Immunological Effects of an Add-On Physical Exercise Therapy in Depressed Adolescents and Its Interplay with Depression Severity
Abstract
1. Introduction
1.1. Neuroimmunology in Pathophysiology and Treatment of Depression
1.2. Immunomodulatory Effects of Exercise in the Treatment of Depression
1.3. Neuroimmunology in Depressed Adolescents
1.4. Exercise-Induced Neuroimmune-Modulation in Depressed Adolescents
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Depression Inventory for Children and Adolescents (DIKJ)
2.4. Structured Clinical Interview for DSM-IV (SKID-I)
2.5. Physical Examinations
2.6. Serum Analytics
2.7. Training Procedures
2.8. Whole Body Vibration
2.9. Ergometer Training
2.10. Treatment as Usual
2.11. Statistical Analysis
3. Results
3.1. Subjects Included
3.2. Drop-Outs and Intention to Treat
3.3. Demographic and Clinical Characteristics
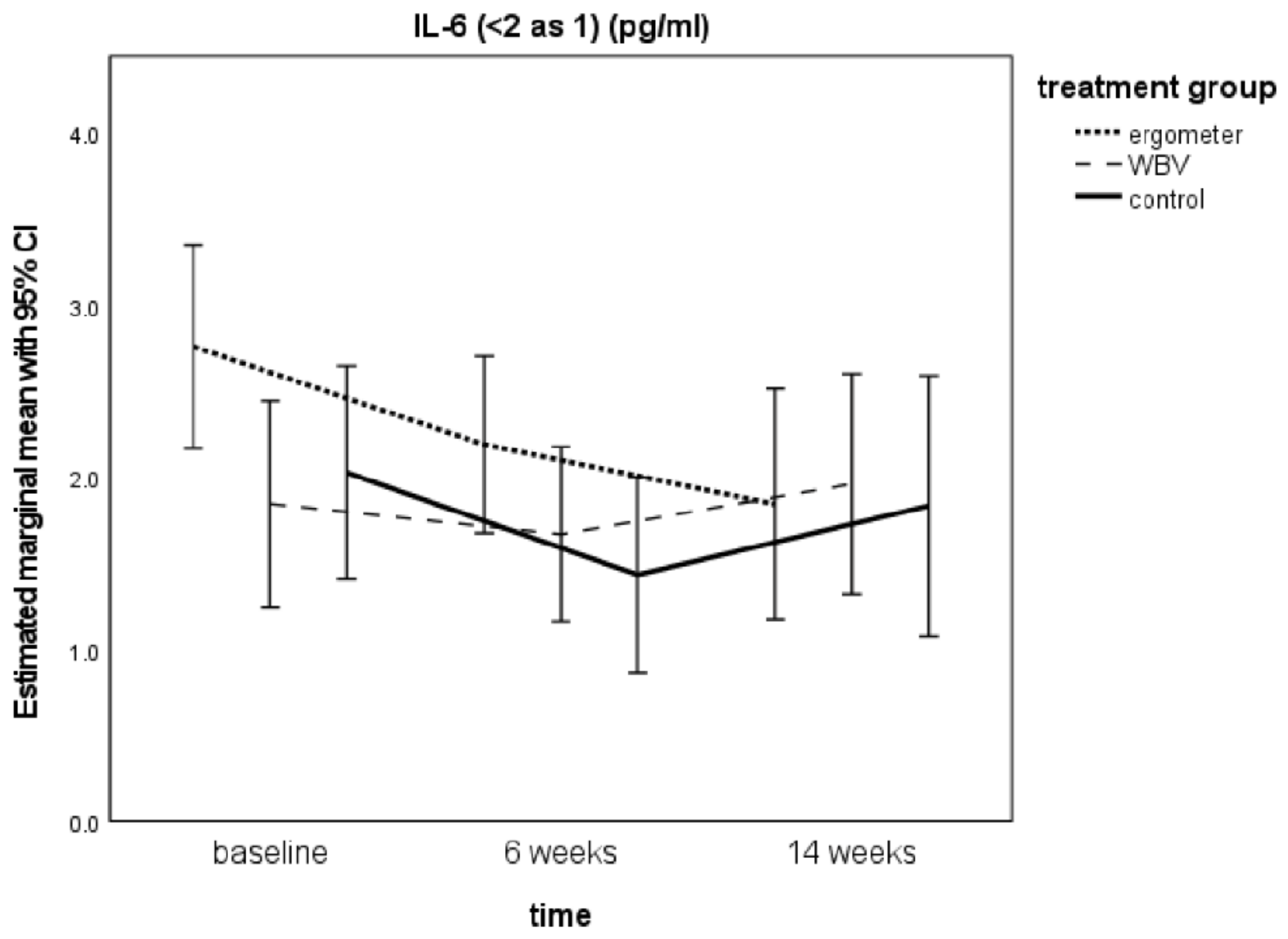
3.4. IL-6 Levels from Baseline to t2
3.5. TNF-α Levels from Baseline to t2
3.6. Influence of Covariates (Age, Sex, BMI, Number of Trainings, Total Therapy Times, Medication) on TNF-α and IL-6
3.7. Influence of IL-6 Levels on TNF-α and Vice Versa
3.8. Influence of IL-6 and TNF-α on DIKJ Scores Over Time
3.9. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CNS | central nervous system |
| CRP | C-reactive protein |
| DIKJ | Depression Inventory for Children and Adolescents |
| DSHS | Sport University Cologne |
| IL-6 | interleukin-6 |
| IMSB | Informatics and Epidemiology of the University of Cologne |
| ITT | intention-to-treat |
| K-ABC | Kaufman Assessment Battery for Children |
| MDD | major depressive disorder |
| MMRM | mixed model for repeated measures |
| PICs | Pro-inflammatory cytokines |
| PRN | pro re nata |
| SKID-I | Structured Clinical Interview for DSM-IV Axis I Disorders, German version |
| SSRI | selective serotonin reuptake inhibitor |
| TAU | treatment-as-usual |
| TNF-α | tumor necrosis factor-α |
| WBV | Whole Body Vibration |
| WISC | Wechsler Intelligence Scale for Children |
References
- McLeod, J.D.; Uemura, R.; Rohrman, S. Adolescent mental health, behavior problems, and academic achievement. J. Health Soc. Behav. 2012, 53, 482–497. [Google Scholar] [CrossRef] [PubMed]
- WHO. Mental Health: New Understanding, New Hope; The World Health Report; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Emslie, G.J. The psychopharmacology of adolescent depression. J. Child Adolesc. Psychopharmacol. 2012, 22, 2–4. [Google Scholar] [CrossRef]
- Dolle, K.; Schulte-Korne, G. The treatment of depressive disorders in children and adolescents. Dtsch. Arztebl. Int. 2013, 110, 854–860. [Google Scholar] [CrossRef][Green Version]
- Cipriani, A.; Zhou, X.; Del Giovane, C.; Hetrick, S.E.; Qin, B.; Whittington, C.; Coghill, D.; Zhang, Y.; Hazell, P.; Leucht, S.; et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: A network meta-analysis. Lancet 2016, 388, 881–890. [Google Scholar] [CrossRef]
- Dolle, K.; Schulte-Korne, G. [Complementary treatment methods for depression in children and adolescents]. Praxis der Kinderpsychologie und Kinderpsychiatrie 2014, 63, 237–263. [Google Scholar] [CrossRef] [PubMed]
- Cooney, G.M.; Dwan, K.; Greig, C.A.; Lawlor, D.A.; Rimer, J.; Waugh, F.R.; McMurdo, M.; Mead, G.E. Exercise for depression. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [PubMed]
- Oberste, M.; Medele, M.; Javelle, F.; Lioba Wunram, H.; Walter, D.; Bloch, W.; Bender, S.; Fricke, O.; Joisten, N.; Walzik, D.; et al. Physical Activity for the Treatment of Adolescent Depression: A Systematic Review and Meta-Analysis. Front. Physiol. 2020, 11, 185. [Google Scholar] [CrossRef]
- Larun, L.; Nordheim, L.V.; Ekeland, E.; Hagen, K.B.; Heian, F. Exercise in prevention and treatment of anxiety and depression among children and young people (Review). Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef]
