Abstract
Sea mayweed (Tripleurospermum maritimum L. syn. Matricaria maritima) is a halophytic species widely distributed along the Atlantic shoreline. Unlike other Tripleurospermum species, the chemical composition and biological activities of this halophyte have received no attention. Here, a hydroalcoholic extract of sea mayweed leaves was evaluated for in vitro antioxidant (DPPH, ABTS, and FRAP bioassays), anti-inflammatory (NO reduction in RAW 264.7 macrophages), anti-diabetic (alpha-glucosidase inhibition), neuroprotective (inhibition of acetylcholinesterase), and skin protective (tyrosinase, melanogenesis, elastase, and collagenase inhibition) activities. Solid–liquid partition chromatography of the extract and NMR characterization of its fractions allowed the identification of some major compounds, including fructo-oligosaccharides in the MeOH20% fraction, a new carbohydrate called tripleurospermine (1), 3-5-dicaffeoylquinic acid (2) in the MeOH40% fraction, and matricaria lactone (3) in the MeOH80% fraction. MeOH40 fraction exhibited strong antioxidant, anti-tyrosinase (thus skin-whitening potential), and anti-glycosidase activities (anti-diabetic potential), whereas MeOH80% fraction showed anti-inflammatory and anti-diabetic potential. Overall, our results suggest that sea mayweed may have dietary or medicinal uses due to its biochemical composition and bioactivities.
1. Introduction
The Matricaria and Tripleurospermum genera have long been considered synonymous within the Asteraceae (Compositae) family. Recently, they have been separated into two closely related taxa in the Anthemideae tribe [1,2]. Together, these two genera consist of about 40 species widely distributed in Europe, North Africa, America, and temperate Asia. Matricaria species are often used for ornamental, medicinal, or feeding purposes, whereas Tripleurospermum do not have uses yet. Over the past decade, many pharmacological properties have been described in the Matricaria genus, including anti-inflammatory [3,4], anti-tumoral, antioxidant, neuroprotective [5], anti-allergic [6], antimicrobial [7,8], anticancer [9], sedative, stomachic, antispasmodic, and antidiarrheal activities [10].
So far, a number of studies have examined the chemical composition of some members of Matricaria or Tripleurospermum, especially of their essential oils [11,12]. In addition, valuable secondary metabolites such as phenolic compounds [13,14,15] and related coumarins [16,17], terpenes [18], and polyacetylenes were identified in Matricaria species (see [19] as a review). However, only a few Tripleurospermum species have been phytochemically investigated up until this point, including T. auriculatum or T. disciforme [20,21,22], whereas little or no information is available about most species, including T. maritimum. Therefore, with the aim of characterizing the chemical composition and bioactivities of this halophyte, we describe here the partial purification of hydroalcoholic leaf extract and the identification of some of its major components, along with several biological properties.
Tripleurospermum maritimum (L.) W.D.J. Koch (previously Matricaria maritima L.) is a biennial halophytic plant. Commonly known as false mayweed or sea mayweed, it grows along the Atlantic coast of Europe, mostly on sandy beaches and pebbles. Alternatively, it can be easily grown in open fields through standard agronomic techniques (Figure 1), yielding about 1.2 TDW/ha (Magné, unpublished data).

Figure 1.
Plants of sea mayweed (Tripleurospermum maritimum L.) on maritime rocks (A) and grown in open field (B) (source: C. Magné).
We previously reported that sea mayweed leaves contain a large amount of fructo-oligosaccharides [23], which are the major components of a water extract. Since no other biochemicals have been reported hitherto in this species, we have investigated the biochemical composition of a polar extract of T. maritimum. We describe here the identification of several major constituents in leaf hydro-ethanolic extract and evaluate in vitro several biological activities of nutraceutical and cosmetic interest in this extract and its fractions.
2. Results
2.1. Total Phenolic Content
The crude extract of Tripleurospermum maritimum leaves exhibited a high phenolic amount, with 22.1 mg GAE/g DW (Figure 2). As the onset of a bioguided fractionation of this extract aimed at identifying the major components of the raw extract, phenolics were detected mainly in the fractions eluted with 20, 40, 60, and 80% methanol, which exhibited 2.85, 3.7, 1.9, and 1.8 times more phenolic compounds than the crude extract, respectively.
