159–165. [Google Scholar]
- Taylor, M.S.; A Kulungowski, M.; Hamelink, J.K. Frostbite injuries during winter maneuvers: A long-term disability. Mil. Med. 1989, 154, 411–412. [Google Scholar] [CrossRef]
- Blair, J.R.; Schatzki, R.; Orr, K.D. Sequelae to cold injury in one hundred patients; follow-up study four years after occurrence of cold injury. J. Am. Med. Assoc. 1957, 163, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.M.; Minson, C.T.; Kellogg, D.L. Cutaneous Vasodilator and Vasoconstrictor Mechanisms in Temperature Regulation. Compr. Physiol. 2014, 4, 33–89. [Google Scholar] [CrossRef] [PubMed]
- Dana, A.S., Jr.; Rex, I.H., Jr.; Samitz, M.H. The Hunting Reaction. Arch. Dermatol. 1969, 99, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Wilson, O.; Goldman, R.F. Role of air temperature and wind in the time necessary for a finger to freeze. J. Appl. Physiol. 1970, 29, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Heggers, J.P.; Robson, M.C.; Manavalen, K.; Weingarten, M.D.; Carethers, J.M.; A Boertman, J.; Smith, D.J.; Sachs, R.J. Experimental and clinical observations on frostbite. Ann. Emerg. Med. 1987, 16, 1056–1062. [Google Scholar] [CrossRef]
- Mazur, P. Cryobiology: The Freezing of Biological Systems. Science 1970, 168, 939–949. [Google Scholar] [CrossRef]
- Meryman, H.; Williams, R.; Douglas, M. Freezing injury from “solution effects” and its prevention by natural or artificial cryoprotection. Cryobiology 1977, 14, 287–302. [Google Scholar] [CrossRef]
- Mazur, P. Causes of injury in frozen and thawed cells. Fed. Proc. 1965, 24, S175–S182. [Google Scholar]
- Manson, P.; Jesudass, R.; Marzella, L.; Bulkley, G.; Im, M.; Narayan, K. Evidence for an early free radical-mediated reperfusion injury in frostbite. Free Radic. Biol. Med. 1991, 10, 7–11. [Google Scholar] [CrossRef]
- Nakae, H.; Endo, S.; Inada, K.; Yamashita, H.; Yamada, Y.; Takakuwa, T.; Kasai, T.; Ogawa, M.; Uchida, K. Plasma concentrations of type II phospholipase A2, cytokines and eicosanoids in patients with burns. Burns 1995, 21, 422–426. [Google Scholar] [CrossRef]
- Huribal, M.; E Cunningham, M.; D’Aiuto, M.L.; E Pleban, W.; A McMillen, M. Endothelin-1 and prostaglandin E2 levels increase in patients with burns. J. Am. Coll. Surg. 1995, 180, 318–322. [Google Scholar]
- Harms, B.A.; Bodai, B.I.; Smith, M.; Gunther, R.; Flynn, J.; Demling, R.H. Prostaglandin release and altered microvascular integrity after burn injury. J. Surg. Res. 1981, 31, 274–280. [Google Scholar] [CrossRef]
- Robson, M.C.; Del Beccaro, E.J.; Heggers, J.P. The effect of prostaglandins on the dermal microcirculation after burning, and the inhibition of the effect by specific pharmacological agents. Plast. Reconstr. Surg. 1979, 63, 781–787. [Google Scholar] [CrossRef]
- Back, N.; Jainchill, M.; Wilkens, H.; Ambrus, J. Effect of Inhibitors of Plasmin, Kallikrein and Kinin on Mortality from Scalding in Mice. Pharmacology 1966, 15, 597–602. [Google Scholar] [CrossRef]
- Tanaka, H.; Wada, T.; Simazaki, S.; Hanumadass, M.; Reyes, H.M.; Matsuda, T. Effects of Cimetidine on Fluid Requirement During Resuscitation of Third-Degree Burns. J. Burn Care Rehabil. 1991, 12, 425–429. [Google Scholar] [CrossRef]
- Alexander, F.; Mathieson, M.; Teoh, K.H.T.; Huval, W.V.; Lelcuk, S.; Valeri, C.R.; Shepro, D.; Hechtman, H.B. Arachidonic Acid Metabolites Mediate Early Burn Edema. J. Trauma Inj. Infect. Crit. Care 1984, 24, 709–712. [Google Scholar] [CrossRef]
- Jackson, D.M. The diagnosis of the depth of burning. BJS 2005, 40, 588–596. [Google Scholar] [CrossRef]