- Wunram, H.L.; Hamacher, S.; Hellmich, M.; Volk, M.; Janicke, F.; Reinhard, F.; Bloch, W.; Zimmer, P.; Graf, C.; Schonau, E.; et al. Whole body vibration added to treatment as usual is effective in adolescents with depression: A partly randomized, three-armed clinical trial in inpatients. Eur. Child Adolesc. Psychiatry 2018, 27, 645–662. [Google Scholar] [CrossRef]
- Himmerich, H.; Patsalos, O.; Lichtblau, N.; Ibrahim, M.A.A.; Dalton, B. Cytokine Research in Depression: Principles, Challenges, and Open Questions. Front. Psychiatry 2019, 10, 30. [Google Scholar] [CrossRef]
- Shariq, A.S.; Brietzke, E.; Rosenblat, J.D.; Barendra, V.; Pan, Z.; McIntyre, R.S. Targeting cytokines in reduction of depressive symptoms: A comprehensive review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 83, 86–91. [Google Scholar] [CrossRef]
- Phillips, C.; Fahimi, A. Immune and Neuroprotective Effects of Physical Activity on the Brain in Depression. Front. Neurosci. 2018, 12, 498. [Google Scholar] [CrossRef] [PubMed]
- Herpertz-Dahlmann, B.; Buhren, K.; Remschmidt, H. Growing up is hard: Mental disorders in adolescence. Dtsch. Arztebl. Int. 2013, 110, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctot, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Pariante, C.M. Why are depressed patients inflamed? A reflection on 20 years of research on depression, glucocorticoid resistance and inflammation. Eur. Neuropsychopharmacol. 2017, 27, 554–559. [Google Scholar] [CrossRef]
- D’Acunto, G.; Nageye, F.; Zhang, J.; Masi, G.; Cortese, S. Inflammatory Cytokines in Children and Adolescents with Depressive Disorders: A Systematic Review and Meta-Analysis. J. Child Adolesc. Psychopharmacol. 2019, 29, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Eyre, H.A.; Papps, E.; Baune, B.T. Treating depression and depression-like behavior with physical activity: An immune perspective. Front. Psychiatry 2013, 4, 3. [Google Scholar] [CrossRef]
- Lavebratt, C.; Herring, M.P.; Liu, J.J.; Wei, Y.B.; Bossoli, D.; Hallgren, M.; Forsell, Y. Interleukin-6 and depressive symptom severity in response to physical exercise. Psychiatry Res. 2017, 252, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, E.M.; Loukov, D.; Bowdish, D.M.E.; Heisz, J.J. Exercise reduces depression and inflammation but intensity matters. Biol. Psychol. 2018, 133, 79–84. [Google Scholar] [CrossRef]
- Smith, R.S. The macrophage theory of depression. Med. Hypotheses 1991, 35, 298–306. [Google Scholar] [CrossRef]
- Maes, M. Evidence for an immune response in major depression: A review and hypothesis. Prog. Neuropsychopharmacol. Biol. Psychiatry 1995, 19, 11–38. [Google Scholar] [CrossRef]
- Capuron, L.; Ravaud, A.; Dantzer, R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. J. Clin. Oncol. 2000, 18, 2143–2151. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Maker, G.L.; Hood, S.D.; Drummond, P.D. A review of peripheral biomarkers in major depression: The potential of inflammatory and oxidative stress biomarkers. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 48, 102–111. [Google Scholar] [CrossRef]
- Gadad, B.S.; Jha, M.K.; Czysz, A.; Furman, J.L.; Mayes, T.L.; Emslie, M.P.; Trivedi, M.H. Peripheral biomarkers of major depression and antidepressant treatment response: Current knowledge and future outlooks. J. Affect. Disord. 2018, 233, 3–14. [Google Scholar] [CrossRef]
- Kohler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Del Grande da Silva, G.; Wiener, C.D.; Barbosa, L.P.; Goncalves Araujo, J.M.; Molina, M.L.; San Martin, P.; Oses, J.P.; Jansen, K.; Dias de Mattos Souza, L.; Azevedo da Silva, R. Pro-inflammatory cytokines and psychotherapy in depression: Results from a randomized clinical trial. J. Psychiatr. Res. 2016, 75, 57–64. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Karson, A.; Demirtas, T.; Bayramgurler, D.; Balci, F.; Utkan, T. Chronic administration of infliximab (TNF-alpha inhibitor) decreases depression and anxiety-like behaviour in rat model of chronic mild stress. Basic Clin. Pharmacol. Toxicol. 2013, 112, 335–340. [Google Scholar] [CrossRef]
