Nudibranchs as Sources of Marine Natural Products with Antitumor Activity: A Comprehensive Review
Abstract
1. Introduction
1.1. Marine-Derived Drugs
1.2. Marine Invertebrates as a Source of MNPs
2. Nudibranchs
3. Nudibranchs as a Source of Antitumor Molecules
3.1. Extraction and Isolation Methods for Obtaining Bioactive Extracts and Compounds from Nudibranchs
3.2. Chemical Structures of Antitumor Compounds Isolated from Nudibranchs
3.2.1. Examples of Antitumor Sesquiterpenes in Nudibranchs
3.2.2. Examples of Antitumor Diterpenes in Nudibranchs
3.2.3. Examples of Antitumor Alkaloids in Nudibranchs
3.2.4. Other Types of Antitumor Compounds in Nudibranchs
3.3. Antitumor Potential of Nudibranch-Derived Extracts and Compounds Against Cancer Cell Lines
3.3.1. Nudibranchs-Derived Extracts with Antitumor Activity
3.3.2. Antitumor Compounds Isolated from Phyllidiella pustulosa and Phyllidia coelestis
3.3.3. Antitumor Compounds Isolated from the Dendrodoris Genus
3.3.4. Antitumor Compounds Isolated from Jorunna funebris
3.3.5. Antitumor Compounds Isolated from Hexabranchus sanguineus
3.3.6. Antitumor Compounds Isolated from the Tambja Genus
3.3.7. Antitumor Compounds Isolated from Other Nudibranch Species
| Material | Extraction and Isolation Methods | Isolated Compounds | Antitumor Potential (IC50 Value) | Mechanisms of Action and Other Bioactive Effects |
|---|---|---|---|---|
| Phyllidiella pustulosa [42] |
| Isothiocyanate sesquiterpene (1R,6R,7R,10S-1-isothiocyano-4-amorphene) (1); isocyano sesquiterpene (1R,6R,7R,10S-1-isocyano-4-amorphene) (2) | Panc-1: 18.8 (1) and 23.7 µg/mL (2) NBT-T2: 17.5 (1) and 20.5 µg/mL (2) HCT-116: 15.6 (1) and 19.7 µg/mL (2) | - |
| Mantle and viscera of Phyllidiella pustulosa or Phyllidia coelestis [44] |
| Bisabolane-type sesquiterpenoids: 3-isocyanotheonellin (3) (P. pustulosa and P. coelestis); theonellin isothiocyanate (4) (P. coelestis), and 7-isocyano-7,8-dihydro-α-bisabolene (5) (P. coelestis) | A549: 8.6 (3) and >50 µM (4, 5) HT-29: 3.35 (3) and >50 µM (4, 5) Capan-1: 1.98 (3) and >50 µM (4, 5) SNU-398: 0.5 (3), 2.15 (4), and 0.5 µM (5) | - |
| Tubercle of Phyllidia coelestis [45] |
| Bridged tricyclic sesquiterpenes: 1-formamido-10(1 → 2)-abeopupukeanane (6) and 2-formamidopupukeanane (7) | HeLa: 0.13 (6) and 0.07 µM (7) MCF-7: 0.65 (6) and 8.2 µM (7) KB: 2.4 (6) and 1.2 µM (7) HT-29: 6.8 (6) and > 20 µM (7) | - |
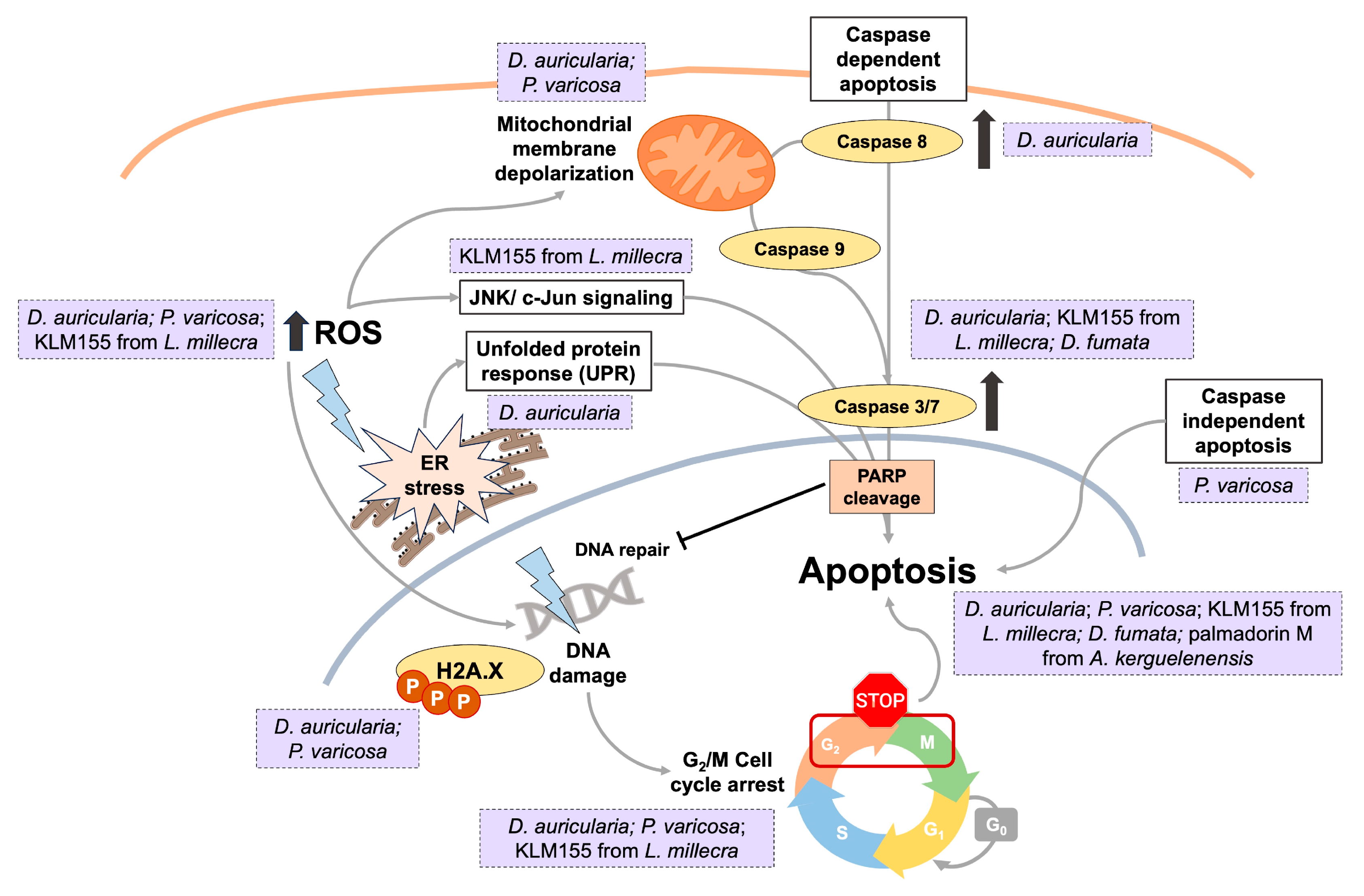
| Phyllidia varicosa; Dolabella auricularia [43] | Extraction with methanol–dichloromethane (1:1, v:v) at 4 °C for 24 h | - | HT-29: 9.3 (P. varicosa) and 0.1 µg/mL (D. auricularia) HGUE-C1: 78.8 (P. varicosa) and 0.1 µg/mL (D. auricularia) SW-480: 13 (P. varicosa) and 0.2 µg/mL (D. auricularia) | Colony formation; cell cycle arrest and apoptosis; ROS generation and mitochondrial membrane depolarization; DNA damage |
| Dolabella auricularia [72] | Extraction with methanol–dichloromethane (1:1, v:v) at 4 °C for 24 h | - | HCT-116: 1.01 µg/mL CCD-18Co: 15.04 µg/mL | ROS generation and activation ER stress; DNA damage; G2/M cell cycle arrest and apoptosis; ↓ colony formation, cell migration and invasion |
| Dendrodoris fumata [46] |
| Steroid: dendrodoristerol (8) | HL-60: 21.63 µM KB: 22.22 µM LU-1: 24.53 µM MCF-7: 41.19 µM LNCaP: 25.34 µM HepG2: 21.59 µM | Apoptosis |
| Dendrodoris carbunculosa [47] |
| Drimane sesquiterpenes: dendocarbin B (9), D (10), H–K (11–14); sesquiterpenoid isodrimeninol (15); and drimane lactone 11-epivaldiviolide (16) | P388: 3.2 (16) and 10–17 μg/mL (9–15) | - |
| Mantle or viscera Jorunna funebris [48] |
| Isoquinolinequinone alkaloid: fennebricin A (17) | A549: 6.2 µM HL-60: 2.5 µM | NF-κB signaling pathway inhibition |
| Mantle, viscera, and egg ribbons of Jorunna funebris [49] |
| Isoquinoline alkaloids: jorunnamycin A (18) and C (19), renieramycin M (20) | HCT-116: 13.0 (18), 1.5 (19), and 7.9 nM (20) QG56: 59.0 (18), 2.8 (19), and 19.0 nM (20) DU145: 29.0 (18) and 0.32 nM (19) | - |
| Mantle and mucus of Jorunna funebris [50] |
| Isoquinoline alkaloid: jorumycin (21) | P388: 12.5 ng/mL A549: 12.5 ng/mL HT-29: 12.5 ng/mL MEL28: 12.5 ng/mL | - |