- Robson, M.C.; Heggers, J.P. Evaluation of hand frostbite blister fluid as a clue to pathogenesis. J. Hand Surg. 1981, 6, 43–47. [Google Scholar] [CrossRef]
- Heggers, J.P.; Ko, F.; Robson, M.C.; Heggers, R.; Craft, K.E. Evaluation of Burn Blister Fluid. Plast. Reconstr. Surg. 1980, 65, 798–804. [Google Scholar] [CrossRef]
- Cauchy, E.; Chetaille, E.; Marchand, V.; Marsigny, B. Retrospective study of 70 cases of severe frostbite lesions: A proposed new classification scheme. Wilderness Environ. Med. 2001, 12, 248–255. [Google Scholar] [CrossRef]
- Mills, W.J., Jr. Clinical Aspects of Freezing Cold Injuries. In Medical Aspects of Harsh Environments; Office of the Surgeon General ; Department of the Army, United States of America: Falls Church, VA, USA, 2001; 35, pp. 429–466. [Google Scholar]
- Lorentzen, A.K.; Davis, C.; Penninga, L. Interventions for frostbite injuries. Cochrane Database Syst. Rev. 2020, 12, CD012980. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Cui, C.-L.; Zhu, H.-Y.; Wang, J.; Xue, Y.; Zhang, N.; Sun, Z.-A.; Gao, X.-X.; Zhou, X.; Yu, J.-A.; et al. The Effects of Recombinant Human Granulocyte–Macrophage Colony-Stimulating Factor Gel on Third-Degree Frostbite Wounds in Northeastern China: A Randomized Controlled Trial. J. Burn Care Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Norheim, A.J.; Mercer, J.; Musial, F.; De Weerd, L. A new treatment for frostbite sequelae; Botulinum toxin. Int. J. Circumpolar Health 2017, 76, 1273677. [Google Scholar] [CrossRef] [PubMed]
- Handford, C.; Buxton, P.; Russell, K.; Imray, C.E.; E McIntosh, S.; Freer, L.; Cochran, A.; Imray, C.H. Frostbite: A practical approach to hospital management. Extreme Physiol. Med. 2014, 3, 7. [Google Scholar] [CrossRef]
- Bates, D.; Schultheis, B.C.; Hanes, M.C.; Jolly, S.M.; Chakravarthy, K.V.; Deer, T.R.; Levy, R.M.; Hunter, C.W. A Comprehensive Algorithm for Management of Neuropathic Pain. Pain Med. 2019, 20, S2–S12. [Google Scholar] [CrossRef]
- Taylor, M.S. Lumbar Epidural Sympathectomy for Frostbite Injuries of the Feet. Mil. Med. 1999, 164, 566–567. [Google Scholar] [CrossRef][Green Version]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic pain: From mechanisms to treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Irsay, L.; Ungur, R.A.; Borda, I.M.; Ciubean, A.D.; Moldovan, I.; Trăistaru, M.R.; Kamal, K.C.; Kamal, D.; Ciortea, V.M. Frostbite arthropathy—A rare case of osteoarthritis, review of the literature and case presentation. Rom. J. Morphol. Embryol. 2019, 60, 1337–1341. [Google Scholar] [PubMed]
- Wang, Y.; Saad, E.; Bonife, T.; Wainapel, S.F. Frostbite Arthritis. Am. J. Phys. Med. Rehabil. 2016, 95, e28. [Google Scholar] [CrossRef] [PubMed]
- E Kahn, J.; Lidove, O.; Laredo, J.D.; Blétry, O. Frostbite arthritis. Ann. Rheum. Dis. 2005, 64, 966–967. [Google Scholar] [CrossRef]
- Pettit, M.T.; Finger, D.R. Frostbite arthropathy. J. Clin. Rheumatol. 1998, 4, 316–318. [Google Scholar] [PubMed]
- Turner, M.; Smith, R.W. Unusual and memorable. Erosive nodal osteoarthritis after frostbite. Ann. Rheum. Dis. 1998, 57, 271. [Google Scholar]
- Crouch, C.; Smith, W.L. Long term sequelae of frostbite. Pediatr. Radiol. 1990, 20, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.; Lai, P.C. Digital deformities from frostbite. Can. Med. Assoc. J. 1985, 132, 14–15. [Google Scholar] [PubMed]
- Nakazato, T.; Ogino, T. Epiphyseal destruction of children’s hands after frostbite: A report of two cases. J. Hand Surg. Am. 1986, 11, 289–292. [Google Scholar] [CrossRef]
