Socio-Economic Status and Health: Evaluation of Human Biomonitored Chemical Exposure to Per- and Polyfluorinated Substances across Status
Abstract
1. Introduction
2. Methodology
2.1. Literature Search
2.2. Meta-Analysis
2.3. Determining Factors
3. Results and Discussion
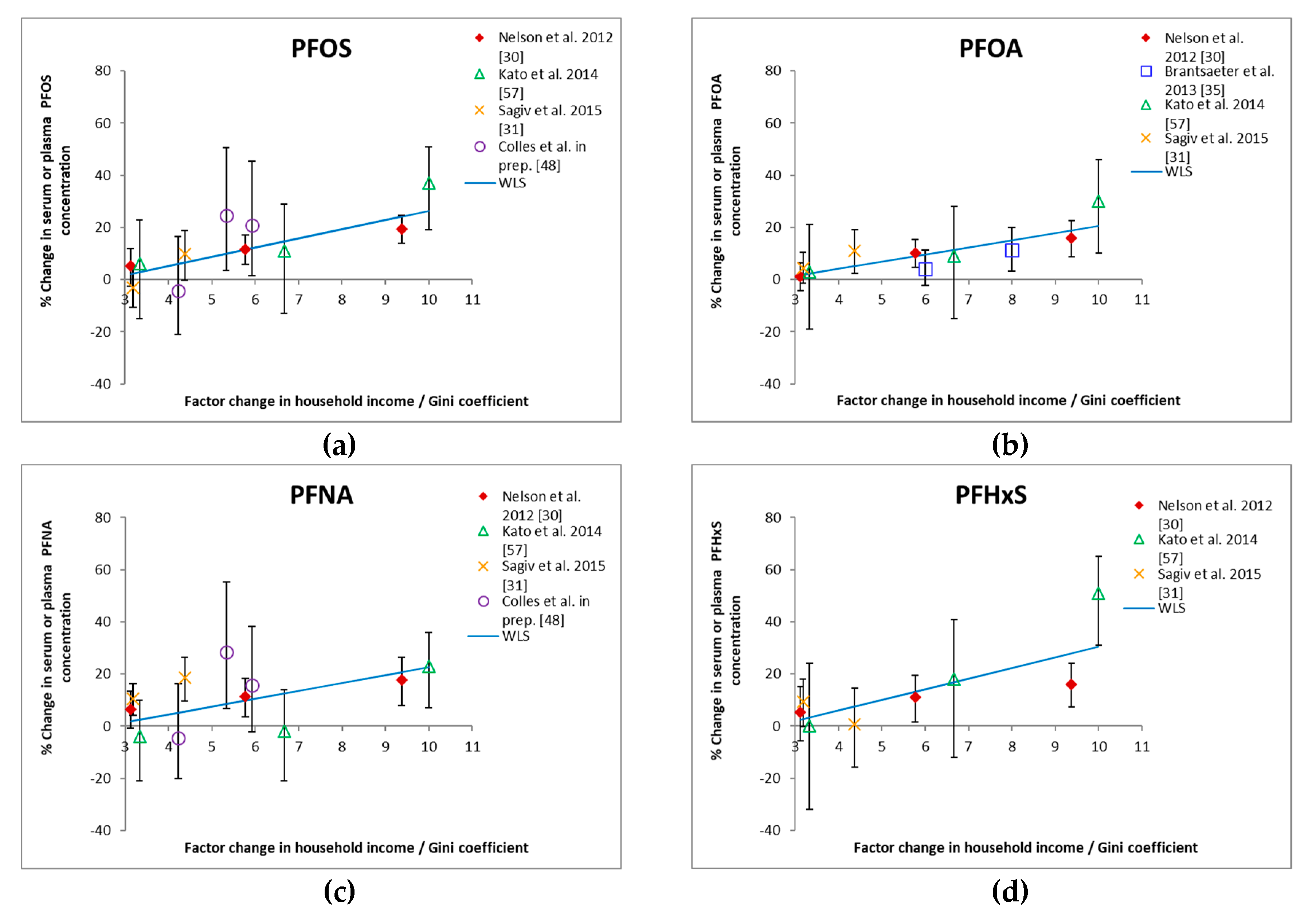
3.1. Income and HBM
3.2. Education and HBM
3.3. PIR (Poverty Income Ratio) and HBM
3.4. Determining Factors
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Environment Agency. EEA Report of the EEA Scientific Committee Seminar on Environment, Human Health and Well-Being: Advancing the Knowledge Database; EEA: Copenhagen, Denmark, 2014. [Google Scholar]
- Dahlgren, G.; Whitehead, M. Policies and Strategies to Promote Social Equity in Health. Background Document to WHO—Stategy Paper for Europe; Institute for Future Studies: Stockholm, Sweden, 1991; ISBN 978-91-85619-18-4. [Google Scholar]
- Briggs, D.J. A framework for integrated environmental health impact assessment of systemic risks. Environ. Health 2008, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- NCI Natural Capital Initiative. Valuing our Life Support Systems; Symposium Report: April 29–May 01 2009. Available online: https://www.naturalcapitalinitiative.org.uk/wp-content/uploads/2014/06/nci_full_lo.pdf (accessed on 3 September 2018).