| Internal organs of Hexabranchus sanguineus [51] |
| Gorgonane-type sesquiterpenoid: 4α-formamidogorgon-11-ene (22) | H1975: 0.87 μM MDA-MB-231: 1.04 μM A549: 1.95 μM H1299: 1.34 μM | - |
| Egg mass of Hexabranchus sanguineus [52] |
| Ulapualide A–C (23–25) | 768-0: 0.27 (23), 0.46 (24), and 1.3 μM (25) DU-145: 0.26 (23), 0.31 (24), and 0.8 μM (25) MDA-MB-231: 0.24 (23), 0.29 (24), and 0.67 μM (25) A549: 0.29 (23), 0.3 (24), and 0.64 μM (25) | - |
| Egg mass of Hexabranchus sanguineus [53] |
| Ulapualide A (23) and B (24) | L1210: 0.01–0.03 μg/mL | - |
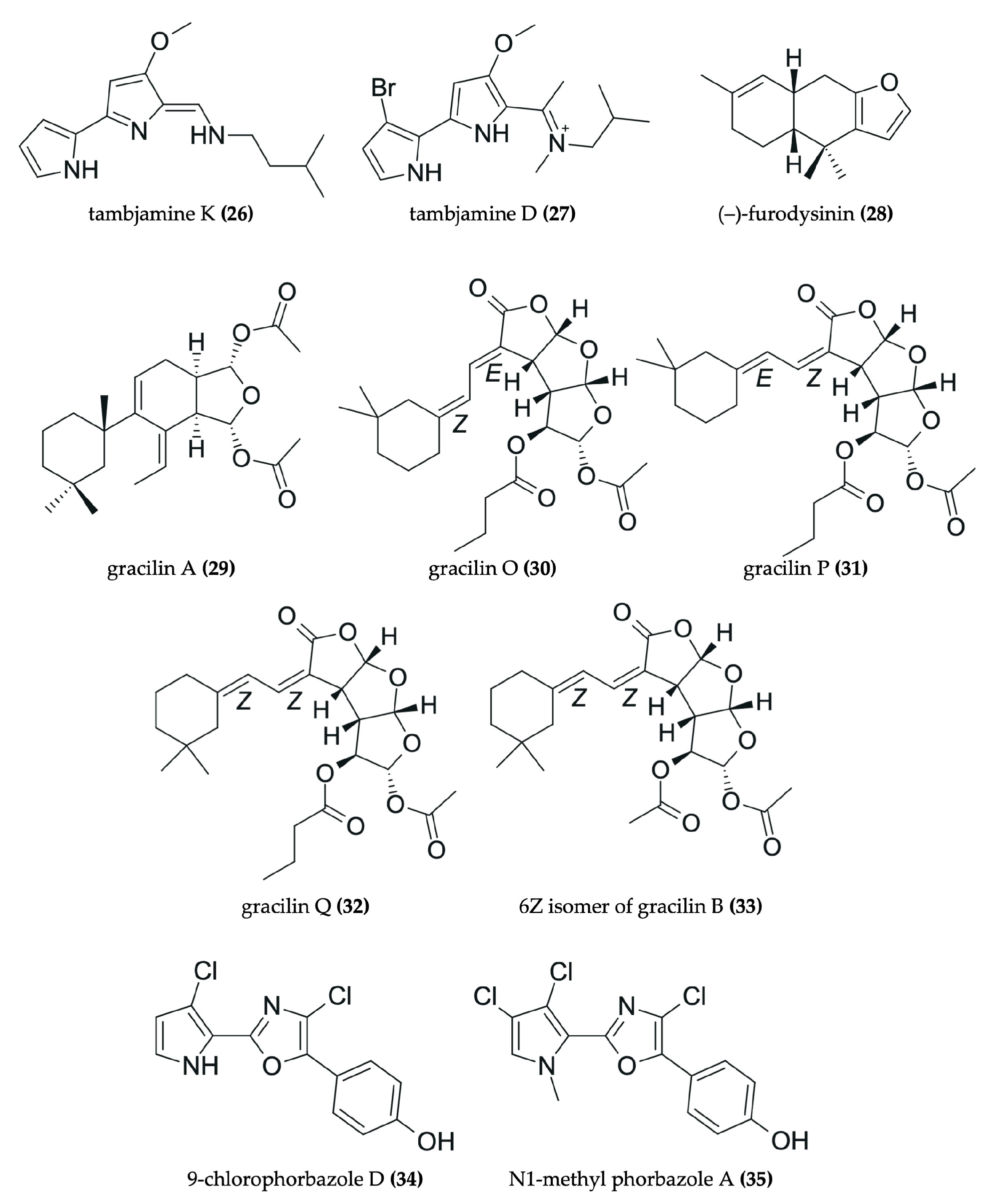
| Tambja ceutae [54] |
| Bromopyrrole alkaloid: tambjamine K (26) | Caco-2: 0.0035 µM HeLa: 14.6 µM C6: 14 µM H9c2: 2.7 µM 3T3-L1: 19 µM | - |
| Tambja eliora [55,56] |
| 4-methoxypyrrolic alkaloid: tambjamine D (27) | V79: 1.2 µg/mL CEM: 12.2 µg/mL HL60: 13.2 µg/mL MCF7: 13.2 µg/mL HCT-8: 10.1 µg/mL B16: 6.7 µg/mL | V79: Apoptosis; ROS generation; ↑ nitrite/nitrate and TBARS production; genotoxicity |
| Armina tigrina; A. maculata; and A. tricolorata [73] | Extraction with acetone | - | AGS: 68.75 (A. tigrina); 220.66 (A. maculata); >500 µg/mL (A. tricolorata) A549: 69.77 (A. tigrina); 250–500 (A. maculata); >500 µg/mL (A. tricolorata) | Anti-inflammatory effect |
| Hypselodoris infucata [62] |
| Sesquiterpene: (–)-furodysinin (28) | HeLa: 102.7 µg/mL | - |
| Goniobranchus splendidus [63] |
| Diterpenoids: gracilins A (29), O (30), P (31), and Q (32); 6Z isomer of gracilin B (33) | HeLa S3: <0.30 (29–32) and 0.32 µg/mL (33) | - |
| Mantle or viscera of Aldisa andersoni [57] |
| Alkaloids: 9-chlorophorbazole D (34) and N1-methyl phorbazole A (35) | A549: 29 (34) and 34 µM (35) MCF-7: 18 (34) and 25 µM (35) SKMEL-28: 22 (34) and 29 µM (35) Hs683: 25 (34) and 25 µM (35) U373: 19 (34) and 19 µM (35) | - |
| Austrodoris kerguelenensis [66] |
| Diterpenoid glyceride esters: palmadorin A (36), B (37), D (38), M (39), N (40), and O (41) | HEL: 8.7 (36), 8.3 (37), 16.5 (38), 4.9 (39), 6.3 (40), and 13.4 μM (41) | Apoptosis (39) |
| Mantle and glands of Tritoniopsis elegans [59] |
| Cladiellane-based diterpene family: tritoniopsin B (42) | C6: no toxicity HeLa: no toxicity Caco-2: 40–65 μM H9c2: 40–65 μM 3T3-L1: 40–65 μM | - |
| Leminda millecra [64] |
| Toluhydroquinone: KLM155 (5-methyl-2-[(2′E,6′E)-3′,7′,11′-trimethyl-2′,6′-dodecadien-9′onyl]-1,4-dihydroxybenzene) (43) | WHCO1: 9.5 µM WHCO6: 5.8 µM ME180: 33.9 µM SiHa: >150 µM MCF12: 32 µM | Cell cycle arrest and apoptosis; ROS generation; JNK/c-Jun signaling |
| Reticulidia fungia [65] |
| Sesquiterpene carbonimidic dichlorides: reticulidins A (44) and B (45) | KB: 0.41 (44) and 0.42 μg/mL (45) L1210: 0.59 (44) and 0.11 μg/mL (45) | - |
| Chromodoris obsoleta [67] |
| Spongian diterpenoids: dorisenone A–D (46–49); 7α-hydroxyspongian-16-one (50); 15α, 16α-diacetoxy-11, 12β-epoxyspongian (51); 7α-acetoxydendrillol-3 (52); 7α-acetoxy- 17β-hydroxy- 15, 17-oxidospongian- 16-one (53); 11β-hydroxyspongi- 12-en- 16- one (54); spongian- 16-one (55); and 7α-acetoxyspongian- 16-one (56) | L1210: 0.21 (46), 1.0 (47), 7.5 (48), 0.8 (49), 7.5 (50), 0.18 (51), 4.8 (52), 1.9 (53), 1.0 (54), 5.0 (55), 2.2 μg/mL (56) KB: 0.22 (46), 1.5 (47), 19.0 (48), 1.4 (49), 10.2 (50), 0.98 (51), 15.0 (52), 2.5 (53), 1.9 (54), 9.2 (55), 16.0 μg/mL (56) | - |
3.4. Exemplary Mechanisms of Action
3.5. Other Biological Activities
4. Discussion and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRC | Colorectal cancer |
| ER | Endoplasmic reticulum |
| FDA | Food and Drug Administration |
| HPLC | High-performance liquid chromatography |
| LLE | Liquid–liquid extraction |
| LPS | Lipopolysaccharide |
| MNPs | Marine natural products |
| NMR | Nuclear magnetic resonance |
| NO | Nitric oxide |
| PUFAs | Polyunsaturated fatty acids |
| ROS | Reactive oxygen species |
| RP | Reversed phase |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Brianna; Lee, S.H. Chemotherapy: How to Reduce Its Adverse Effects While Maintaining the Potency? Med. Oncol. 2023, 40, 88. [Google Scholar] [CrossRef] [PubMed]
- Halbrook, C.J.; Lyssiotis, C.A.; Pasca di Magliano, M.; Maitra, A. Pancreatic Cancer: Advances and Challenges. Cell 2023, 186, 1729–1754. [Google Scholar] [CrossRef] [PubMed]