- Rossis, C.G.; Yiacoumettis, A.M.; Elemenoglou, J. Squamous cell carcinoma of the heel developing at site of previous frostbite1. J. R. Soc. Med. 1982, 75, 715–718. [Google Scholar]
- Carrera, G.F.; Kozin, F.; Flaherty, L.; Mccarty, D.J. Radiographic changes in the hands following childhood frostbite injury. Skelet. Radiol. 1981, 6, 33–37. [Google Scholar] [CrossRef]
- McKendry, R.J. Frostbite arthritis. Can. Med. Assoc. J. 1981, 125, 1128–1130. [Google Scholar]
- Solomon, S.D. Frostbite arthritis. Arthritis Rheum. 1980, 23, 1332. [Google Scholar] [CrossRef]
- Carrera, G.F.; Kozin, F.; Mccarty, D.J. Arthritis after frostbite injury in children. Arthritis Rheum. 1979, 22, 1082–1087. [Google Scholar] [CrossRef]
- Ellis, R.; Short, J.G.; Simonds, B.D. Unilateral osteoarthritis of the distal interphalangeal joints following frostbite: A case report. Radiology 1969, 93, 857–858. [Google Scholar] [CrossRef] [PubMed]
- Selke, A.C. Destruction of Phalangeal Epiphyses by Frostbite. Radiology 1969, 93, 859–860. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, A.; Nilsson, O.; Svartholm, F. Epiphyseal destruction following frostbite. Report of three cases. Acta Chir. Scand. 1968, 134, 37–40. [Google Scholar] [PubMed]
- Wenzl, J.E.; Burke, E.C.; Bianco, A.J., Jr. Epiphyseal destruction from frostbite of the hands. Am. J. Dis. Child. 1967, 114, 668–670. [Google Scholar] [CrossRef]
- Bigelow, D.R.; Ritchie, G.W. The effects of frostbite in childhood. J. Bone Jt. Surg. Br. Vol. 1963, 45-B, 122–131. [Google Scholar] [CrossRef]
- Florkiewicz, L.; Kozlowski, K. Symmetrical Epiphyseal Destruction by Frostbite. Arch. Dis. Child. 1962, 37, 51–52. [Google Scholar] [CrossRef]
- Dreyfuss, J.R.; Glimcher, M.J. Epiphyseal Injury Following Frostbite. N. Engl. J. Med. 1955, 253, 1065–1068. [Google Scholar] [CrossRef]
- Thelander, H. Epiphyseal destruction by frostbite. J. Pediatr. 1950, 36, 105–106. [Google Scholar] [CrossRef]
- Bennett, R.B.; Blount, W.P. Destruction of epiphyses by freezing. JAMA 1935, 105, 661–662. [Google Scholar] [CrossRef]
- Murphy, J.V.; Banwell, P.E.; Roberts, A.H.N.; McGrouther, D.A. Frostbite: Pathogenesis and Treatment. J. Trauma Inj. Infect. Crit. Care 2000, 48, 171–178. [Google Scholar] [CrossRef]
- Khan, M.I.; Tariq, M.; Rehman, A.; Zafar, A.; Sheen, S.N. Efficacy of cervicothoracic sympathectomy versus conservative management in patients suffering from incapacitating Raynaud’s syndrome after frost bite. J. Ayub. Med. Coll. Abbottabad. 2008, 20, 21–24. [Google Scholar]
- Tercan, M.; Bekerecioglu, M. Decreased Serum Nitric Oxide Level in Experimental Frostbite Injury: A Preliminary Study. Ann. Plast. Surg. 2002, 48, 107–108. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Haroutounian, S.; Kamerman, P.; Baron, R.; Bennett, D.; Bouhassira, D.; Cruccu, G.; Freeman, R.; Hansson, P.; Nurmikko, T.; et al. Neuropathic pain: An updated grading system for research and clinical practice. Pain 2016, 157, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Mumenthaler, M.; Schliack, H.; Eichenberger, M. Läsionen Peripherer Nerven: Diagnostik und Therapie; Thieme-Verlag: New York, NY, USA, 1977; ISBN 10:3133802038. [Google Scholar]
- Arvesen, A.; Rosén, L.; Eltvik, L.P.; Kroese, A.; Stranden, E. Skin microcirculation in patients with sequelae from local cold injuries. Int. J. Microcirc. 1994, 14, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.R.; Heckman, H.M.; Powell, H.C. Axonal viability and the persistence of thermal hyperalgesia after partial freeze lesions of nerve. J. Neurol. Sci. 1996, 139, 28–38. [Google Scholar] [CrossRef]