- Danese, A.; Pariante, C.M.; Caspi, A.; Taylor, A.; Poulton, R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl. Acad. Sci. USA 2007, 104, 1319–1324. [Google Scholar] [CrossRef]
- Baumeister, D.; Akhtar, R.; Ciufolini, S.; Pariante, C.M.; Mondelli, V. Childhood trauma and adulthood inflammation: A meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-alpha. Mol. Psychiatry 2016, 21, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, D.; Ciufolini, S.; Mondelli, V. Effects of psychotropic drugs on inflammation: Consequence or mediator of therapeutic effects in psychiatric treatment? Psychopharmacology 2016, 233, 1575–1589. [Google Scholar] [CrossRef] [PubMed]
- Dishman, R.K.; Berthoud, H.R.; Booth, F.W.; Cotman, C.W.; Edgerton, V.R.; Fleshner, M.R.; Gandevia, S.C.; Gomez-Pinilla, F.; Greenwood, B.N.; Hillman, C.H.; et al. Neurobiology of exercise. Obesity 2006, 14, 345–356. [Google Scholar] [CrossRef]
- Mead, G.E.; Morley, W.; Campbell, P.; Greig, C.A.; McMurdo, M.; Lawlor, D.A. Exercise for depression. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef]
- Rimer, J.; Dwan, K.; Lawlor, D.A.; Greig, C.A.; McMurdo, M.; Morley, W.; Mead, G.E. Exercise for depression. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Gleeson, M.; Woods, J.A.; Bishop, N.C.; Fleshner, M.; Green, C.; Pedersen, B.K.; Hoffman-Goetz, L.; et al. Position statement. Part one: Immune function and exercise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar]
- Schuch, F.B.; Deslandes, A.C.; Stubbs, B.; Gosmann, N.P.; Silva, C.T.; Fleck, M.P. Neurobiological effects of exercise on major depressive disorder: A systematic review. Neurosci. Biobehav. Rev. 2016, 61, 1–11. [Google Scholar] [CrossRef]
- Deslandes, A.; Moraes, H.; Ferreira, C.; Veiga, H.; Silveira, H.; Mouta, R.; Pompeu, F.A.; Coutinho, E.S.; Laks, J. Exercise and mental health: Many reasons to move. Neuropsychobiology 2009, 59, 191–198. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Steensberg, A.; Schjerling, P. Muscle-derived interleukin-6: Possible biological effects. J. Physiol. 2001, 536, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V.; Borkoles, E.; Polman, R.; Stojanovska, L. Physical and immunological aspects of exercise in chronic diseases. Immunotherapy 2014, 6, 1145–1157. [Google Scholar] [CrossRef]
- Starkie, R.; Ostrowski, S.R.; Jauffred, S.; Febbraio, M.; Pedersen, B.K. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003, 17, 884–886. [Google Scholar] [CrossRef]
- Fischer, C.P. Interleukin-6 in acute exercise and training: What is the biological relevance? Exerc. Immunol. Rev. 2006, 12, 6–33. [Google Scholar]
- Lira, F.S.; Koyama, C.H.; Yamashita, A.S.; Rosa, J.C.; Zanchi, N.E.; Batista, M.L., Jr.; Seelaender, M.C. Chronic exercise decreases cytokine production in healthy rat skeletal muscle. Cell Biochem. Funct. 2009, 27, 458–461. [Google Scholar] [CrossRef]
- Petersen, A.M.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Smart, N.A.; Larsen, A.I.; Le Maitre, J.P.; Ferraz, A.S. Effect of exercise training on interleukin-6, tumour necrosis factor alpha and functional capacity in heart failure. Cardiol. Res. Pract. 2011, 2011, 532620. [Google Scholar] [CrossRef]
- Jolly, M.; Verbeke, W. Immune System Function and its Relation to Depression: How Exercise can Alter the Immune SystemDepression Dynamics. J. Depress. Anxiety 2018, 7, 4. [Google Scholar] [CrossRef]
- Paula Martins, R.; Lim, C.K.; Ghisoni, K.; Staats, A.; Dallagnol, K.; Solano, A.; Guillemin, G.; Aguiar, A.S., Jr.; Latini, A. Treating depression with exercise: The inflammasome inhibition perspective. J. Syst. Integr. Neurosci. 2016, 3, 1–9. [Google Scholar] [CrossRef][Green Version]
- Euteneuer, F.; Dannehl, K.; Del Rey, A.; Engler, H.; Schedlowski, M.; Rief, W. Immunological effects of behavioral activation with exercise in major depression: An exploratory randomized controlled trial. Transl. Psychiatry 2017, 7, e1132. [Google Scholar] [CrossRef] [PubMed]