- Nooreldeen, R.; Bach, H. Current and Future Development in Lung Cancer Diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Ganesh, K.; Massagué, J. Targeting Metastatic Cancer. Nat. Med. 2021, 27, 34–44. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer Chemotherapy and beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Papon, N.; Copp, B.R.; Courdavault, V. Marine Drugs: Biology, Pipelines, Current and Future Prospects for Production. Biotechnol. Adv. 2022, 54, 107871. [Google Scholar] [CrossRef]
- Haque, N.; Parveen, S.; Tang, T.; Wei, J.; Huang, Z. Marine Natural Products in Clinical Use. Mar. Drugs 2022, 20, 528. [Google Scholar] [CrossRef]
- Loria, R.; Laquintana, V.; Bon, G.; Trisciuoglio, D.; Frapolli, R.; Covello, R.; Amoreo, C.A.; Ferraresi, V.; Zoccali, C.; Novello, M.; et al. HMGA1/E2F1 Axis and NFkB Pathways Regulate LPS Progression and Trabectedin Resistance. Oncogene 2018, 37, 5926–5938. [Google Scholar] [CrossRef]
- El-Tanani, M.; Rabbani, S.A.; Satyam, S.M.; Rangraze, I.R.; Wali, A.F.; El-Tanani, Y.; Aljabali, A.A.A. Deciphering the Role of Cancer Stem Cells: Drivers of Tumor Evolution, Therapeutic Resistance, and Precision Medicine Strategies. Cancers 2025, 17, 382. [Google Scholar] [CrossRef]
- Romano, G.; Almeida, M.; Varela Coelho, A.; Cutignano, A.; Gonçalves, L.G.; Hansen, E.; Khnykin, D.; Mass, T.; Ramšak, A.; Rocha, M.S.; et al. Biomaterials and Bioactive Natural Products from Marine Invertebrates: From Basic Research to Innovative Applications. Mar. Drugs 2022, 20, 219. [Google Scholar] [CrossRef]
- Ilhan, H.A.; Pulat, Ç.Ç. Cytotoxic and Antitumor Compounds from Marine Invertebrates. In Encyclopedia of Marine Biotechnology; Kim, S.-K., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 2529–2584. ISBN 978-1-119-14380-2. [Google Scholar]
- Leal, M.C.; Puga, J.; Serôdio, J.; Gomes, N.C.M.; Calado, R. Trends in the Discovery of New Marine Natural Products from Invertebrates over the Last Two Decades—Where and What Are We Bioprospecting? PLoS ONE 2012, 7, e30580. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Yang, D.; Cao, Y.; Peng, Z.; Wang, Y.; Xi, J.; Yan, C.; Li, X. Anticancer Potential of Active Alkaloids and Synthetic Analogs Derived from Marine Invertebrates. Eur. J. Med. Chem. 2024, 279, 116850. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.M.; Abdel-Hameid, M.K.; El-Nassan, H.B.; El-Khouly, E.A. Synthesis and Cytotoxic Activity of Novel Mono- and Bis-Indole Derivatives: Analogues of Marine Alkaloid Nortopsentin. Med. Chem. 2021, 17, 779–789. [Google Scholar] [CrossRef]
- Pecoraro, C.; Terrana, F.; Panzeca, G.; Parrino, B.; Cascioferro, S.; Diana, P.; Giovannetti, E.; Carbone, D. Nortopsentins as Leads from Marine Organisms for Anticancer and Anti-Inflammatory Agent Development. Molecules 2023, 28, 6450. [Google Scholar] [CrossRef]
- Li, C.-S.; Liu, L.-T.; Yang, L.; Li, J.; Dong, X. Chemistry and Bioactivity of Marine-Derived Bisabolane Sesquiterpenoids: A Review. Front. Chem. 2022, 10, 881767. [Google Scholar] [CrossRef]
- Shi, Q.; Yu, S.; Zhou, M.; Wang, P.; Li, W.; Jin, X.; Pan, Y.; Sheng, Y.; Li, H.; Qin, L.; et al. Diterpenoids of Marine Organisms: Isolation, Structures, and Bioactivities. Mar. Drugs 2025, 23, 131. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Balandin, S.V.; Belogurova-Ovchinnikova, O.Y.; Ovchinnikova, T.V. Marine Invertebrate Antimicrobial Peptides and Their Potential as Novel Peptide Antibiotics. Mar. Drugs 2023, 21, 503. [Google Scholar] [CrossRef]
- Raymond, M.J.F.; Rakotondraibe, H.L. Recent Updates on Terpenoids and Other Bioactive Constituents of Marine Sponges. Molecules 2025, 30, 1112. [Google Scholar] [CrossRef] [PubMed]
- Santhiravel, S.; Dave, D.; Shahidi, F. Bioactives from Marine Resources as Natural Health Products: A Review. Pharmacol. Rev. 2025, 77, 100006. [Google Scholar] [CrossRef] [PubMed]
- Gallimore, W. Marine Metabolites: Oceans of Opportunity. In Pharmacognosy; Badal, S., Delgoda, R., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 377–400. ISBN 978-0-12-802104-0. [Google Scholar]