- Kobayashi, K.; Fukuoka, T.; Obata, K.; Yamanaka, H.; Dai, Y.; Tokunaga, A.; Noguchi, K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J. Comp. Neurol. 2005, 493, 596–606. [Google Scholar] [CrossRef]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nat. Cell Biol. 2002, 416, 52–58. [Google Scholar] [CrossRef]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.J.; et al. A TRP Channel that Senses Cold Stimuli and Menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef]
- Wasner, G.; Schattschneider, J.; Binder, A.; Baron, R. Topical menthol-a human model for cold pain by activation and sensitization of C nociceptors. Brain 2004, 127, 1159–1171. [Google Scholar] [CrossRef]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.-E.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nat. Cell Biol. 2007, 448, 204–208. [Google Scholar] [CrossRef]
- Colburn, R.W.; Lubin, M.L.; Stone, D.J.; Wang, Y.; Lawrence, D.; D’Andrea, M.R.; Brandt, M.R.; Liu, Y.; Flores, C.M.; Qin, N. Attenuated Cold Sensitivity in TRPM8 Null Mice. Neuron 2007, 54, 379–386. [Google Scholar] [CrossRef]
- Dhaka, A.; Murray, A.N.; Mathur, J.; Earley, T.J.; Petrus, M.J.; Patapoutian, A. TRPM8 Is Required for Cold Sensation in Mice. Neuron 2007, 54, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, W.M.; Palkar, R.; Lippoldt, E.K.; McCoy, D.D.; Baluch, F.; Chen, J.; McKemy, D.D. A Sensory-Labeled Line for Cold: TRPM8-Expressing Sensory Neurons Define the Cellular Basis for Cold, Cold Pain, and Cooling-Mediated Analgesia. J. Neurosci. 2013, 33, 2837–2848. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Hosokawa, H.; Hori, A.; Matsumura, K.; Kobayashi, S. Cold sensitivity of recombinant TRPA1 channels. Brain Res. 2007, 1160, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, K. Physiological significance of TRPV2 as a mechanosensor, thermosensor and lipid sensor. J. Physiol. Sci. 2016, 66, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Thapa, D.; Baldissera, L.; Argunhan, F.; Aubdool, A.; Brain, S.D. Relevance of TRPA1 and TRPM8 channels as vascular sensors of cold in the cutaneous microvasculature. Pflügers Archiv—Eur. J. Physiol. 2018, 470, 779–786. [Google Scholar] [CrossRef]
- Austin, P.; Wu, A.; Moalem-Taylor, G. Chronic Constriction of the Sciatic Nerve and Pain Hypersensitivity Testing in Rats. J. Vis. Exp. 2012, 2012, e3393. [Google Scholar] [CrossRef]
- Bennett, G.J.; Xie, Y.-K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Frederick, J.; Buck, M.; Matson, D.; Cortright, D. Increased TRPA1, TRPM8, and TRPV2 expression in dorsal root ganglia by nerve injury. Biochem. Biophys. Res. Commun. 2007, 358, 1058–1064. [Google Scholar] [CrossRef]
- Su, L.; Wang, C.; Yu, Y.-H.; Ren, Y.-Y.; Xie, K.-L.; Wang, G.-L. Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain. BMC Neurosci. 2011, 12, 120. [Google Scholar] [CrossRef] [PubMed]
- Calvo, R.R.; Meegalla, S.K.; Parks, D.J.; Parsons, W.H.; Ballentine, S.K.; Lubin, M.L.; Schneider, C.; Colburn, R.W.; Flores, C.M.; Player, M.R. Discovery of vinylcycloalkyl-substituted benzimidazole TRPM8 antagonists effective in the treatment of cold allodynia. Bioorg. Med. Chem. Lett. 2012, 22, 1903–1907. [Google Scholar] [CrossRef] [PubMed]
- Namer, B.; Kleggetveit, I.P.; Handwerker, H.; Schmelz, M.; Jorum, E. Role of TRPM8 and TRPA1 for cold allodynia in patients with cold injury. Pain 2008, 139, 63–72. [Google Scholar] [CrossRef]