- European Environment Agency—Joint Research Centre. Environment and Human Health; EEA: Copenhagen, Denmark, 2013. [Google Scholar]
- WHO. WHO Declaration of the Sixth Ministerial Conference on Environment and Health; WHO Regional Office for Europe: Copenhagen, Denmark, 2017. [Google Scholar]
- European Parliament and the Council. EU Regulation No 282/2014 of the European Parliament and of the Council of 11 March 2014 on the Establishment of a Third Programme for the Union’s Action in the Field of Health (2014–2020) and Repealing Decision No 1350/2007/EC 2014. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2014.086.01.0001.01.ENG (accessed on 12 December 2018).
- Brainard, J.S.; Jones, A.P.; Bateman, I.J.; Lovett, A.A.; Fallon, P.J. Modelling Environmental Equity: Access to Air Quality in Birmingham, England. Environ. Plan. A 2002, 34, 695–716. [Google Scholar] [CrossRef]
- Marmot, M.; Allen, J.; Goldblatt, P.; Boyce, T.; McNeish, D.; Grady, M.; Geddes, I. Fair Society, Healthy Lives. The Marmot Review. Strategic Review of Health Inequalities in England Post-2010; University College London: London, UK, 2010; ISBN 978-0-9564870-0-1. [Google Scholar]
- Llop, S.; Ballester, F.; Estarlich, M.; Iñiguez, C.; Ramón, R.; Gonzalez, M.C.; Murcia, M.; Esplugues, A.; Rebagliato, M. Social factors associated with nitrogen dioxide (NO2) exposure during pregnancy: The INMA-Valencia project in Spain. Soc. Sci. Med. 2011, 72, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Walker, G. Environmental Justice: Concepts, Evidence and Politics; Routledge: Abingdon Oxon, UK, 2012; ISBN 978-0-415-58973-4. [Google Scholar]
- Fecht, D.; Fischer, P.; Fortunato, L.; Hoek, G.; de Hoogh, K.; Marra, M.; Kruize, H.; Vienneau, D.; Beelen, R.; Hansell, A. Associations between air pollution and socioeconomic characteristics, ethnicity and age profile of neighbourhoods in England and The Netherlands. Environ. Pollut. 2015, 198, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Morrens, B.; Den Hond, E.; Schoeters, G.; Coertjens, D.; Colles, A.; Nawrot, T.S.; Baeyens, W.; De Henauw, S.; Nelen, V.; Loots, I. Human biomonitoring from an environmental justice perspective: Supporting study participation of women of Turkish and Moroccan descent. Environ. Health 2017, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, N. Community Capacity for Environmental Health Promotion: Determinants and Implications for Practice. Health Educ. Behav. 2004, 31, 472–490. [Google Scholar] [CrossRef]
- Universiteit Antwerpen (Faculteit Politieke en Sociale Wetenschappen) en Vlaams Instituut Gezond Leven. Pilootproject Sociale Ongelijkheid en Milieu—Studie Uitgevoerd in Opdracht van Vlaams Planbureau voor Omgeving. 2016. Available online: https://www.milieuinfo.be/dms/d/d/workspace/SpacesStore/2a7a59e8-fa40-453c-bec5-6c7883a4031e/Pilootprojectsocialeongelijkheidenmilieu_EINDRAPPORT.pdf (accessed on 22 November 2018).