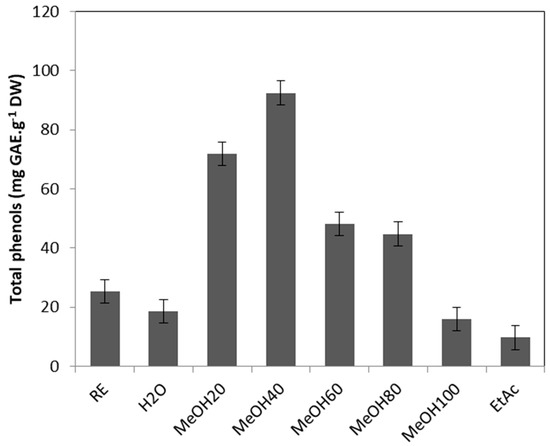
Figure 2.
Total phenolic content (mg GAE/g DW) of T. maritimum raw extract and fractions. RE: Raw extract; H2O: Water fraction; MeOH20: MeOH20% fraction; MeOH40: MeOH40% fraction; MeOH60: MeOH60% fraction; MeOH80: MeOH80% fraction; MeOH100: MeOH100% fraction; EtAc: Ethyl acetate fraction. Means ± standard deviations of three replicates are represented, and different letters above the bars indicate significantly different means (p < 0.05).
2.2. Antioxidant Activities
The crude extract of T. maritimum exhibited high reducing activity, with an EC50 value of 0.155 mg/mL for the FRAP bioassay (Figure 3A). After fractionating the extract, the MeOH20%, MeOH40%, MeOH60%, and MeOH80% fractions exhibited the highest reducing capacity, with EC50 values 5, 8.9, 7.5, and 2.6 times lower than that of the crude extract, respectively. The same trend was observed in the DPPH assay, where MeOH20%, MeOH40%, MeOH60%, and MeOH80% fractions showed 3.9, 4.4, 6.5, and 3 times lower IC50 values than that of the crude extract (0.116 mg/mL), respectively (Figure 3B). Similarly, the ABTS radical scavenging capacity was distributed among the fractions eluted with methanol solutions. Thus, MeOH20%, MeOH40%, MeOH60%, MeOH80%, and MeOH100% fractions exhibited 7.2, 14.8, 6.85, 7.3, and 4.5 times higher activities than the crude extract (55.3 mg TE/g DW), respectively (Figure 3C). Conversely, the last fraction (eluted with ethyl acetate) showed negligible or no activities using every antioxidant bioassay.
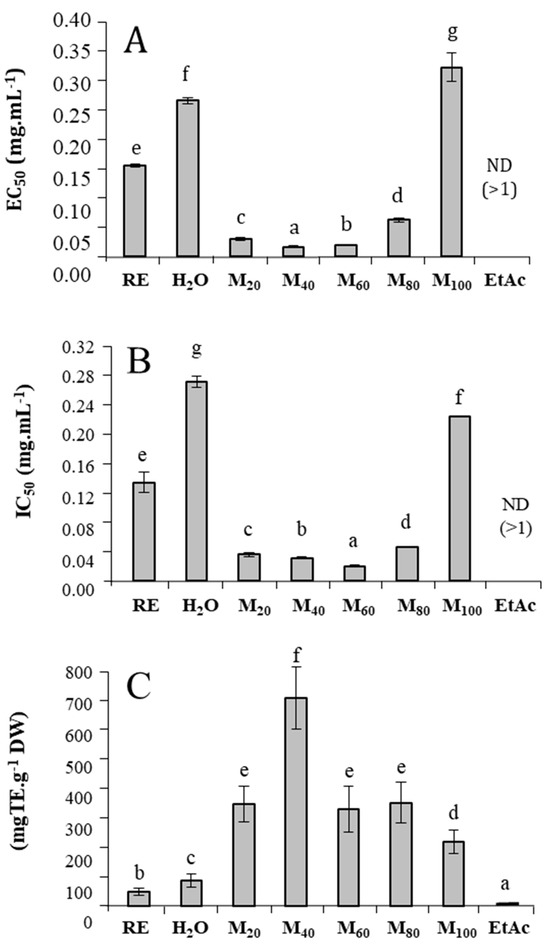
Figure 3.