- E Brown, F.; Spiegel, P.K.; E Boyle, W. Digital deformity: An effect of frostbite in children. Pediatrics 1983, 71, 955–959. [Google Scholar]
- Jackson, T.P.; Gaeta, R. Neurolytic blocks revisited. Curr. Pain Headache Rep. 2008, 12, 7–13. [Google Scholar] [CrossRef]
- Golant, A.; Nord, R.M.; Paksima, N.; Posner, M.A. Cold Exposure Injuries to the Extremities. J. Am. Acad. Orthop. Surg. 2008, 16, 704–715. [Google Scholar] [CrossRef]
- Iorio, M.L.; Masden, D.L.; Higgins, J.P. Botulinum toxin A treatment of Raynaud’s phenomenon: A review. Semin. Arthritis Rheum. 2012, 41, 599–603. [Google Scholar] [CrossRef]
- Morris, J.L.; Jobling, P.; Gibbins, I.L. Differential inhibition by botulinum neurotoxin A of cotransmitters released from autonomic vasodilator neurons. Am. J. Physiol. Circ. Physiol. 2001, 281, H2124–H2132. [Google Scholar] [CrossRef]
- Neumeister, M.W.; Chambers, C.B.; Herron, M.S.; Webb, K.; Wietfeldt, J.; Gillespie, J.N.; Bueno, R.A.; Cooney, C.M. Botox Therapy for Ischemic Digits. Plast. Reconstr. Surg. 2009, 124, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, G.; De Andrés, J.; Villanueva-Pérez, V.L.; Asensio-Samper, J.M. Subcutaneous and perineural botulinum toxin type a for neuropathic pain: A descriptive review. Clin. J. Pain 2013, 29, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Ranoux, D.; Attal, N.; Morain, F.; Bouhassira, D. Botulinum toxin type a induces direct analgesic effects in chronic neuropathic pain. Ann. Neurol. 2008, 64, 274–283. [Google Scholar] [CrossRef] [PubMed]

| Classification Proposed by Cauchy et al. | Classification Analogous to Burn Injury | |||
|---|---|---|---|---|
| Extent of Initial Lesion Immediately after Rewarming | Lesion Characteristics * | Time to Onset After Rewarming | ||
| first Grade | Absence of initial lesion | first Degree | Partial skin freezing with: Erythema, oedema Skin desquamation | 2–3 h 5–10 days |
| second Grade | Initial lesion on distal phalanx | second Degree | Full thickness skin freezing with: Clear blister formation Intensive pain | 12–24 h 3–10 days |
| third Grade | Initial lesion on intermediate or proximal phalanx | third Degree | Subcutaneous freezing with: Haemorrhagic blister formation Skin necrosis | 12–24 h 5 days–5 weeks |
| fourth Grade | Initial carpal/tarsal lesion | fourth Degree | Freezing deeper than the subcutis with: Cyanotic and insensitive tissue Tissue mummification | Immediately up to 3 months |
| Article | Population and Follow Up Timing | Frostbite Grade/Degree | Long-Term Sequelae |
|---|---|---|---|
| Norheim et al., 2018 [15] | Self-reported data of 397 Norwegian soldiers in 2017 having suffered frostbite from 2010–2014 | 1–2 | 70 % with long-term sequelae 21% unable to work and undertake usual leisure activities |
| Carlsson et al., 2014 [16] | Self-reported data of 12 patients; 4 patients with hand frostbite, 6 patients with feet frostbite, and 2 patients with hand and feet frostbite; hand frostbite was followed-up after 4 month and 4 years, foot frostbite only after 4 years | 1–2 | 4 months after frostbite of the hands (n = 6): 100% with discomfort when exposed to cold 67% with cold sensation 67% with white fingers/toes 4 years after frostbite of the hands (n = 6): 100% with discomfort when exposed to cold 83% with cold sensation 17% with white fingers/toes 4 years after frostbite of the feet (n = 8): 89% with discomfort when exposed to cold 100% with cold sensation 100% with white fingers/toes |
