Dietary Exposure to Acrylamide and Associated Health Risks for the Korean Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Samples
2.2. Sample Preparation and Analysis
2.3. Food Intake Data
2.4. Dietary Exposure to AA
- Daily dietary exposure: Estimated daily dietary exposure to AA (μg/kg BW/day)
- Ci: the concentrations of AA in a food composite sample (μg/kg)
- Ai: the consumption amount of a food group for the corresponding population groups of different ages and genders, as in the KNHANES (g/day)
- B: the body weight (kg) of the corresponding population groups obtained from KNHANES
- n: the total number of food groups consumed.
2.5. Risk Assessment of AA
3. Results and Discussion
3.1. Contents of AA in the Food Sample
3.2. Dietary Exposure to AA
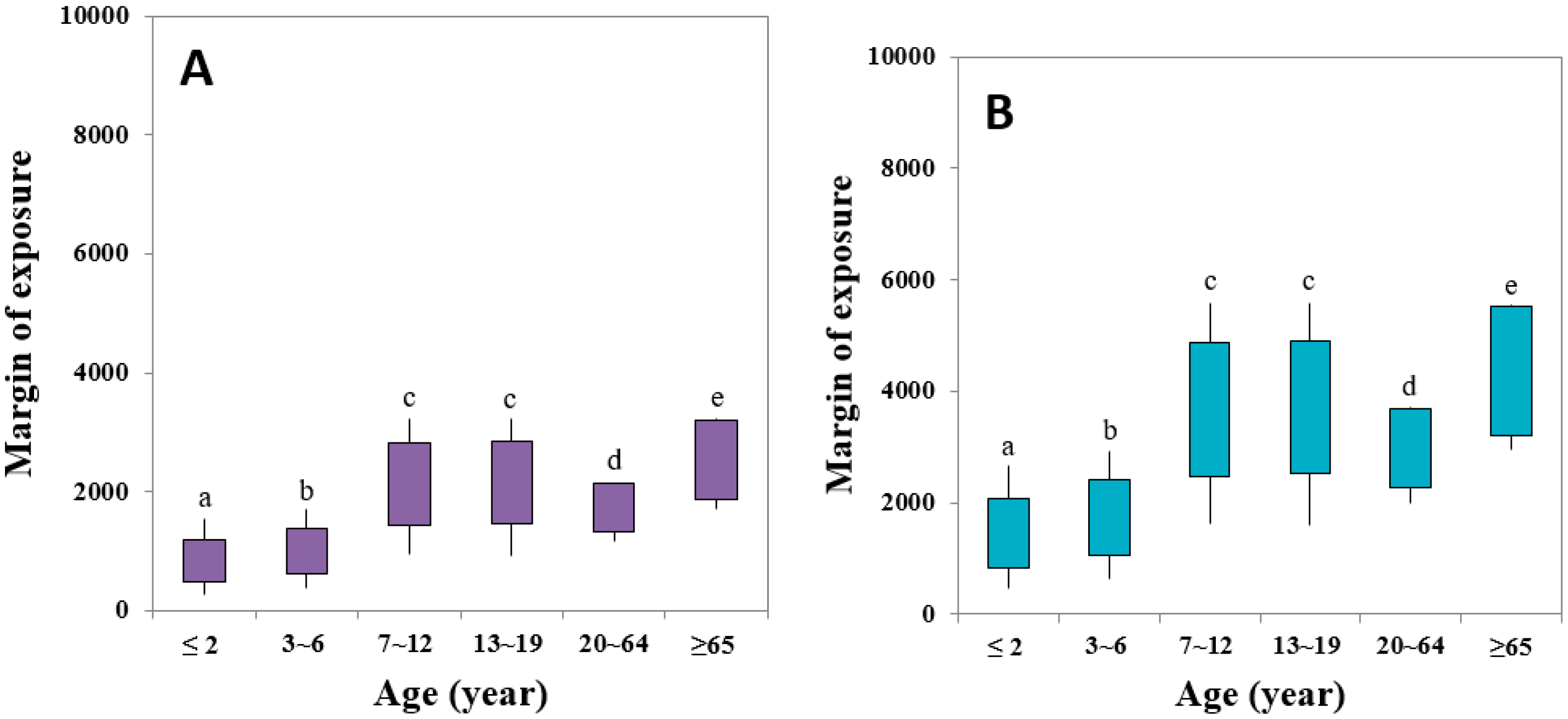
3.3. Risk Assessment of AA
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Taeymans, D.; Wood, J.; Ashby, P.; Blank, I.; Studer, A.; Stadler, R.H.; Gonde, P.; Eijck, P.; Lalljie, S.; Lingnert, H. A review of acrylamide: An industry perspective on research, analysis, formation, and control. Crit. Rev. Food Sci. Nutr. 2004, 44, 323–347. [Google Scholar] [CrossRef]
- European Food Safety Authority. A Report of the Data Collection and Exposure Unit in Response to a Request from the European Commission; European Food Safety Authority: Parma, Italy, 2009.