- Clougherty, J.E.; Kubzansky, L.D. A Framework for Examining Social Stress and Susceptibility to Air Pollution in Respiratory Health. Environ. Health Perspect. 2009, 117, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Vineis, P.; Stringhini, S.; Porta, M. The environmental roots of non-communicable diseases (NCDs) and the epigenetic impacts of globalization. Environ. Res. 2014, 133, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Duncan, G.J.; Daly, M.C.; McDonough, P.; Williams, D.R. Optimal indicators of socioeconomic status for health research. Am. J. Public Health 2002, 92, 1151–1157. [Google Scholar] [CrossRef]
- Dockery, D.W.; Pope, C.A.; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G.; Speizer, F.E. An association between air pollution and mortality in six U.S. cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef]
- Pope, C.A.; Thun, M.J.; Namboodiri, M.M.; Dockery, D.W.; Evans, J.S.; Speizer, F.E.; Heath, C.W. Particulate Air-Pollution As A Predictor of Mortality in A Prospective-Study of US Adults. Am. J. Respir. Crit. Care Med. 1995, 151, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, J.; Melzer, D.; Henley, W.; Galloway, T.S.; Osborne, N.J. Associations between socioeconomic status and environmental toxicant concentrations in adults in the USA: NHANES 2001–2010. Environ. Int. 2013, 59, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Morello-Frosch, R.; Zuk, M.; Jerrett, M.; Shamasunder, B.; Kyle, A.D. Understanding the cumulative impacts of inequalities in environmental health: Implications for policy. Health Aff. Millwood 2011, 30, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Frumkin, H. Environmental Health: From Global to Local, 3rd ed.; WILEY: San Francisco, CA, USA, 2016; p. 260. ISBN 978-0-470-56778-4. [Google Scholar]
- Phelan, J.C.; Link, B.G.; Tehranifar, P. Social Conditions as Fundamental Causes of Health Inequalities: Theory, Evidence, and Policy Implications. J. Health Soc. Behav. 2010, 51, S28–S40. [Google Scholar] [CrossRef] [PubMed]
- Vesterinen, H.M.; Morello-Frosch, R.; Sen, S.; Zeise, L.; Woodruff, T.J. Cumulative effects of prenatal-exposure to exogenous chemicals and psychosocial stress on fetal growth: Systematic-review of the human and animal evidence. PLoS ONE 2017, 12, e0176331. [Google Scholar] [CrossRef] [PubMed]
- Senier, L.; Brown, P.; Shostak, S.; Hanna, B. The socio-exposome: Advancing exposure science and environmental justice in a postgenomic era. Environ. Sociol. 2016, 3, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Neidell, M.J. Air pollution, health, and socio-economic status: The effect of outdoor air quality on childhood asthma. J. Health Econ. 2004, 23, 1209–1236. [Google Scholar] [CrossRef]
- Human Biomonitoring for Europe. HBM4EU. Available online: https://www.hbm4eu.eu/about-hbm4eu/ (accessed on 3 September 2018).
- IPCHEM portal. IPCHEM—EU Information Platform for Chemical Monitoring. Available online: https://ipchem.jrc.ec.europa.eu/RDSIdiscovery/ipchem/index.html (accessed on 3 September 2018).
- Nelson, J.W.; Scammell, M.K.; Hatch, E.E.; Webster, T.F. Social disparities in exposures to bisphenol A and polyfluoroalkyl chemicals: A cross-sectional study within NHANES 2003–2006. Environ. Health 2012, 11, 10. [Google Scholar] [CrossRef]
- Sagiv, S.K.; Rifas-Shiman, S.L.; Webster, T.F.; Mora, A.M.; Harris, M.H.; Calafat, A.M.; Ye, X.; Gillman, M.W.; Oken, E. Sociodemographic and Perinatal Predictors of Early Pregnancy Per- and Polyfluoroalkyl Substance (PFAS) Concentrations. Environ. Sci. Technol. 2015, 49, 11849–11858. [Google Scholar] [CrossRef]
- Lindim, C.; van Gils, J.; Cousins, I.T. Europe-wide estuarine export and surface water concentrations of PFOS and PFOA. Water Res. 2016, 103, 124–132. [Google Scholar] [CrossRef]
- Haug, L.S.; Thomsen, C.; Becher, G. Time trends and the influence of age and gender on serum concentrations of perfluorinated compounds in archived human samples. Environ. Sci. Technol. 2009, 43, 2131–2136. [Google Scholar] [CrossRef] [PubMed]
- Glynn, A.; Berger, U.; Bignert, A.; Ullah, S.; Aune, M.; Lignell, S.; Darnerud, P.O. Perfluorinated alkyl acids in blood serum from primiparous women in Sweden: Serial sampling during pregnancy and nursing, and temporal trends 1996–2010. Environ. Sci. Technol. 2012, 46, 9071–9079. [Google Scholar] [CrossRef]