- Dean, L.J.; Prinsep, M.R. The Chemistry and Chemical Ecology of Nudibranchs. Nat. Prod. Rep. 2017, 34, 1359–1390. [Google Scholar] [CrossRef] [PubMed]
- Caso, A.; da Silva, F.B.; Esposito, G.; Teta, R.; Sala, G.D.; Cavalcanti, L.P.A.N.; Valverde, A.L.; Martins, R.C.C.; Costantino, V. Exploring Chemical Diversity of Phorbas Sponges as a Source of Novel Lead Compounds in Drug Discovery. Mar. Drugs 2021, 19, 667. [Google Scholar] [CrossRef]
- Cheney, K.L.; White, A.; Mudianta, I.W.; Winters, A.E.; Quezada, M.; Capon, R.J.; Mollo, E.; Garson, M.J. Choose Your Weaponry: Selective Storage of a Single Toxic Compound, Latrunculin A, by Closely Related Nudibranch Molluscs. PLoS ONE 2016, 11, e0145134. [Google Scholar] [CrossRef]
- Carbone, M.; Gavagnin, M.; Haber, M.; Guo, Y.-W.; Fontana, A.; Manzo, E.; Genta-Jouve, G.; Tsoukatou, M.; Rudman, W.B.; Cimino, G.; et al. Packaging and Delivery of Chemical Weapons: A Defensive Trojan Horse Stratagem in Chromodorid Nudibranchs. PLoS ONE 2013, 8, e62075. [Google Scholar] [CrossRef]
- Cabeza, L.; Peña, M.; Martínez, R.; Mesas, C.; Galisteo, M.; Perazzoli, G.; Prados, J.; Porres, J.M.; Melguizo, C. Anemonia sulcata and Its Symbiont Symbiodinium as a Source of Anti-Tumor and Anti-Oxidant Compounds for Colon Cancer Therapy: A Preliminary In Vitro Study. Biology 2021, 10, 134. [Google Scholar] [CrossRef]
- Patra, S.; Praharaj, P.P.; Panigrahi, D.P.; Panda, B.; Bhol, C.S.; Mahapatra, K.K.; Mishra, S.R.; Behera, B.P.; Jena, M.; Sethi, G.; et al. Bioactive Compounds from Marine Invertebrates as Potent Anticancer Drugs: The Possible Pharmacophores Modulating Cell Death Pathways. Mol. Biol. Rep. 2020, 47, 7209–7228. [Google Scholar] [CrossRef]
- Bouchet, P.; Rocroi, J.-P.; Hausdorf, B.; Kaim, A.; Kano, Y.; Nützel, A.; Parkhaev, P.; Schrödl, M.; Strong, E.E. Revised Classification, Nomenclator and Typification of Gastropod and Monoplacophoran Families. Malacologia 2017, 61, 1–526. [Google Scholar] [CrossRef]
- Beckmann, A.; Özbek, S. The Nematocyst: A Molecular Map of the Cnidarian Stinging Organelle. Int. J. Dev. Biol. 2012, 56, 577–582. [Google Scholar] [CrossRef]
- Greenwood, P.G. Acquisition and Use of Nematocysts by Cnidarian Predators. Toxicon 2009, 54, 1065–1070. [Google Scholar] [CrossRef]
- Goodheart, J.A.; Barone, V.; Lyons, D.C. Movement and Storage of Nematocysts across Development in the Nudibranch Berghia stephanieae (Valdés, 2005). Front. Zool. 2022, 19, 16. [Google Scholar] [CrossRef]
- Vorobyeva, O.A.; Ekimova, I.A.; Malakhov, V.V. Morphological Organization of Cerata in the Nudibranch Pteraeolidia semperi (Gastropoda, Nudibranchia). Dokl. Biol. Sci. 2023, 508, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, G.; Kanai, N.; Miura, T.; Oguchi, K. Blue Angels Have Devil Hands: Predatory Behavior Using Cerata in Glaucus atlanticus. Ecology 2025, 106, e70062. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.; Giménez, F.; Marin, A.; Mollo, E.; Cimino, G. Transfer of Secondary Metabolites from the sponges Dysidea fragilis and Pleraplysilla spinifera to the Mantle Dermal Formations (MDFs) of the nudibranch Hypserlodoris webbi. Experientia 1994, 50, 510–516. [Google Scholar] [CrossRef]
- Wägele, H.; Klussmann-Kolb, A. Opisthobranchia (Mollusca, Gastropoda)—More than Just Slimy Slugs. Shell Reduction and Its Implications on Defence and Foraging. Front. Zool. 2005, 2, 3. [Google Scholar] [CrossRef]
- Winters, A.E.; White, A.M.; Dewi, A.S.; Mudianta, I.W.; Wilson, N.G.; Forster, L.C.; Garson, M.J.; Cheney, K.L. Distribution of Defensive Metabolites in Nudibranch Molluscs. J. Chem. Ecol. 2018, 44, 384–396. [Google Scholar] [CrossRef]
- Prieto-Baños, S.; Layton, K.K.S. Tracing the Evolution of Key Traits in Dorid Nudibranchs. PLoS ONE 2025, 20, e0317704. [Google Scholar] [CrossRef]
- Gomes, N.G.M.; Dasari, R.; Chandra, S.; Kiss, R.; Kornienko, A. Marine Invertebrate Metabolites with Anticancer Activities: Solutions to the “Supply Problem” Mar. Drugs 2016, 14, 98. [Google Scholar] [CrossRef]
- Kurnianda, V.; Hirade, H.; Jansen, R.; Tanaka, J. Two Nitrogenous Sesquiterpenoids from the Nudibranch Phyllidiella pustulosa. J. Asian Nat. Prod. Res. 2022, 24, 39–44. [Google Scholar] [CrossRef]