| Koljonen et al., 2004 [17] | Self-reported data form 14 patients with frostbite during the previous 7 years | Not specified | 15% with daily, intolerable pain 50% chronic pain 50% with limitations in their social life 36% with poor emotional well being |
| Ervasti et al., 2000 [18] | Clinical examination of 30 patients with frostbite 4–11 years earlier | 2 | 63% with sequelae of any kind 66% with increased tendency for vasospasm 53% with hypersensitivity to cold 40% with numbness of fingers 33% with declined sensitivity to touch 13% with lowered working ability |
| Arvesen et al., 1996 * [19] | Clinical examination of 40 Norwegian soldiers with frostbite in the previous 21–78 months; 16 with involvement of the hands and 24 with involvement of the feet | 1–3 | 38% with disturbed sense of cold 38% with disturbed sense of heat 33% skin and nail dystrophia 20% with hyperhidrosis 18% with reduced light-touch perception 18% with reduced pain perceptions 10% with reduced blunt-touch perception 8% with pain on deep pressure5% with paraesthesia 3% with reduced muscle power |
| Rosen et al., 1991 * [20] | Self-reported data of 40 Norwegian soldiers with frostbite at least 2 years prior; 18 with involvement of the hands and 28 with involvement of the feet | 1–3 | Hands: 100% with cold hypersensitivity 50% with paraesthesia 61% with hypaesthesia 56% skin and nail dystrophia 44% with pain 6% with hyperaesthesia 6% with hyperhidrosis 6% with arthralgia Feet: 93% with cold hypersensitivity 64% skin and nail dystrophia 54% with pain 46% with paraesthesia 54% with hypaesthesia 14% with hyperhidrosis 11% with hyperaesthesia 7% pain when walking 4% with arthralgia |
| Taylor et al., 1989 [21] | 40 US soldiers examined 6 months after frostbite | 1–4 | 65% with neurovascular sequelae (cold sensitivity, paraesthesia, pain, and hyperaesthesia) 8% had to be reassigned to new functions due to symptom severity |
| Blair et al., 1957 [22] | Self-reported data of 97 US soldiers with frostbite in previous 4 years; 50 were examined clinically | 2–4 | Self-reported sequelae in winter 71% with numbness 70% with pain 69% with cold feet 58% with abnormal colour 53% with hyperhidrosis 40% with pathology in joints Self-reported sequelae in summer 31% with numbness 45% with pain 24% with cold feet 31% with abnormal colour 78% with hyperhidrosis 25% with pathology in joints Sequelae detected on physical examination 58% with abnormal nails 48% with abnormal colour 42% with hyperhidrosis 28% with joint stiffness |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regli, I.B.; Strapazzon, G.; Falla, M.; Oberhammer, R.; Brugger, H. Long-Term Sequelae of Frostbite—A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 9655. https://doi.org/10.3390/ijerph18189655
Regli IB, Strapazzon G, Falla M, Oberhammer R, Brugger H. Long-Term Sequelae of Frostbite—A Scoping Review. International Journal of Environmental Research and Public Health. 2021; 18(18):9655. https://doi.org/10.3390/ijerph18189655
Chicago/Turabian StyleRegli, Ivo B., Giacomo Strapazzon, Marika Falla, Rosmarie Oberhammer, and Hermann Brugger. 2021. "Long-Term Sequelae of Frostbite—A Scoping Review" International Journal of Environmental Research and Public Health 18, no. 18: 9655. https://doi.org/10.3390/ijerph18189655
APA StyleRegli, I. B., Strapazzon, G., Falla, M., Oberhammer, R., & Brugger, H. (2021). Long-Term Sequelae of Frostbite—A Scoping Review. International Journal of Environmental Research and Public Health, 18(18), 9655. https://doi.org/10.3390/ijerph18189655