- Brantsæter, A.L.; Whitworth, K.W.; Ydersbond, T.A.; Haug, L.S.; Haugen, M.; Knutsen, H.K.; Thomsen, C.; Meltzer, H.M.; Becher, G.; Sabaredzovic, A.; et al. Determinants of plasma concentrations of perfluoroalkyl substances in pregnant Norwegian women. Environ. Int. 2013, 54, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Schoeters, G.; Govarts, E.; Bruckers, L.; Den Hond, E.; Nelen, V.; De Henauw, S.; Sioen, I.; Nawrot, T.S.; Plusquin, M.; Vriens, A.; et al. Three cycles of human biomonitoring in Flanders—Time trends observed in the Flemish Environment and Health Study. Int. J. Hyg. Environ. Health 2017, 220, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.W.; Mabury, S.A.; Solomon, K.R.; Muir, D.C.G. Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 2003, 22, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Olsen, G.W.; Burris, J.M.; Ehresman, D.J.; Froehlich, J.W.; Seacat, A.M.; Butenhoff, J.L.; Zobel, L.R. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007, 115, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Anitole, K.; Hodes, C.; Lai, D.; Pfahles-Hutchens, A.; Seed, J. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol. Sci. 2007, 99, 366–394. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Andersen, E.W.; Budtz-Jørgensen, E.; Nielsen, F.; Mølbak, K.; Weihe, P.; Heilmann, C. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 2012, 307, 391–397. [Google Scholar] [CrossRef]
- Grandjean, P.; Budtz-Jørgensen, E. Immunotoxicity of perfluorinated alkylates: Calculation of benchmark doses based on serum concentrations in children. Environ. Health 2013, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Haug, L.S.; Huber, S.; Becher, G.; Thomsen, C. Characterisation of human exposure pathways to perfluorinated compounds–comparing exposure estimates with biomarkers of exposure. Environ. Int. 2011, 37, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, C.; D’Hollander, W.; Roosens, L.; Covaci, A.; Smolders, R.; Van Den Heuvel, R.; Govarts, E.; Van Campenhout, K.; Reynders, H.; Bervoets, L. First assessment of population exposure to perfluorinated compounds in Flanders, Belgium. Chemosphere 2012, 86, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Ode, A.; Rylander, L.; Lindh, C.H.; Källén, K.; Jönsson, B.A.G.; Gustafsson, P.; Olofsson, P.; Ivarsson, S.A.; Rignell-Hydbom, A. Determinants of maternal and fetal exposure and temporal trends of perfluorinated compounds. Environ. Sci. Pollut. Res. Int. 2013, 20, 7970–7978. [Google Scholar] [CrossRef] [PubMed]
- Beesoon, S.; Webster, G.M.; Shoeib, M.; Harner, T.; Benskin, J.P.; Martin, J.W. Isomer profiles of perfluorochemicals in matched maternal, cord, and house dust samples: Manufacturing sources and transplacental transfer. Environ. Health Perspect. 2011, 119, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Arbuckle, T.E.; Kubwabo, C.; Walker, M.; Davis, K.; Lalonde, K.; Kosarac, I.; Wen, S.W.; Arnold, D.L. Umbilical cord blood levels of perfluoroalkyl acids and polybrominated flame retardants. Int. J. Hyg. Environ. Health 2013, 216, 184–194. [Google Scholar] [CrossRef]
- Jain, R.B. Contribution of diet and other factors to the levels of selected polyfluorinated compounds: Data from NHANES 2003–2008. Int. J. Hyg. Environ. Health 2014, 217, 52–61. [Google Scholar] [CrossRef]
- Colles, A.; Bruckers, L.; Den, H.E.; Govarts, E.; Morrens, B.; Schettgen, T.; Buekers, J.; Coertjens, D.; Nawrot, T.; Loots, I.; et al. Perfluorinated substances in the Flemish population (Belgium): Levels and determinants of exposure. Chemosphere. under review.
- Lauritzen, H.B.; Larose, T.L.; Øien, T.; Odland, J.Ø.; van de Bor, M.; Jacobsen, G.W.; Sandanger, T.M. Factors Associated with Maternal Serum Levels of Perfluoroalkyl Substances and Organochlorines: A Descriptive Study of Parous Women in Norway and Sweden. PLoS ONE 2016, 11, e0166127. [Google Scholar] [CrossRef]
- Sochorová, L.; Hanzlíková, L.; Černá, M.; Drgáčová, A.; Fialová, A.; Švarcová, A.; Gramblička, T.; Pulkrabová, J. Perfluorinated alkylated substances and brominated flame retardants in serum of the Czech adult population. Int. J. Hyg. Environ. Health 2017, 220, 235–243. [Google Scholar] [CrossRef]