- Ruiz-Torres, V.; Rodríguez-Pérez, C.; Herranz-López, M.; Martín-García, B.; Gómez-Caravaca, A.-M.; Arráez-Román, D.; Segura-Carretero, A.; Barrajón-Catalán, E.; Micol, V. Marine Invertebrate Extracts Induce Colon Cancer Cell Death via ROS-Mediated DNA Oxidative Damage and Mitochondrial Impairment. Biomolecules 2019, 9, 771. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, W.-T.; Li, S.-W.; Ye, J.-Y.; Huan, X.-J.; Gavagnin, M.; Yao, L.-G.; Wang, H.; Miao, Z.-H.; Li, X.-W.; et al. Cytotoxic Nitrogenous Terpenoids from Two South China Sea Nudibranchs Phyllidiella pustulosa, Phyllidia coelestis, and Their Sponge-Prey Acanthella cavernosa. Mar. Drugs 2019, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Jaisamut, S.; Prabpai, S.; Tancharoen, C.; Yuenyongsawad, S.; Hannongbua, S.; Kongsaeree, P.; Plubrukarn, A. Bridged Tricyclic Sesquiterpenes from the Tubercle Nudibranch Phyllidia coelestis Bergh. J. Nat. Prod. 2013, 76, 2158–2161. [Google Scholar] [CrossRef]
- Huong, P.T.M.; Phong, N.V.; Thao, N.P.; Binh, P.T.; Thao, D.T.; Thanh, N.V.; Cuong, N.X.; Nam, N.H.; Thung, D.C.; Minh, C.V. Dendrodoristerol, a Cytotoxic C20 Steroid from the Vietnamese Nudibranch Mollusk Dendrodoris fumata. J. Asian Nat. Prod. Res. 2020, 22, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Sakio, Y.; Hirano, Y.J.; Hayashi, M.; Komiyama, K.; Ishibashi, M. Dendocarbins A−N, New Drimane Sesquiterpenes from the Nudibranch Dendrodoris carbunculosa. J. Nat. Prod. 2001, 64, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-Y.; Chen, W.-T.; Kurtán, T.; Mándi, A.; Ding, J.; Li, J.; Li, X.-W.; Guo, Y.-W. Bioactive Isoquinolinequinone Alkaloids from the South China Sea Nudibranch Jorunna funebris and Its Sponge-Prey Xestospongia Sp. Future Med. Chem. 2016, 8, 17–27. [Google Scholar] [CrossRef]
- Charupant, K.; Suwanborirux, K.; Amnuoypol, S.; Saito, E.; Kubo, A.; Saito, N. Jorunnamycins A—C, New Stabilized Renieramycin-Type Bistetrahydroisoquinolines Isolated from the Thai Nudibranch Jorunna funebris. Chem. Pharm. Bull. 2007, 55, 81–86. [Google Scholar] [CrossRef]
- Fontana, A.; Cavaliere, P.; Wahidulla, S.; Naik, C.G.; Cimino, G. A New Antitumor Isoquinoline Alkaloid from the Marine Nudibranch Jorunna funebris. Tetrahedron 2000, 56, 7305–7308. [Google Scholar] [CrossRef]
- Shen, S.-M.; Su, M.-Z.; Yu, D.-D.; Luo, H.; Li, X.-W.; Guo, Y.-W. Chemistry, Chemo-Ecology, and Biological Activities of Uncommon and Structurally Diverse Sesquiterpenoids from the Sanya Bay Nudibranch Hexabranchus sanguineus. Chem.—Eur. J. 2023, 29, e202300457. [Google Scholar] [CrossRef]
- Parrish, S.M.; Yoshida, W.; Yang, B.; Williams, P.G. Ulapualides C–E Isolated from a Hawaiian Hexabranchus sanguineus Egg Mass. J. Nat. Prod. 2017, 80, 726–730. [Google Scholar] [CrossRef]
- Roesener, J.A.; Scheuer, P.J. Ulapualide A and B, Extraordinary Antitumor Macrolides from Nudibranch Eggmasses. J. Am. Chem. Soc. 1986, 108, 846–847. [Google Scholar] [CrossRef]
- Carbone, M.; Irace, C.; Costagliola, F.; Castelluccio, F.; Villani, G.; Calado, G.; Padula, V.; Cimino, G.; Lucas Cervera, J.; Santamaria, R.; et al. A New Cytotoxic Tambjamine Alkaloid from the Azorean Nudibranch Tambja ceutae. Bioorg. Med. Chem. Lett. 2010, 20, 2668–2670. [Google Scholar] [CrossRef]
- Cavalcanti, B.C.; Júnior, H.V.N.; Seleghim, M.H.R.; Berlinck, R.G.S.; Cunha, G.M.A.; Moraes, M.O.; Pessoa, C. Cytotoxic and Genotoxic Effects of Tambjamine D, an Alkaloid Isolated from the Nudibranch Tambja eliora, on Chinese Hamster Lung Fibroblasts. Chem. Biol. Interact. 2008, 174, 155–162. [Google Scholar] [CrossRef]
- Granato, A.C.; de Oliveira, J.H.H.L.; Seleghim, M.H.R.; Berlinck, R.G.S.; Macedo, M.L.; Ferreira, A.G.; da Rocha, R.M.; Hajdu, E.; Peixinho, S.; Pessoa, C.O.; et al. Produtos naturais da ascídia Botrylloides giganteum, das esponjas Verongula gigantea, Ircinia felix, Cliona delitrix e do nudibrânquio Tambja eliora, da costa do Brasil. Quím. Nova 2005, 28, 192–198. [Google Scholar] [CrossRef]
- Nuzzo, G.; Ciavatta, M.L.; Kiss, R.; Mathieu, V.; Leclercqz, H.; Manzo, E.; Villani, G.; Mollo, E.; Lefranc, F.; D’Souza, L.; et al. Chemistry of the Nudibranch Aldisa andersoni: Structure and Biological Activity of Phorbazole Metabolites. Mar. Drugs 2012, 10, 1799–1811. [Google Scholar] [CrossRef] [PubMed]