- Mills, N.T.; Scott, J.G.; Wray, N.R.; Cohen-Woods, S.; Baune, B.T. Research review: The role of cytokines in depression in adolescents: A systematic review. J. Child Psychol. Psychiatry 2013, 54, 816–835. [Google Scholar] [CrossRef] [PubMed]
- Angold, A.; Costello, E.J. Puberty and depression. Child Adolesc. Psychiatr. Clin. N. Am. 2006, 15, 919–937, ix. [Google Scholar] [CrossRef]
- Brotman, M.A.; Schmajuk, M.; Rich, B.A.; Dickstein, D.P.; Guyer, A.E.; Costello, E.J.; Egger, H.L.; Angold, A.; Pine, D.S.; Leibenluft, E. Prevalence, clinical correlates, and longitudinal course of severe mood dysregulation in children. Biol. Psychiatry 2006, 60, 991–997. [Google Scholar] [CrossRef]
- Brooks, S.J.; Kutcher, S. Diagnosis and measurement of adolescent depression: A review of commonly utilized instruments. J. Child Adolesc. Psychopharmacol. 2001, 11, 341–376. [Google Scholar] [CrossRef]
- Brambilla, F.; Monteleone, P.; Maj, M. Interleukin-1beta and tumor necrosis factor-alpha in children with major depressive disorder or dysthymia. J. Affect. Disord. 2004, 78, 273–277. [Google Scholar] [CrossRef]
- Gabbay, V.; Klein, R.G.; Alonso, C.M.; Babb, J.S.; Nishawala, M.; De Jesus, G.; Hirsch, G.S.; Hottinger-Blanc, P.M.; Gonzalez, C.J. Immune system dysregulation in adolescent major depressive disorder. J. Affect. Disord. 2009, 115, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Gariup, M.; Gonzalez, A.; Lazaro, L.; Torres, F.; Serra-Pages, C.; Morer, A. IL-8 and the innate immunity as biomarkers in acute child and adolescent psychopathology. Psychoneuroendocrinology 2015, 62, 233–242. [Google Scholar] [CrossRef]
- Pallavi, P.; Sagar, R.; Mehta, M.; Sharma, S.; Subramanium, A.; Shamshi, F.; Sengupta, U.; Pandey, R.M.; Mukhopadhyay, A.K. Serum cytokines and anxiety in adolescent depression patients: Gender effect. Psychiatry Res. 2015, 229, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Henje Blom, E.; Lekander, M.; Ingvar, M.; Asberg, M.; Mobarrez, F.; Serlachius, E. Pro-inflammatory cytokines are elevated in adolescent females with emotional disorders not treated with SSRIs. J. Affect. Disord. 2012, 136, 716–723. [Google Scholar] [CrossRef]
- Guyer, A.E.; Silk, J.S.; Nelson, E.E. The neurobiology of the emotional adolescent: From the inside out. Neurosci. Biobehav. Rev. 2016, 70, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.M.; Sisk, C.L. The organizing actions of adolescent gonadal steroid hormones on brain and behavioral development. Neurosci. Biobehav. Rev. 2016, 70, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Sisk, C.L. Hormone-dependent adolescent organization of socio-sexual behaviors in mammals. Curr. Opin. Neurobiol. 2016, 38, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Hannestad, J.; DellaGioia, N.; Bloch, M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: A meta-analysis. Neuropsychopharmacology 2011, 36, 2452–2459. [Google Scholar] [CrossRef]
- Amitai, M.; Taler, M.; Carmel, M.; Michaelovsky, E.; Eilat, T.; Yablonski, M.; Orpaz, N.; Chen, A.; Apter, A.; Weizman, A.; et al. The Relationship Between Plasma Cytokine Levels and Response to Selective Serotonin Reuptake Inhibitor Treatment in Children and Adolescents with Depression and/or Anxiety Disorders. J. Child Adolesc. Psychopharmacol. 2016, 26, 727–732. [Google Scholar] [CrossRef]
- Carter, T.; Morres, I.D.; Meade, O.; Callaghan, P. The Effect of Exercise on Depressive Symptoms in Adolescents: A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 580–590. [Google Scholar] [CrossRef]
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders: DSM-V; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- WHO. ICD-10 Classification of Mental and Behavioural Disorders. Available online: www.who.int/classifications/icd/en/GRNBOOK.pdf (accessed on 29 June 2016).