- Fisher, M.; Arbuckle, T.E.; Liang, C.L.; LeBlanc, A.; Gaudreau, E.; Foster, W.G.; Haines, D.; Davis, K.; Fraser, W.D. Concentrations of persistent organic pollutants in maternal and cord blood from the maternal-infant research on environmental chemicals (MIREC) cohort study. Environ. Health 2016, 15, 59. [Google Scholar] [CrossRef]
- Berg, V.; Nøst, T.H.; Huber, S.; Rylander, C.; Hansen, S.; Veyhe, A.S.; Fuskevåg, O.M.; Odland, J.Ø.; Sandanger, T.M. Maternal serum concentrations of per- and polyfluoroalkyl substances and their predictors in years with reduced production and use. Environ. Int. 2014, 69, 58–66. [Google Scholar] [CrossRef]
- Apelberg, B.J.; Goldman, L.R.; Calafat, A.M.; Herbstman, J.B.; Kuklenyik, Z.; Heidler, J.; Needham, L.L.; Halden, R.U.; Witter, F.R. Determinants of fetal exposure to polyfluoroalkyl compounds in Baltimore, Maryland. Environ. Sci. Technol. 2007, 41, 3891–3897. [Google Scholar] [CrossRef]
- Calafat, A.M.; Wong, L.-Y.; Kuklenyik, Z.; Reidy, J.A.; Needham, L.L. Polyfluoroalkyl chemicals in the U.S. population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ. Health Perspect. 2007, 115, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.W.; Hoffman, K.; Thayer, K.A.; Daniels, J.L. Polyfluoroalkyl chemicals and menopause among women 20–65 years of age (NHANES). Environ. Health Perspect. 2014, 122, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Lewin, A.; Arbuckle, T.E.; Fisher, M.; Liang, C.L.; Marro, L.; Davis, K.; Abdelouahab, N.; Fraser, W.D. Univariate predictors of maternal concentrations of environmental chemicals: The MIREC study. Int. J. Hyg. Environ. Health 2017, 220, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Wong, L.-Y.; Chen, A.; Dunbar, C.; Webster, G.M.; Lanphear, B.P.; Calafat, A.M. Changes in serum concentrations of maternal poly- and perfluoroalkyl substances over the course of pregnancy and predictors of exposure in a multiethnic cohort of Cincinnati, Ohio pregnant women during 2003–2006. Environ. Sci. Technol. 2014, 48, 9600–9608. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-operation and Development. OECD Ginicoefficient for Income Inequality. Available online: https://data.oecd.org/inequality/income-inequality.htm (accessed on 3 September 2018).
- Berkman, L.; Kawachi, I.; Glymour, M.M. Social Epidemiology; Oxford University Press: New York, NY, USA, 2000; ISBN 978-0-19-939533-0. [Google Scholar]
- DEMOCOPHES. DEMOCOPHES. Available online: http://www.eu-hbm.info/democophes (accessed on 3 September 2018).
- International Standard Classification of Education. Eurostat ISCED. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/International_Standard_Classification_of_Education_(ISCED) (accessed on 3 September 2018).
- Webster, J.B.; Bishaw, A. Income, Earnings, and Poverty Data from the 2005 American Community Survey; US Census: Washington, DC, USA, 2005.
- National Research Council. Human Biomonitoring for Environmental Chemicals; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Halldorsson, T.I.; Fei, C.; Olsen, J.; Lipworth, L.; McLaughlin, J.K.; Olsen, S.F. Dietary predictors of perfluorinated chemicals: A study from the Danish National Birth Cohort. Environ. Sci. Technol. 2008, 42, 8971–8977. [Google Scholar] [CrossRef] [PubMed]
- Haug, L.S.; Thomsen, C.; Brantsæter, A.L.; Kvalem, H.E.; Haugen, M.; Becher, G.; Alexander, J.; Meltzer, H.M.; Knutsen, H.K. Diet and particularly seafood are major sources of perfluorinated compounds in humans. Environ. Int. 2010, 36, 772–778. [Google Scholar] [CrossRef]
- Turrell, G.; Hewitt, B.; Patterson, C.; Oldenburg, B. Measuring socio-economic position in dietary research: Is choice of socio-economic indicator important? Public Health Nutr. 2003, 6, 191–200. [Google Scholar] [CrossRef]
- Popkin, B.M.; Zizza, C.; Siega-Riz, A.M. Who is leading the change?. U.S. dietary quality comparison between 1965 and 1996. Am. J. Prev. Med. 2003, 25, 1–8. [Google Scholar] [CrossRef]
- Vlismas, K.; Stavrinos, V.; Panagiotakos, D.B. Socio-economic status, dietary habits and health-related outcomes in various parts of the world: A review. Cent. Eur. J. Public Health 2009, 17, 55–63. [Google Scholar] [CrossRef]
- Perfood. PERFOOD. Available online: https://ibed.fnwi.uva.nl/perfood/ (accessed on 20 November 2018).