- Forster, L.C.; Clegg, J.K.; Cheney, K.L.; Garson, M.J. Expanding the Repertoire of Spongian-16-One Derivatives in Australian Nudibranchs of the Genus Goniobranchus and Evaluation of Their Anatomical Distribution. Mar. Drugs 2021, 19, 680. [Google Scholar] [CrossRef] [PubMed]
- Ciavatta, M.L.; Manzo, E.; Mollo, E.; Mattia, C.A.; Tedesco, C.; Irace, C.; Guo, Y.-W.; Li, X.-B.; Cimino, G.; Gavagnin, M. Tritoniopsins A–D, Cladiellane-Based Diterpenes from the South China Sea Nudibranch Tritoniopsis elegans and Its Prey Cladiella krempfi. J. Nat. Prod. 2011, 74, 1902–1907. [Google Scholar] [CrossRef]
- Rawlings, T.A. Encapsulation of Eggs by Marine Gastropods: Effect of Variation in Capsule from on the Vulnerability of Embryos to Predation. Evolution 1994, 48, 1301–1313. [Google Scholar] [CrossRef]
- Benkendorff, K.; Davis, A.R.; Bremner, J.B. Chemical Defense in the Egg Masses of Benthic Invertebrates: An Assessment of Antibacterial Activity in 39 Mollusks and 4 Polychaetes. J. Invertebr. Pathol. 2001, 78, 109–118. [Google Scholar] [CrossRef]
- Mudianta, W.; Ni, W.; Martiningsih; Nyoman, I.; Prasetia, D.; Nursid, M. Bioactive Terpenoid from the Balinese Nudibranch Hypselodoris infucata. Indones. J. Pharm. 2016, 27, 104–110. [Google Scholar] [CrossRef]
- Hirayama, Y.; Katavic, P.L.; White, A.M.; Pierens, G.K.; Lambert, L.K.; Winters, A.E.; Kigoshi, H.; Kita, M.; Garson, M.J. New Cytotoxic Norditerpenes from the Australian Nudibranchs Goniobranchus splendidus and Goniobranchus daphne. Aust. J. Chem. 2015, 69, 136–144. [Google Scholar] [CrossRef]
- Whibley, C.E.; McPhail, K.L.; Keyzers, R.A.; Maritz, M.F.; Leaner, V.D.; Birrer, M.J.; Davies-Coleman, M.T.; Hendricks, D.T. Reactive Oxygen Species Mediated Apoptosis of Esophageal Cancer Cells Induced by Marine Triprenyl Toluquinones and Toluhydroquinones. Mol. Cancer Ther. 2007, 6, 2535–2543. [Google Scholar] [CrossRef]
- Tanaka, J.; Higa, T. Two New Cytotoxic Carbonimidic Dichlorides from the Nudibranch Reticulidia fungia. J. Nat. Prod. 1999, 62, 1339–1340. [Google Scholar] [CrossRef]
- Maschek, J.A.; Mevers, E.; Diyabalanage, T.; Chen, L.; Ren, Y.; McClintock, J.B.; Amsler, C.D.; Wu, J.; Baker, B.J. Palmadorin Chemodiversity from the Antarctic Nudibranch Austrodoris kerguelenensis and Inhibition of Jak2/STAT5-Dependent HEL Leukemia Cells. Tetrahedron 2012, 68, 9095–9104. [Google Scholar] [CrossRef]
- Miyamoto, T.; Sakamoto, K.; Arao, K.; Komori, T.; Higuchi, R.; Sasaki, T. Dorisenones, Cytotoxic Spongian Diterpenoids, from the Nudibranch Chromodoris obsoleta. Tetrahedron 1996, 52, 8187–8198. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Shang, X.; Keum, Y.-S. Advances in Lipid Extraction Methods—A Review. Int. J. Mol. Sci. 2021, 22, 13643. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Tian, C.; Deng, H.; Ming, Z.; Zhang, Z.; Fu, W.; Li, J. Separation of Chemical Groups from Wood Tar via Sequential Organic Solvent Extraction and Glycerol-Assisted Distillation. Sep. Purif. Technol. 2025, 357, 130019. [Google Scholar] [CrossRef]
- Shang, J.; Hu, B.; Wang, J.; Zhu, F.; Kang, Y.; Li, D.; Sun, H.; Kong, D.-X.; Hou, T. Cheminformatic Insight into the Differences between Terrestrial and Marine Originated Natural Products. J. Chem. Inf. Model. 2018, 58, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Menna, M.; Imperatore, C.; D’Aniello, F.; Aiello, A. Meroterpenes from Marine Invertebrates: Structures, Occurrence, and Ecological Implications. Mar. Drugs 2013, 11, 1602–1643. [Google Scholar] [CrossRef]
- Ruiz-Torres, V.; Forsythe, N.; Pérez-Sánchez, A.; Van Schaeybroeck, S.; Barrajón-Catalán, E.; Micol, V. A Nudibranch Marine Extract Selectively Chemosensitizes Colorectal Cancer Cells by Inducing ROS-Mediated Endoplasmic Reticulum Stress. Front. Pharmacol. 2021, 12, 625946. [Google Scholar] [CrossRef]
- Gomes, N.G.M.; Fernandes, F.; Madureira-Carvalho, Á.; Valentão, P.; Lobo-da-Cunha, A.; Calado, G.; Andrade, P.B. Profiling of Heterobranchia Sea Slugs from Portuguese Coastal Waters as Producers of Anti-Cancer and Anti-Inflammatory Agents. Molecules 2018, 23, 1027. [Google Scholar] [CrossRef]