- Swedish National Food Administration. Information about Acrylamide in Food; Swedish National Food Administration: Uppsala, Sweden, 2002.
- Rannou, C.; Laroque, D.; Renault, E.; Prost, C.; Sérot, T. Mitigation strategies of acrylamide, furans, heterocyclic amines and browning during the Maillard reaction in foods. Food Res. Int. 2016, 90, 154–176. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Levin, C.E. Review of methods for the reduction of dietary content and toxicity of acrylamide. J. Agric. Food Chem. 2008, 56, 6113–6140. [Google Scholar] [CrossRef]
- Friedman, M. Acrylamide: Inhibition of formation in processed food and mitigation of toxicity in cells, animals, and humans. Food Funct. 2015, 6, 1752–1772. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific opinion on acrylamide in food. EFSA Panel on Contaminants in the Food Chain (CONTAM). EFSA J. 2015, 13, 4104. [Google Scholar]
- European Union. Commission Regulation (EU) 2017/2158 of 20 November 2017 Establishing Mitigation Measures and Benchmark Levels for the Reduction of the Presence of Acrylamide in Food; European Union: Brussels, Belgium, 2017. [Google Scholar]
- World Health Organization. Health Implications of Acrylamide in Food: Report of a Joint FAO/WHO Consultation, WHO Headquarters, Geneva, Switzerland, 25–27 June 2002; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Dybing, E.; Sanner, T. Risk assessment of acrylamide in foods. Toxicol. Sci. 2003, 75, 7–15. [Google Scholar] [CrossRef]
- Adani, G.; Filippini, T.; Wise, L.A.; Halldorsson, T.; Blaha, L.; Vinceti, M. Dietary intake of acrylamide and risk of breast, endometrial, and ovarian cancers: A systematic review and dose-response meta-analysis. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1095–1106. [Google Scholar] [CrossRef]
- Olsen, A.; Christensen, J.; Outzen, M.; Olesen, P.T.; Frandsen, H.; Overvad, K.; Halkjær, J. Pre-diagnostic acrylamide exposure and survival after breast cancer among postmenopausal Danish women. Toxicology 2012, 296, 67–72. [Google Scholar] [CrossRef]
- Jeong, H.; Hwang, S.; Kwon, H. Survey for acrylamide in processed foods from Korean market and individual exposure estimation using a non-parametric probabilistic model. Food Addit. Contam. 2020, 37, 916–930. [Google Scholar] [CrossRef]
- Kawahara, J.; Zheng, Y.; Terui, M.; Shinohara, A.; Uyama, K.; Yoneyama, M.; Nakajima, D.; Shibata, Y.; Adachi, S. Dietary exposure to acrylamide in a group of Japanese adults based on 24-h duplicate diet samples. Food Addit. Contam. 2019, 36, 15–25. [Google Scholar] [CrossRef]
- Zhou, P.P.; Zhao, Y.F.; Liu, H.L.; Ma, Y.J.; Li, X.Y.; Wu, Y.N. Dietary exposure of the Chinese population to acrylamide. Biomed. Environ. Sci. 2013, 26, 421–429. [Google Scholar]
- European Food Safety Authority. Results on acrylamide levels in food from monitoring years 2007–2009 and Exposure assessment. EFSA J. 2011, 9, 2133. [Google Scholar] [CrossRef]
- FAO; WHO. The Joint FAO/WHO Expert Committee on Food Additives (JECFA) Evaluation of Certain Contaminants in Food: Seventy-Second Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- European Food Safety Authority. Draft Opinion of the Scientific Committee on a harmonised approach for risk assessment of compounds which are both genotoxic and carcinogenic. EFSA J. 2005, 282, 1–31. [Google Scholar]
- Nematollahi, A.; Kamankesh, M.; Hosseini, H.; Ghasemi, J.; Hosseini-Esfahani, F.; Mohammadi, A.; Khaneghah, A.M. Acrylamide content of collected food products from Tehran’s market: A risk assessment study. Environ. Sci. Pollut. Res. Int. 2020, 27, 30558–30570. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, S.H.; Koo, M.S. Acrylamide contamination of food products in Korea. Safe Food 2011, 6, 34–39. [Google Scholar]
- U.S. Food and Drug Administration. Detection and Quantitation of Acrylamide in Foods; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2003.