- Herzke, D.; Huber, S.; Bervoets, L.; D’Hollander, W.; Hajslova, J.; Pulkrabova, J.; Brambilla, G.; De Filippis, S.P.; Klenow, S.; Heinemeyer, G.; et al. Perfluorinated alkylated substances in vegetables collected in four European countries; occurrence and human exposure estimations. Environ. Sci. Pollut. Res. Int. 2013, 20, 7930–7939. [Google Scholar] [CrossRef]
- Hlouskova, V.; Hradkova, P.; Poustka, J.; Brambilla, G.; De Filipps, S.P.; D’Hollander, W.; Bervoets, L.; Herzke, D.; Huber, S.; de Voogt, P.; et al. Occurrence of perfluoroalkyl substances (PFASs) in various food items of animal origin collected in four European countries. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2013, 30, 1918–1932. [Google Scholar] [CrossRef] [PubMed]
- D’Hollander, W.; Herzke, D.; Huber, S.; Hajslova, J.; Pulkrabova, J.; Brambilla, G.; De Filippis, S.P.; Bervoets, L.; de Voogt, P. Occurrence of perfluorinated alkylated substances in cereals, salt, sweets and fruit items collected in four European countries. Chemosphere 2015, 129, 179–185. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Opinion of the scientific panel on contaminants in the food chain on perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and their salts. EFSA J. 2008, 653, 1–131. [Google Scholar]
- Klenow, S.; Heinemeyer, G.; Brambilla, G.; Dellatte, E.; Herzke, D.; de Voogt, P. Dietary exposure to selected perfluoroalkyl acids (PFAAs) in four European regions. Food Addit. Contam. Part A 2013, 30, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- De Ridder, K.; Bel, S.; Brocatus, L.; Cuypers, K.; Lebacq, T.; Moyersoen, I.; Ost, C.; Teppers, E. Voedselconsumptiepeiling 2014–2015. Rapport 4: De Consumptie van Voedingsmiddelen en de Inname van Voedingsstoffen; WIV-ISP: Brussel, Belgium, 2015. [Google Scholar]
- STATBEL. De Belgische Lonen Onder de Loep. Available online: https://statbel.fgov.be/nl/nieuws/de-belgische-lonen-onder-de-loep (accessed on 21 November 2018).
- Tittlemier, S.A.; Pepper, K.; Seymour, C.; Moisey, J.; Bronson, R.; Cao, X.-L.; Dabeka, R.W. Dietary exposure of Canadians to perfluorinated carboxylates and perfluorooctane sulfonate via consumption of meat, fish, fast foods, and food items prepared in their packaging. J. Agric. Food Chem. 2007, 55, 3203–3210. [Google Scholar] [CrossRef] [PubMed]
- Schecter, A.; Colacino, J.; Haffner, D.; Patel, K.; Opel, M.; Päpke, O.; Birnbaum, L. Perfluorinated compounds, polychlorinated biphenyls, and organochlorine pesticide contamination in composite food samples from Dallas, Texas, USA. Environ. Health Perspect. 2010, 118, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Canfax Research Services. Fact Sheet Consumer Demand. 2015. Available online: http://www.canfax.ca/Samples/Consumer Demand May 2015.pdf (accessed on 22 November 2018).
- United States Department of Agriculture. USDA Food Demand Analysis. 2012. Available online: https://www.ers.usda.gov/topics/food-choices-health/food-consumption-demand/food-demand-analysis/ (accessed on 22 November 2018).
- Tonsor, G.; Mintert, J.; Schroeder, T. US Meat Demand: Household Dynamics and Media Information Impacts. J. Agric. Resour. Econ. 2010, 35, 1–17. [Google Scholar]
- Begley, T.H.; Hsu, W.; Noonan, G.; Diachenko, G. Migration of fluorochemical paper additives from food-contact paper into foods and food simulants. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2008, 25, 384–390. [Google Scholar] [CrossRef]
- Trier, X.; Granby, K.; Christensen, J.H. Polyfluorinated surfactants (PFS) in paper and board coatings for food packaging. Environ. Sci. Pollut. Res. Int. 2011, 18, 1108–1120. [Google Scholar] [CrossRef]
- Xie, W.; Wu, Q.; Kania-Korwel, I.; Tharappel, J.C.; Telu, S.; Coleman, M.C.; Glauert, H.P.; Kannan, K.; Mariappan, S.V.S.; Spitz, D.R.; et al. Subacute exposure to N-ethyl perfluorooctanesulfonamidoethanol results in the formation of perfluorooctanesulfonate and alters superoxide dismutase activity in female rats. Arch. Toxicol. 2009, 83, 909–924. [Google Scholar] [CrossRef]
- Office of Environmental Health Hazard Assessment (OEHHA). Potential Designated Chemicals: Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs). 2015. Available online: https://biomonitoring.ca.gov/downloads/potential-designated-chemicals-perfluoroalkyl-and-polyfluoroalkyl-substances-pfass (accessed on 22 November 2018).