- Wittchen, H.; Zaudig, T. Strukturiertes Klinisches Interview für DSM-IV; Hogrefe: Göttingen, Germany, 1997. [Google Scholar]
- Stiensmeier-Pelster, J.; Schürmann, M.; Duda, K. DIKJ Depressionsinventar für Kinder und Jugendliche, 2nd ed.; Hogrefe: Göttingen, Germany, 1989. [Google Scholar]
- Melchers, P.; Preuß, U. Kaufman Assessment Battery for Children, 8th ed.; Pearson Assessment: Frankfurt, Germany, 2009. [Google Scholar]
- Petermann, F.; Daseking, M. HAWIK-IV, 3rd ed.; Huber Verlag: Bern, Germany, 2010. [Google Scholar]
- Petermann, F.; Daseking, M. WISC-IV; Pearson Assessment: Frankfurt am Main, Germany, 2011. [Google Scholar]
- Deutsche Gesellschaft für Sportmedizin (DGSP). S1-Leitlinie Vorsorgeuntersuchung im Sport. Available online: http://www.dgsp.de/_downloads/allgemein/leitlinie_vorsorgeuntersuchung_4.10.2007-1-19.pdf (accessed on 29 June 2016).
- Bohus, M.; Limberger, M.F.; Frank, U.; Chapman, A.L.; Kuhler, T.; Stieglitz, R.D. Psychometric properties of the Borderline Symptom List (BSL). Psychopathology 2007, 40, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, M. The Children’s Depression, Inventory (CDI). Psychopharmacol. Bull. 1985, 21, 995–998. [Google Scholar]
- Trappe, H.J.; Löllgen, H. Leitlinien zur Ergometrie (Deutsche Ges. für Kardiologie). Zeitschrift für Kardiologie 2000, 89, 821–837. [Google Scholar]
- Veilleux, L.N.; Rauch, F. Reproducibility of jumping mechanography in healthy children and adults. J. Musculoskelet. Neuronal Interact. 2010, 10, 256–266. [Google Scholar]
- Novotec. Leonardo Mechanography Ground Reaction Force Platform (GRFP): Getting Started v4.1; Novotec Medical GmbH: Pforzheim, Germany, 2006. [Google Scholar]
- R&D Systems, Inc. Quantikine ELISA Human TNF-α Immunoassay. Available online: https://resources.rndsystems.com/pdfs/datasheets/dta00c.pdf?v=20210526&_ga=2.26931572.893794815.1622046480-926488157.1622046480 (accessed on 26 May 2021).
- Roche. Diagnostics. Steckbrief IL 6 Analysekit. Available online: http://www.uk-koeln.de/institute/kchemie/Diagnostik/Parameter/Testkits/Roche/IL6.pdf (accessed on 22 November 2019).
- Roche. Elecsys IL-6 Cobas. Available online: http://www.unsere-uniklinik.de/institute/kchemie/Zentrallabor/Diagnostik/Parameter/Testkits/Roche/IL-6.pdf (accessed on 26 May 2021).