- Lee, S.; Yoo, M.; Koo, M.; Kim, H.J.; Kim, M.; Park, S.K.; Shin, D. In-house-validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for survey of acrylamide in various processed foods from Korean market. Food Sci. Nutr. 2013, 1, 402–407. [Google Scholar] [CrossRef]
- Korea Food & Drug Administration. Acrylamide Monitoring in Food Products and Its Intake Estimation; Korea Food & Drug Administration: Cheongju, Korea, 2011.
- Lee, B.K.; Kim, Y. Relationship between blood manganese and blood pressure in the Korean general population according to KNHANES 2008. Environ. Res. 2011, 111, 797–803. [Google Scholar] [CrossRef]
- Zając, J.; Bojar, I.; Helbin, J.; Kolarzyk, E.; Potocki, A.; Strzemecka, J.; Owoc, A. Dietary acrylamide exposure in chosen population of South Poland. Ann. Agric. Environ. Med. 2013, 20, 351–355. [Google Scholar]
- Ono, H.; Chuda, Y.; Ohnishi-Kameyama, M.; Yada, H.; Ishizaka, M.; Kobayashi, H.; Yoshida, M. Analysis of acrylamide by LC-MS/MS and GC-MS in processed Japanese foods. Food Addit. Contam. 2003, 20, 215–220. [Google Scholar] [CrossRef]
- Elias, A.; Roasto, M.; Reinik, M.; Nelis, K.; Nurk, E.; Elias, T. Acrylamide in commercial foods and intake by infants in Estonia. Food Addit. Contam. 2017, 34, 1875–1884. [Google Scholar] [CrossRef]
- European Food Safety Authority. Update on acrylamide levels in food from monitoring years 2007 to 2010. EFSA J. 2012, 10, 2938. [Google Scholar] [CrossRef]
- Mizukami, Y.; Kohata, K.; Yamaguchi, Y.; Hayashi, N.; Sawai, Y.; Chuda, Y.; Ono, H.; Yada, H.; Yoshida, M. Analysis of acrylamide in green tea by gas chromatography-mass spectrometry. J. Agric. Food Chem. 2006, 54, 7370–7377. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yuan, Y.; Liu, J.; Zhao, G.; Hu, X. Survey of acrylamide levels in Chinese foods. Food. Addit. Contam. 2008, 1, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Sirot, V.; Hommet, F.; Tard, A.; Leblanc, J. Dietary acrylamide exposure of the French population: Results of the second French Total Diet Study. Food Chem. Toxicol. 2012, 50, 889–894. [Google Scholar] [CrossRef]
- Mojska, H.; Gielecińska, I.; Szponar, L.; Ołtarzewski, M. Estimation of the dietary acrylamide exposure of the Polish population. Food Chem. Toxicol. 2010, 48, 2090–2096. [Google Scholar] [CrossRef]
- Normandin, L.; Bouchard, M.; Ayotte, P.; Blanchet, C.; Becalski, A.; Bonvalot, Y.; Phaneuf, D.; Lapointe, C.; Gagné, M.; Courteau, M. Dietary exposure to acrylamide in adolescents from a Canadian urban center. Food Chem. Toxicol. 2013, 57, 75–83. [Google Scholar] [CrossRef]
- Kim, H.J.; Ha, J.H.; Chun, H.S.; Cho, E.J. Estimation of Daily exposure to 3-monochloropropane-1,2-diol from commercial soy sauces in Korea. Food Sci. Biotechnol. 2006, 15, 768–772. [Google Scholar]