- Trombetta, A.; Maggiora, M.; Martinasso, G.; Cotogni, P.; Canuto, R.A.; Muzio, G. Arachidonic and Docosahexaenoic Acids Reduce the Growth of A549 Human Lung-Tumor Cells Increasing Lipid Peroxidation and PPARs. Chem. Biol. Interact. 2007, 165, 239–250. [Google Scholar] [CrossRef]
- Yao, Q.; Fu, T.; Wang, L.; Lai, Y.; Wang, Y.; Xu, C.; Huang, L.; Guo, Y. Role of Autophagy in the Ω-3 Long Chain Polyunsaturated Fatty Acid-Induced Death of Lung Cancer A549 Cells. Oncol. Lett. 2015, 9, 2736–2742. [Google Scholar] [CrossRef]
- Takaki, M.; Freire, V.F.; Nicacio, K.J.; Bertonha, A.F.; Nagashima, N.; Sarpong, R.; Padula, V.; Ferreira, A.G.; Berlinck, R.G.S. Metabolomics Reveals Minor Tambjamines in a Marine Invertebrate Food Chain. J. Nat. Prod. 2021, 84, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetti, M.; Ferraro, G.A.; Nicoletti, G.F.; Filosa, R.; Caraglia, M. Anti-Inflammatory Drugs as Anticancer Agents. Int. J. Mol. Sci. 2020, 21, 2605. [Google Scholar] [CrossRef] [PubMed]
- Ambrozova, G.; Pekarova, M.; Lojek, A. Effect of Polyunsaturated Fatty Acids on the Reactive Oxygen and Nitrogen Species Production by Raw 264.7 Macrophages. Eur. J. Nutr. 2010, 49, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.-M.; Li, S.-W.; Su, M.-Z.; Yao, L.-G.; Appendino, G.; Guo, Y.-W. Structurally Diverse Diterpenoids from the Sanya Bay Nudibranch Hexabranchus sanguineus and Its Sponge-Prey Chelonaplysilla Sp. Chem.—Eur. J. 2023, 29, e202203858. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and Cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in Biology and Targeted Therapy: New Insights and Translational Implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Roberti, A.; Chaffey, L.E.; Greaves, D.R. NF-κB Signaling and Inflammation—Drug Repurposing to Treat Inflammatory Disorders? Biology 2022, 11, 372. [Google Scholar] [CrossRef]
- Zhang, R.; Ren, Y.; Ren, T.; Yu, Y.; Li, B.; Zhou, X. Marine-Derived Antioxidants: A Comprehensive Review of Their Therapeutic Potential in Oxidative Stress-Associated Diseases. Mar. Drugs 2025, 23, 223. [Google Scholar] [CrossRef]
- Lopes, D.; Cunha, E.; Conde, T.; Moreira, A.; Cruz, S.; Domingues, P.; Oliveira, M.; Cartaxana, P. Antimicrobial, Antioxidant and Anti-Inflammatory Activities of the Mucus of the Tropical Sea Slug Elysia crispata. Molecules 2024, 29, 4593. [Google Scholar] [CrossRef]
- Boulebd, H. Mechanistic Insights into the Antioxidant and Pro-Oxidant Activities of Bromophenols from Marine Algae: A DFT Investigation. J. Org. Chem. 2024, 89, 8168–8177. [Google Scholar] [CrossRef]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C—Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Rajashekar, C.B. Dual Role of Plant Phenolic Compounds as Antioxidants and Prooxidants. Am. J. Plant Sci. 2023, 14, 15–28. [Google Scholar] [CrossRef]
- Jomori, T.; Higa, N.; Hokama, S.; Tyas, T.A.; Matsuura, N.; Ueda, Y.; Kimura, R.; Arizono, S.; de Voogd, N.J.; Hayashi, Y.; et al. A Novel Sesterterpenoid, Petrosaspongin and γ-Lactone Sesterterpenoids with Leishmanicidal Activity from Okinawan Marine Invertebrates. Mar. Drugs 2024, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Hertzer, C.; Kehraus, S.; Böhringer, N.; Kaligis, F.; Bara, R.; Erpenbeck, D.; Wörheide, G.; Schäberle, T.F.; Wägele, H.; König, G.M. Antibacterial Scalarane from Doriprismatica stellata Nudibranchs (Gastropoda, Nudibranchia), Egg Ribbons, and Their Dietary Sponge Spongia Cf. Agaricina (Demospongiae, Dictyoceratida). Beilstein J. Org. Chem. 2020, 16, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Costa, E.; Abreu, M.; Gargiulo, D.; Rocha, E.; Ramos, A.A. Anticancer Effects of Seaweed Compounds Fucoxanthin and Phloroglucinol, Alone and in Combination with 5-Fluorouracil in Colon Cells. J. Toxicol. Environ. Health A 2017, 80, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.A.; Almeida, T.; Lima, B.; Rocha, E. Cytotoxic Activity of the Seaweed Compound Fucosterol, Alone and in Combination with 5-Fluorouracil, in Colon Cells Using 2D and 3D Culturing. J. Toxicol. Environ. Health A 2019, 82, 537–549. [Google Scholar] [CrossRef]