- Semler, O.; Fricke, O.; Vezyroglou, K.; Stark, C.; Stabrey, A.; Schoenau, E. Results of a prospective pilot trial on mobility after whole body vibration in children and adolescents with osteogenesis imperfecta. Clin. Rehabilit. 2008, 22, 387–394. [Google Scholar] [CrossRef]
- Rauch, F.; Sievanen, H.; Boonen, S.; Cardinale, M.; Degens, H.; Felsenberg, D.; Roth, J.; Schoenau, E.; Verschueren, S.; Rittweger, J. Reporting whole-body vibration intervention studies: Recommendations of the International Society of Musculoskeletal and Neuronal Interactions. J. Musculoskelet. Neuronal Interact. 2010, 10, 193–198. [Google Scholar]
- Gibbons, M.W. Effect of Whole Body Vibration on Sterotypy of Young Children with Autism; Utah State University: Logan, UT, USA, 2011. [Google Scholar]
- Semler, O.; Fricke, O.; Vezyroglou, K.; Stark, C.; Schoenau, E. Preliminary results on the mobility after whole body vibration in immobilized children and adolescents. J. Musculoskelet. Neuronal Interact. 2007, 7, 77–81. [Google Scholar]
- Stark, C.; Nikopoulou-Smyrni, P.; Stabrey, A.; Semler, O.; Schoenau, E. Effect of a new physiotherapy concept on bone mineral density, muscle force and gross motor function in children with bilateral cerebral palsy. J. Musculoskelet. Neuronal Interact. 2010, 10, 151–158. [Google Scholar] [PubMed]
- Bhattacharya, A.; Derecki, N.C.; Lovenberg, T.W.; Drevets, W.C. Role of neuro-immunological factors in the pathophysiology of mood disorders. Psychopharmacology 2016, 233, 1623–1636. [Google Scholar] [CrossRef]
- Byrne, M.L.; O’Brien-Simpson, N.M.; Reynolds, E.C.; Walsh, K.A.; Laughton, K.; Waloszek, J.M.; Woods, M.J.; Trinder, J.; Allen, N.B. Acute phase protein and cytokine levels in serum and saliva: A comparison of detectable levels and correlations in a depressed and healthy adolescent sample. Brain Behav. Immun. 2013, 34, 164–175. [Google Scholar] [CrossRef]
- Pedraz-Petrozzi, B.; Neumann, E.; Sammer, G. Pro-inflammatory markers and fatigue in patients with depression: A case-control study. Sci. Rep. 2020, 10, 9494. [Google Scholar] [CrossRef]
- Duivis, H.E.; Vogelzangs, N.; Kupper, N.; de Jonge, P.; Penninx, B.W. Differential association of somatic and cognitive symptoms of depression and anxiety with inflammation: Findings from the Netherlands Study of Depression and Anxiety (NESDA). Psychoneuroendocrinology 2013, 38, 1573–1585. [Google Scholar] [CrossRef] [PubMed]
- McElroy, E.; Casey, P.; Adamson, G.; Filippopoulos, P.; Shevlin, M. A comprehensive analysis of the factor structure of the Beck Depression Inventory-II in a sample of outpatients with adjustment disorder and depressive episode. Ir. J. Psychol. Med. 2018, 35, 53–61. [Google Scholar] [CrossRef]
- Perez-Sanchez, G.; Becerril-Villanueva, E.; Arreola, R.; Martinez-Levy, G.; Hernandez-Gutierrez, M.E.; Velasco-Velasquez, M.A.; Alvarez-Herrera, S.; Cruz-Fuentes, C.; Palacios, L.; de la Pena, F.; et al. Inflammatory Profiles in Depressed Adolescents Treated with Fluoxetine: An 8-Week Follow-up Open Study. Mediat. Inflamm. 2018, 2018, 4074051. [Google Scholar] [CrossRef] [PubMed]
| Screening | Randomization | |
| Inpatient treatment Major depression (SKID I) DIKJ > 18 raw points No exclusion criteria Meeting inclusion criteria | Ergometer training + TAU WBV training + TAU Non-randomized controls (TAU) | |
| 3 Measure Points | Laboratory and physical measurements at t0, t1, t2 | Psychological Parameters at t0, t1, t2 |
| t0= inclusion t1 = after 6 weeks intervention t2 = 8 weeks after t1 (no further intervention) | Serum analytics: IL-6, TNF-α Physical measurements: BMI; Spiroergometry, Jump mechanography; Calipermetry | Clinical interview (SKID I) Depression questionnaire DIKJ |