- Cieślik, I.; Cieslik, E.; Topolska, K.; Surma, M. Dietary acrylamide exposure from traditional food products in Lesser Poland and associated risk assessment. Ann. Agric. Environ. Med. 2020, 27, 225–230. [Google Scholar] [CrossRef]
| Food Category | Sample (n) | Acrylamide (μg/kg) | Benchmark Level (2) (μg/kg) | Estimated Daily Exposure (μg/kg BW/day) | |
|---|---|---|---|---|---|
| Mean ± SD (1) | Range | ||||
| Potato crisps | 40 | 546 ± 353 | 14–1435 | 750 | 0.002 |
| Crisps (except potato crisps) | 30 | 135 ± 176 | <LOQ–693 | 400 | 0.004 |
| Biscuits | 70 | 178 ± 201 | <LOQ–861 | 350 | 0.009 |
| French fries | 40 | 372 ± 220 | 93–1080 | 500 | 0.001 |
| Chocolate products | 20 | 58 ± 71 | <LOQ–232 | - (3) | 0.001 |
| Cocoa products | 20 | 4 ± 17 | <LOQ–74 | - | ≈0 (4) |
| Breakfast cereals | 40 | 80 ± 82 | <LOQ–370 | 150–300 | 0.001 |
| Tea products | 25 | 245 ± 314 | <LOQ–889 | - | ≈0 |
| Dried and roasted seaweed | 20 | 114 ± 110 | <LOQ–335 | - | 0.002 |
| Nut products | 20 | 23 ± 38 | <LOQ–135 | - | ≈0 |
| Coffee (5) | 60 | 353 ± 270 | 57–989 | 400–850 | 0.021 |
| Bread | 20 | 1 ± 4 | <LOQ–17 | 50–100 | ≈0 |
| Cakes | 40 | 1 ± 5 | <LOQ–27 | 50 | ≈0 |
| Juice | 20 | 8 ± 37 | <LOQ–170 | - | 0.001 |
| Kimchi | 20 | <LOQ | <LOQ | - | 0 |
| Subpopulation of Different Ages (years) | Dietary Exposure to Acrylamide (mg/kg BW/day) | ||||
|---|---|---|---|---|---|
| Mean | 50th Percentile | 90th Percentile | 95th (1) Percentile | Maximum | |
| ≤2 | 0.150 | 0.116 | 0.273 | 0.371 | 0.659 |
| 3–6 | 0.129 | 0.106 | 0.226 | 0.293 | 0.493 |
| 7–12 | 0.064 | 0.056 | 0.102 | 0.125 | 0.192 |
| 13–19 | 0.063 | 0.056 | 0.099 | 0.122 | 0.194 |
| 20–64 | 0.084 | 0.084 | 0.128 | 0.136 | 0.156 |
| ≥65 | 0.056 | 0.056 | 0.091 | 0.096 | 0.105 |
| Total | 0.077 | 0.076 | 0.112 | 0.122 | 0.153 |
| Food Category | Median Values of Dietary Exposure to Acrylamide (10−3 μg/kg BW/day) among the Subpopulations of Korea (1) | |||||
|---|---|---|---|---|---|---|
| Age (≤2) | Age (3–6) | Age (7–12) | Age (13–19) | Age (20–64) | Age (≥65) | |
| Potato crisps | 2.9 (2) | 3.8 | 7.6 | 7.9 | 1.1 | 0.0 |
| Crisps (except potato crisps) | 18.3 | 19.0 | 10.0 | 6.7 | 1.3 | 0.2 |
| Biscuits | 34.0 | 24.0 | 8.1 | 8.5 | 3.1 | 0.6 |
| French fries | 1.5 | 0.0 | 2.2 | 2.6 | 0.9 | 0.0 |
| Coffee | 0.0 | 0.4 | 0.0 | 2.1 | 63.3 | 50.8 |
| Bread | 0.5 | 0.7 | 0.6 | 0.4 | 0.2 | 0.0 |
| Cakes | 4.1 | 4.8 | 2.7 | 2.5 | 1.1 | 0.3 |
| Cocoa products | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Chocolates | 3.9 | 4.5 | 3.7 | 3.3 | 0.8 | 0.4 |
| Cereals | 2.6 | 4.1 | 2.0 | 1.4 | 0.4 | 0.0 |