- Herzke, D.; Olsson, E.; Posner, S. Perfluoroalkyl and polyfluoroalkyl substances (PFASs) in consumer products in Norway—A pilot study. Chemosphere 2012, 88, 980–987. [Google Scholar] [CrossRef]
- Fraser, A.J.; Webster, T.F.; Watkins, D.J.; Nelson, J.W.; Stapleton, H.M.; Calafat, A.M.; Kato, K.; Shoeib, M.; Vieira, V.M.; McClean, M.D. Polyfluorinated compounds in serum linked to indoor air in office environments. Environ. Sci. Technol. 2012, 46, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, J.-P.; Parent, A.-S.; Kleinjans, J.C.S.; Nawrot, T.S.; Schoeters, G.; Van Larebeke, N. Rationale for Environmental Hygiene towards global protection of fetuses and young children from adverse lifestyle factors. Environ. Health 2018, 17, 42. [Google Scholar] [CrossRef] [PubMed]

| Search Term 1 in All Fields | Search Term 2 in All Fields | Number of Studies Found | Number of Studies Selected Based on Title and Abstract Relevance | Selected Studies a,b |
|---|---|---|---|---|
| PFAS | / | 504 | 0 | Too many results. Search term added. |
| SES | 2 | 0 | / | |
| Socio-economic c | 10 | 1 | [31] | |
| Education | 35 | 3 | [49,50,51] | |
| Income | 4 | 0 | / | |
| Predictor | 7 | 1 | [52] | |
| Polyfluoroalkyl | / | 265 | 0 | Too many results. Search term added. |
| SES | 0 | 0 | / | |
| Socio-economic | 3 | 2 | [30,53] | |
| Education | 13 | 2 | [54,55] | |
| Income | 1 | 0 | / | |
| Predictor | 3 | 0 | / | |
| Perfluoroalkyl | / | 1453 | 0 | Too many results. Search term added. |
| SES | 2 | 1 | [56] | |
| Socio-economic | 6 | 0 | / | |
| Education | 66 | 0 | / | |
| Income | 7 | 1 | [57] | |
| Predictor | 5 | 0 | / | |
| Total selected | 11 |
| Reference (Sampling Time) | Country | Age Category (Years) | Size | Household Income | GM or Median Concentration (ng/mL) | % Change per Income Category from Regression Models | Remark | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFOS | PFOA | PFNA | PFHxS | PFOS | PFOA | PFNA | PFHxS | ||||||
| Nelson et al. [30] (sampling period 2003–2006) | US | Adolescents and adults (>12 y) | 3953 | $0–19,999 | 16.5 | 3.4 | 0.9 | 1.7 | −19.3(−24.6, −13.8) | −15.9(−22.5, −8.7) | −17.8(−26.5, −8.0) | −16.1(−24.1, −7.3) | NHANES (2003–2006); Multivariable linear regression model adjusted for NHANES cycle, age, gender, race/ethnicity, creatinine. |
| $20,000–44,999 | 17.9 | 3.7 | 0.9 | 1.8 | −11.6(−17.1, −5.8) | −10.1(−15.2, −4.6) | −11.3(−18.4, −3.5) | −11.0(−19.4, −1.7) | |||||
| $45,000–74,999 | 18.5 | 4 | 1 | 1.8 | −5.4(−11.8, 2.4) | −1.3(−6.5, 4.2) | −6.6(−13.5, 0.8) | −5.3(−15.1, 5.6) | |||||
| ≥$75,000 | 19.8 | 4.2 | 1.1 | 2 | Ref | Ref | Ref | Ref | |||||
| Brantsæter et al. [35] (sampling period 2003–2004) | Norway | Pregnant women (<25 y to >35 y) | 487 | Both < 300,000 NOK | 12.5 | 2.16 | 0.36 | 0.59 | Ref | Maternal education and household income both reflect socio-economic status and were not selected in the same multiple linear regression model. | |||
| One ≥ 300,000 NOK | 12.8 | 1.99 | 0.38 | 0.57 | 4.7(−2.6, 12.6) | ||||||||
| Both ≥300,000 NOK | 13.3 | 2.41 | 0.44 | 0.67 | 12.6(3.6, 22.3) | ||||||||
| Kato et al. [57] (sampling period 2003–2006) | US (Cincinnati) | Pregnant women (≥18 y) | 180 | <$20,000 | 9.44 | 4.1 | 0.64 | 0.84 | −37(−51, −19) | −30(−46, −10) | −23(−36, −7) | −51(−65, −31) | Univariate linear regression model. |
| $20,000–40,000 | 13.29 | 5.35 | 0.84 | 1.4 | −11(−29, 13) | −9(−28, 15) | 2(−14, 21) | −18(−41, 12) | |||||
| $40,000–80,000 | 13.98 | 5.69 | 0.86 | 1.72 | −6(−23, 15) | −3(−21, 19) | 4(−10, 21) | 0(−24, 32) | |||||
| >$80,000 | 14.87 | 5.89 | 0.83 | 1.71 | Ref | Ref | Ref | Ref | |||||