- Elbanna, A.H.; Khalil, Z.G.; Bernhardt, P.V.; Capon, R.J. Chrysosporazines A-E: P-Glycoprotein Inhibitory Piperazines from an Australian Marine Fish Gastrointestinal Tract-Derived Fungus, Chrysosporium Sp. CMB-F214. Org. Lett. 2019, 21, 8097–8100. [Google Scholar] [CrossRef]
- Elbanna, A.H.; Agampodi Dewa, A.; Khalil, Z.G.; Capon, R.J. Precursor-Directed Biosynthesis Mediated Amplification of Minor Aza Phenylpropanoid Piperazines in an Australian Marine Fish-Gut-Derived Fungus, Chrysosporium Sp. CMB-F214. Mar. Drugs 2021, 19, 478. [Google Scholar] [CrossRef]
- Mohamed, O.G.; Salim, A.A.; Khalil, Z.G.; Elbanna, A.H.; Bernhardt, P.V.; Capon, R.J. Chrysosporazines F–M: P-Glycoprotein Inhibitory Phenylpropanoid Piperazines from an Australian Marine Fish Derived Fungus, Chrysosporium Sp. CMB-F294. J. Nat. Prod. 2020, 83, 497–504. [Google Scholar] [CrossRef]
- Kristiana, R.; Bedoux, G.; Pals, G.; Mudianta, I.W.; Taupin, L.; Marty, C.; Asagabaldan, M.A.; Ayuningrum, D.; Trianto, A.; Bourgougnon, N.; et al. Bioactivity of Compounds Secreted by Symbiont Bacteria of Nudibranchs from Indonesia. PeerJ 2020, 8, e8093. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, S.M.; Patin, N.V.; Hanora, A.; Aboseidah, A.; Desoky, S.; Desoky, S.G.; Stewart, F.J.; Lopanik, N.B. The Natural Product Biosynthetic Potential of Red Sea Nudibranch Microbiomes. PeerJ 2021, 9, e10525. [Google Scholar] [CrossRef]
- Elfeky, H.H.; Hanora, A.; Solyman, S.M. Bioactivity of Bacteria Associated with Red Sea Nudibranchs and Whole Genome Sequence of Nocardiopsis dassonvillei RACA-4. Mar. Genomics 2023, 67, 101004. [Google Scholar] [CrossRef]
- Abdelrahman, S.M.; Dosoky, N.S.; Hanora, A.M.; Lopanik, N.B. Metabolomic Profiling and Molecular Networking of Nudibranch-Associated Streptomyces Sp. SCSIO 001680. Molecules 2022, 27, 4542. [Google Scholar] [CrossRef]
- Kristiana, R.; Sibero, M.T.; Farisa, M.Y.; Ayuningrum, D.; Dirgantara, D.; Hanafi, M.; Radjasa, O.K.; Sabdono, A.; Trianto, A. Antibacterial Potential of Nudibranch-Associated Bacteria from Saparua and Nusa Laut Islands, Indonesia. Biodivers. J. Biol. Divers. 2019, 20, 1811–1819. [Google Scholar] [CrossRef]






| Drug Name | Type of Compound | Source Organism | Mechanism of Action | Cancer Treatment Indications |
|---|---|---|---|---|
| Cytarabine | Nucleoside | Tectitethya crypta (sponge) | Cell cycle arrest in S phase by inhibiting DNA synthesis | Acute leukemia |
| Trabectedin | Alkaloid | Ecteinascidia turbinata (sea squirt) | DNA alkylating agent, disruption of association of DNA-binding proteins | Ovarian cancer, soft tissue sarcoma, unresectable or metastatic liposarcoma, or leiomyosarcoma |
| Eribulin mesylate | Macrolide | Halichondria okadai (sponge) | Cell cycle arrest in G2/M phase by inhibiting microtubule growth | Metastatic breast cancer, unresectable or metastatic liposarcoma |
| Brentuximab vedotin | Antibody drug conjugate (MMAE) | Dolabella auricularia (mollusk) | The antibody targets CD30 and MMAE disrupts microtubule formation | Hodgkin lymphoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Servillera, M.; Peña, M.; Cabeza, L.; Pula, H.J.; Prados, J.; Melguizo, C. Nudibranchs as Sources of Marine Natural Products with Antitumor Activity: A Comprehensive Review. Mar. Drugs 2025, 23, 319. https://doi.org/10.3390/md23080319
Servillera M, Peña M, Cabeza L, Pula HJ, Prados J, Melguizo C. Nudibranchs as Sources of Marine Natural Products with Antitumor Activity: A Comprehensive Review. Marine Drugs. 2025; 23(8):319. https://doi.org/10.3390/md23080319
Chicago/Turabian StyleServillera, Máximo, Mercedes Peña, Laura Cabeza, Héctor J. Pula, Jose Prados, and Consolación Melguizo. 2025. "Nudibranchs as Sources of Marine Natural Products with Antitumor Activity: A Comprehensive Review" Marine Drugs 23, no. 8: 319. https://doi.org/10.3390/md23080319
APA StyleServillera, M., Peña, M., Cabeza, L., Pula, H. J., Prados, J., & Melguizo, C. (2025). Nudibranchs as Sources of Marine Natural Products with Antitumor Activity: A Comprehensive Review. Marine Drugs, 23(8), 319. https://doi.org/10.3390/md23080319