| Teas | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.1 |
| Kimchi | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Juices | 14.0 | 9.7 | 4.5 | 5.7 | 2.9 | 0.4 |
| Dried and roasted seaweed | 2.9 | 2.8 | 1.3 | 0.8 | 0.7 | 0.5 |
| Nuts and nut products | 0.3 | 0.5 | 0.2 | 0.3 | 0.3 | 0.1 |
| Total | 85.0 | 74.2 | 42.6 | 42.3 | 76.4 | 53.5 |
| Food Category | 95th Percentile Values of Dietary Exposure to Acrylamide (10−3 μg/kg BW/day) among the Subpopulations of Korea (1) | |||||
|---|---|---|---|---|---|---|
| Age (≤2) | Age (3–6) | Age (7–12) | Age (13–19) | Age (20–64) | Age (≥65) | |
| Potato crisps | 11.0 (2) | 13.2 | 18.0 | 18.9 | 2.7 | 0.1 |
| Crisps (except potato crisps) | 84.8 | 86.7 | 45.1 | 30.5 | 5.7 | 1.3 |
| Biscuits | 281.5 | 199.2 | 66.3 | 71.1 | 25.3 | 5.2 |
| French fries | 5.8 | 0.0 | 7.0 | 8.9 | 2.5 | 0.0 |
| Coffee | 0.0 | 2.0 | 0.0 | 4.4 | 112.4 | 91.0 |
| Bread | 1.2 | 1.7 | 1.4 | 1.0 | 0.5 | 0.1 |
| Cakes | 7.4 | 8.2 | 4.5 | 4.5 | 1.8 | 0.6 |
| Cocoa products | 0.2 | 1.3 | 0.4 | 0.1 | 0.0 | 0.0 |
| Chocolates | 10.2 | 10.4 | 8.0 | 7.4 | 1.7 | 1.0 |
| Cereals | 12.3 | 18.2 | 8.7 | 6.3 | 1.6 | 0.1 |
| Teas | 0.0 | 0.4 | 0.0 | 0.6 | 1.7 | 0.7 |
| Kimchi | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Juices | 60.4 | 61.1 | 17.7 | 26.1 | 10.5 | 2.3 |
| Dried and roasted seaweed | 33.6 | 32.2 | 14.5 | 9.4 | 8.3 | 5.5 |
| Nuts and nut products | 0.8 | 1.3 | 0.7 | 0.9 | 0.8 | 0.4 |
| Total | 509.3 | 436.1 | 192.4 | 190.3 | 175.5 | 108.4 |
| Percentile | Margin of Exposure (MOE) | |
|---|---|---|
| Harderian Gland Tumors | Mammary Tumors | |
| Mean | 2347 | 4042 |
| 50th | 2371 | 4084 |
| 90th | 1602 | 2759 |
| 95th | 1471 | 2533 |
| 99th | 1177 | 2027 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Kim, H.J. Dietary Exposure to Acrylamide and Associated Health Risks for the Korean Population. Int. J. Environ. Res. Public Health 2020, 17, 7619. https://doi.org/10.3390/ijerph17207619
Lee S, Kim HJ. Dietary Exposure to Acrylamide and Associated Health Risks for the Korean Population. International Journal of Environmental Research and Public Health. 2020; 17(20):7619. https://doi.org/10.3390/ijerph17207619
Chicago/Turabian StyleLee, Sanghee, and Hyun Jung Kim. 2020. "Dietary Exposure to Acrylamide and Associated Health Risks for the Korean Population" International Journal of Environmental Research and Public Health 17, no. 20: 7619. https://doi.org/10.3390/ijerph17207619
APA StyleLee, S., & Kim, H. J. (2020). Dietary Exposure to Acrylamide and Associated Health Risks for the Korean Population. International Journal of Environmental Research and Public Health, 17(20), 7619. https://doi.org/10.3390/ijerph17207619