| Sagiv et al. [31] (sampling period 1999–2002) | US (Boston) | Pregnant women (<20 y to >35 y) | 1645 | <$40,000 | 24.3 | 5.3 | 0.6 | 2.3 | −9.8(−18.9, 0.3) | −11.1(−19.1, −2.4) | −18.5(−26.5, −9.7) | −0.7(−14.7, 15.7) | Fully adjusted multivariable linear regression model; adjusted for year, age, race/ethnicity, education, marital status, smoking, parity, breastfeeding, BMI, gestational age, albumin, GFR. |
| $40,000–70,000 | 26.9 | 5.7 | 0.6 | 2.4 | 3.2(−3.8, 10.6) | −4.5(−10.3, 1.5) | −10.4(−16.3, −4.1) | −9.4(−18.0, 0.1) | |||||
| >$70,000 | 24.9 | 5.7 | 0.7 | 2.6 | Ref | Ref | Ref | Ref | |||||
| Colles et al. [48] (sampling period 2012–2015) | Belgium (Flanders) | Adults (50–65 y) | 168 | ≤€1250 | 6.348 | 0.729 | Ref | Not included in model a | Ref | Not included in model | Income is equivalent income b: household income corrected for the number of persons in the household. Stepwise multiple linear regression model with age, BMI and gender forced into model. | ||
| €1250–1600 | 6.066 | 0.702 | −6(−29, 23) | −6(−27, 22) | |||||||||
| €1600–2000 | 9.077 | 1.056 | 34(5, 70) | 38(9, 74) | |||||||||
| >€2000 | 8.792 | 0.975 | 29(2, 63) | 21(−3, 51) | |||||||||
| Nr. | Educational Level | Potatoes and Potato Products | Fish, Fish Preparations, Shellfish | Meat & Meat Preparations | Vegetables | Fruit | Eggs |
|---|---|---|---|---|---|---|---|
| Group 1 | ISCED0-2 | 44 | 25 | 131 | 152 | 71 | 8 |
| Group 2 | ISCED3-6 | 43 | 26 | 125 | 172 | 101 | 7 |
| Group 3 | ISCED7-8 | 36 | 29 | 107 | 205 | 115 | 8 |
| Nr. | Educational Level | Unit | Potatoes and Potato Products | Fish and Seafood | Meat & Meat Preparations | Vegetables | Fruit | Eggs | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Fish (Fresh and Marine) | Seafood | |||||||||
| Group 1 | ISCED0-2 | ng PFOS/day | 4.4 | 5.1 | 5.6 | 0.5 | 6.9 | 0.5 | 23 | |
| Group 2 | ISCED3-6 | - | 4.7 | 5.4 | 5.3 | 0.6 | 9.9 | 0.4 | 26 | |
| Group 3 | ISCED7-8 | 5.2 | 6.0 | 4.6 | 0.7 | 11.3 | 0.5 | 28 | ||
| Percent change between group 1 and group 3 a | % | - | +17 | +17 | −18 | +34 | +63 | <1 | +22 | |
| Average contribution to total intake in percent | % | - | 18 | 21 | 20 | 2 | 36 | 2 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buekers, J.; Colles, A.; Cornelis, C.; Morrens, B.; Govarts, E.; Schoeters, G. Socio-Economic Status and Health: Evaluation of Human Biomonitored Chemical Exposure to Per- and Polyfluorinated Substances across Status. Int. J. Environ. Res. Public Health 2018, 15, 2818. https://doi.org/10.3390/ijerph15122818
Buekers J, Colles A, Cornelis C, Morrens B, Govarts E, Schoeters G. Socio-Economic Status and Health: Evaluation of Human Biomonitored Chemical Exposure to Per- and Polyfluorinated Substances across Status. International Journal of Environmental Research and Public Health. 2018; 15(12):2818. https://doi.org/10.3390/ijerph15122818
Chicago/Turabian StyleBuekers, Jurgen, Ann Colles, Christa Cornelis, Bert Morrens, Eva Govarts, and Greet Schoeters. 2018. "Socio-Economic Status and Health: Evaluation of Human Biomonitored Chemical Exposure to Per- and Polyfluorinated Substances across Status" International Journal of Environmental Research and Public Health 15, no. 12: 2818. https://doi.org/10.3390/ijerph15122818
APA StyleBuekers, J., Colles, A., Cornelis, C., Morrens, B., Govarts, E., & Schoeters, G. (2018). Socio-Economic Status and Health: Evaluation of Human Biomonitored Chemical Exposure to Per- and Polyfluorinated Substances across Status. International Journal of Environmental Research and Public Health, 15(12), 2818. https://doi.org/10.3390/ijerph15122818