| Data at t0 | Total N = 64 | Ergometer N = 20 | WBV N = 21 | Controls N = 23 | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Sex ♂ | 18 | (28.1) | 8 | (40) | 6 | (28.6) | 4 | (17.4) | 0.268 |
| Age | 15.9 | ±1.1 | 16.1 | ±1.2 | 15.9 | ±1.2 | 15.7 | ±1.1 | 0.531 |
| BMI | 24.6 | ±6.2 | 26 | ±7.6 | 24.7 | ±5.9 | 23.3 | ±5.0 | 0.361 |
| IQ | 100.1 | ±11.9 (4) | 100.4 | ±8.4 (1) | 100.4 | ±14.1 (1) | 99.6 | ±13.0 (2) | 0.968 |
| DIKJ score | 27.6 | ±6.4 | 27.0 | ±6.2 | 26.9 | ±6.2 | 28.8 | ±6.9 | 0.560 |
| IL-6 (med) | 2.31 | ±1.33 (5) | 2.80 | ±1.47 | 1.95 | ±1.1 | 2.16 | ±1.30 | 0.108 |
| TNF-α | 1.64 | ±0.68 (16) | 1.70 | ±0.7 (3) | 1.55 | ±0.66 (5) | 1.68 | ±0.73 (8) | 0.787 |
| Data at t1 | |||||||||
| Length of stay | 68 | ±33 | 80 | ±36 | 60 | ±20 | 64 | ±39 | 0.123 |
| Dropouts | 12 | (19) | 3 | (4.8) | 3 | (4.7) | 6 | (9.3) | 0.565 |
| Medication | |||||||||
| None | 47 | (73.4) | 14 | (70) | 18 | (85.7) | 15 | (65.2) | 0.224 |
| PRN or SSRI < 3 weeks | 9 | (14.1) | 5 | (25) | 2 | (9.5) | 2 | (8.7) | 0.224 |
| > 3 weeks | 8 | (12.5) | 1 | (5) | 1 | (4.8) | 6 | (26.1) | 0.224 |
| Number of Trainings | 23.5 | ±2.47 (3) | 22.1 | ±3.7 (3) | 0.690 | ||||
| Additional Sports/minutes | 476 | ±567 | 298 | ±415 (13) | 650 | ±456(15) | 480 | ±0 (22) | 0.013 |
| Total therapy time/minutes | 1077 | ±419(13) | 1149 | ±419 (4) | 979 | ±381 (3) | 1113 | ±463 (6) | 0.698 |
| Treatment Group | Time | IL-6 Med | SE | CI Upper B.–Lower B. | p-Value | IL-6 Max | SE | CI Upper B.-Lower B. | p-Value | IL-6 Min | SE | CI Upper B.–Lower B. | p-Value | TNF-α | SE | CI Upper B.–Lower B. | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ergometer | Baseline | 2.760 b | 0.295 | 2.016–3.547 | 0.087 | 2.913 b | 0.242 | 2.306–3.503 | 0.263 | 2.608 b | 0.365 | 1.678–3.677 | 0.046 | 1.805 c | 0.162 | 0.756–2.512 | 0.516 |
| 6 Weeks | 2.190 b | 0.256 | 1.485–2.857 | 0.288 | 2.560 b | 0.190 | 2.045–3.076 | 0.316 | 1.824 b | 0.343 | 0.884–2.748 | 0.275 | 1.681 c | 0.184 | 0.621–2.413 | 0.664 | |
| 14 Weeks | 1.843 b | 0.333 | 0.922–2.638 | 0.026 | 2.313 b | 0.244 | 1.655–2.888 | 0.065 | 1.371 b | 0.434 | 0.159–2.463 | 0.017 | 1.848 c | 0.200 | 0.705–2.502 | 0.896 | |
| WBV | Baseline | 1.845 b | 0.300 | 1.314–2.791 | 0.535 | 2.250 b | 0.245 | 1.768–2.922 | 0.647 | 1.443 b | 0.371 | 0.796–2.727 | 0.506 | 1.669 c | 0.167 | 0.769–2.356 | 0.420 |
| 6 Weeks | 1.668 b | 0.253 | 1.216–2.469 | 0.417 | 2.119 b | 0.187 | 1.745–2.676 | 0.116 | 1.217 b | 0.339 | 0.628–2.351 | 0.760 | 1.811 c | 0.185 | 0.906–2.507 | 0.703 | |
| 14 Weeks | 1.960 b | 0.318 | 1.335–2.901 | 0.334 | 2.553 b | 0.233 | 2.070–3.193 | 0.372 | 1.368 b | 0.416 | 0.563–2.674 | 0.787 | 1.739 c | 0.197 | 0.827–2.442 | 0.746 | |
| Control | Baseline | 2.026 b | 0.310 | 1.766–3.816 | 0.361 | 2.460 b | 0.253 | 1.973–3.550 | 0.237 | 1.596 b | 0.385 | 1.390–4.079 | 0.121 | 1.925 c | 0.182 | 0.515–3.974 | 0.112 |
| 6 Weeks | 1.433 b | 0.283 | 1.300–3.202 | 0.392 | 1.998 b | 0.211 | 1.685–3.105 | 0.202 | 0.885 b | 0.378 | 0.760–3.324 | 0.333 | 1.727 c | 0.204 | 0.198–3.676 | 0.597 | |
| 14 Weeks | 1.833 b | 0.376 | 1.606–3.795 | 0.460 | 2.403 b | 0.276 | 1.992–3.589 | 0.934 | 1.277 b | 0.490 | 1.062–3.989 | 0.723 | 1.791 c | 0.251 | 0.305–3.822 | 0.500 |
| Source | IL6 Med p-Value | IL-6 Max p-Value | IL-6 Min p-Value | TNF-α p-Value |
|---|---|---|---|---|
| Intercept | 0.712 | 0.051 | 0.668 | 0.099 |
| Treatment Group | 0.396 | 0.485 | 0.406 | 0.914 |
| Time | 0.093 | 0.246 | 0.053 | 0.668 |
| Treatment Group * Time | 0.379 | 0.256 | 0.446 | 0.536 |
| Sex | 0.674 | 0.835 | 0.462 | <0.001 |
| Age | 0.782 | 0.294 | 0.877 | 0.236 |
| Medication | 0.020 | 0.024 | 0.031 | 0.406 |
| BMI | <0.001 | <0.001 | <0.001 | 0.125 |
| Number_Trainings | 0.418 | 0.847 | 0.322 | 0.772 |
| Total therapy time week 1–6 | 0.082 | 0.108 | 0.099 | 0.625 |
| Total therapy time week 7–14 | 0.449 | 0.453 | 0.489 | 0.653 |
| IL6 Med t0 | IL6 Med t1 | IL6 Med t2 | TNF Alpha t0 | TNF Alpha t1 | TNF Alpha t2 | DIKJ Raw Score t0 | DIKJ Raw Score t1 | DIKJ Raw Score t2 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| IL6 med t0 | Pearson Correlation | 1 | 0.361 * | 0.193 | 0.010 | 0.227 | 0.086 | −0.103 | 0.026 | −0.256 |
| p-value | 0.013 | 0.210 | 0.946 | 0.125 | 0.587 | 0.438 | 0.861 | 0.111 | ||
| IL6 med t1 | Pearson Correlation | 1 | 0.312 * | −0.051 | 0.115 | 0.467 ** | 0.117 | 0.049 | −0.088 | |
| p-value | 0.035 | 0.733 | 0.438 | 0.002 | 0.418 | 0.734 | 0.586 | |||
| IL6 med t2 | Pearson Correlation | 1 | −0.098 | −0.086 | 0.106 | 0.145 | 0.171 | −0.117 | ||
| p-value | 0.528 | 0.576 | 0.498 | 0.331 | 0.251 | 0.462 | ||||
| TNF alpha t0 | Pearson Correlation | 1 | 0.497 ** | 0.427 ** | −0.182 | −0.243 | −0.241 | |||
| p-value | <0.001 | 0.005 | 0.215 | 0.096 | 0.139 | |||||
| TNF alpha t1 | Pearson Correlation | 1 | 0.564 ** | 0.033 | −0.042 | −0.031 | ||||
| p-value | <0.001 | 0.823 | 0.777 | 0.847 | ||||||
| TNF alpha t2 | Pearson Correlation | 1 | −0.054 | −0.139 | −0.194 | |||||
| p-value | 0.729 | 0.374 | 0.243 | |||||||
| DIKJ raw score t0 | Pearson Correlation | 1 | 0.725 ** | 0.510 ** | ||||||
| p-value | <0.001 | <0.001 | ||||||||
| DIKJ raw score t1 | Pearson Correlation | 1 | 0.707 ** | |||||||
| p-value | <0.001 | |||||||||
| DIKJ raw score t2 | Pearson Correlation | 1 | ||||||||
| p-value | ||||||||||
| Ergometer | WBV | Controls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | |
| Time | 0.005 | 0.006 | 0.005 | 0.016 | 0.013 | 0.022 | 0.010 | 0.078 | 0.022 | 0.024 | 0.022 | 0.102 |
| Gender | 0.034 | 0.034 | 0.036 | 0.082 | 0.284 | 0.288 | 0.309 | 0.988 | 0.070 | 0.102 | 0.058 | 0.175 |
| Age | 0.547 | 0.515 | 0.561 | 0.720 | 0.987 | 0.716 | 0.872 | 0.864 | 0.037 | 0.042 | 0.031 | 0.157 |
| IL-6 med | 0.175 | 0.008 | 0.610 | |||||||||
| IL-6 max | 0.276 | 0.013 | 0.982 | |||||||||
| IL-6 min | 0.130 | 0.009 | 0.430 | |||||||||
| TNF-α | 0.835 | 0.182 | 0.364 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wunram, H.L.; Oberste, M.; Hamacher, S.; Neufang, S.; Grote, N.; Krischer, M.K.; Bloch, W.; Schönau, E.; Bender, S.; Fricke, O. Immunological Effects of an Add-On Physical Exercise Therapy in Depressed Adolescents and Its Interplay with Depression Severity. Int. J. Environ. Res. Public Health 2021, 18, 6527. https://doi.org/10.3390/ijerph18126527
Wunram HL, Oberste M, Hamacher S, Neufang S, Grote N, Krischer MK, Bloch W, Schönau E, Bender S, Fricke O. Immunological Effects of an Add-On Physical Exercise Therapy in Depressed Adolescents and Its Interplay with Depression Severity. International Journal of Environmental Research and Public Health. 2021; 18(12):6527. https://doi.org/10.3390/ijerph18126527
Chicago/Turabian StyleWunram, Heidrun Lioba, Max Oberste, Stefanie Hamacher, Susanne Neufang, Nils Grote, Maya Kristina Krischer, Wilhelm Bloch, Eckhard Schönau, Stephan Bender, and Oliver Fricke. 2021. "Immunological Effects of an Add-On Physical Exercise Therapy in Depressed Adolescents and Its Interplay with Depression Severity" International Journal of Environmental Research and Public Health 18, no. 12: 6527. https://doi.org/10.3390/ijerph18126527
APA StyleWunram, H. L., Oberste, M., Hamacher, S., Neufang, S., Grote, N., Krischer, M. K., Bloch, W., Schönau, E., Bender, S., & Fricke, O. (2021). Immunological Effects of an Add-On Physical Exercise Therapy in Depressed Adolescents and Its Interplay with Depression Severity. International Journal of Environmental Research and Public Health, 18(12), 6527. https://doi.org/10.3390/ijerph18126527