Antioxidant activities of T. maritimum raw extract and its fractions. (A) ferric reducing capacity (EC50 in mg/mL); (B) radical scavenging activity against DPPH (IC50 in mg/mL); (C) radical scavenging activity against ABTS (mg TE/g DW). RE: Raw extract; H2O: Water fraction; M20: MeOH20% fraction; M40: MeOH40% fraction; M60: MeOH60% fraction; M80: MeOH80% fraction; M100: MeOH100% fraction; EtAc: Ethyl acetate fraction. Means ± standard deviations of three replicates are represented, and different letters above the bars indicate significantly different means (p < 0.05).
2.3. Other Biological Activities
2.3.1. Anti-Ageing Activity
Sea mayweed crude extract exhibited an appreciable activity against tyrosinase, with a value of 234 mg KAE/g DW. Of the seven fractions eluted from this extract, the last five exhibited a significantly higher activity than that of the crude extract (p < 0.05) (Table 1). Among them, the MeOH100% and ethyl acetate fractions were the most active, with around 700 mg KAE/g DW. Moreover, a significant anti-melanogenic action on B16 4A5 melanoma cells was found in the last fraction (eluted with ethyl acetate), being 50% more active than the standard (arbutin). However, neither the raw extract nor its fractions exhibited any activities against melanogenesis or other ageing-related enzymes like elastase and collagenase until a concentration of 0.5 g/L.

Table 1.
Skin-whitening (tyrosinase inhibition), anti-inflammatory (inhibition of NO production in RAW 264.7 macrophages), anti-melanogenic (inhibition of melanin production in RAW 264.7 macrophages), neuroprotective (acetylcholine esterase inhibition), and anti-diabetic (alpha-glucosidase inhibition) activities of sea mayweed polar extract and its fractions. Means ± SEM of three replicates are presented, and different letters indicate significantly different means (p < 0.05). ND: not determined.
2.3.2. Anti-Inflammatory Activity
Sea mayweed raw extract did not show any capacity to inhibit NO production by RAW 264.7 macrophages. However, two of its fractions (namely MeOH60% and MeOH80%) were able to strongly reduce this indicator of inflammation (Table 1). Accordingly, fractions eluted with 60% and 80% MeOH inhibited NO production five and three times more intensely than the positive control L-NAME (27.81 μg/mL), respectively.
2.3.3. Neuroprotective Activity
Sea mayweed extract and fractions were evaluated for their capacity to inhibit AChE, the primary enzyme responsible for the hydrolytic metabolism of the neurotransmitter acetylcholine. However, only the MeOH60% fraction exhibited a slight inhibitory effect on AChE (Table 1).
2.3.4. Anti-Diabetic and Anti-Obesity Activities
Pancreatic lipase is the enzyme responsible for the digestion and absorption of triglycerides, and its inhibition is one of the most widely studied methods to determine the potential activity of natural products to prevent and treat obesity. Here, neither extracts nor fractions showed inhibitory activity on rat lipase enzyme in vitro. Then, their capacity to inhibit α-amylase and α-glucosidase was assessed. Although sea mayweed raw extract was inactive on these enzymes, four fractions strongly inhibited α-glucosidase: MeOH40%, MeOH60%, MeOH80%, and ethyl acetate fractions (Table 1). Accordingly, these four fractions exhibited a remarkable inhibitory effect, with 15-, 50-, 6-, and 150-fold lower IC50 values than the standard glucosidase inhibitor acarbose. In addition, no amylase inhibition could be detected in these fractions.
2.4. Structural Determination
Following purification of the extract, the characterization of the active fractions was performed by NMR spectroscopy. The 1H-NMR spectrum of the water fraction (effluent) mainly showed the presence of numerous signals from oses (including specific signals of glucose and sucrose between 4.8 and 5.5 ppm) and amino acids (mainly alanine, proline, and glutamine between 1.5 and 2.5 ppm) (Figure 4A). Major signals in the 3.6–4.3 ppm range, assigned to fructo-oligosaccharides, were found in the MeOH20% fraction (Figure 4B).
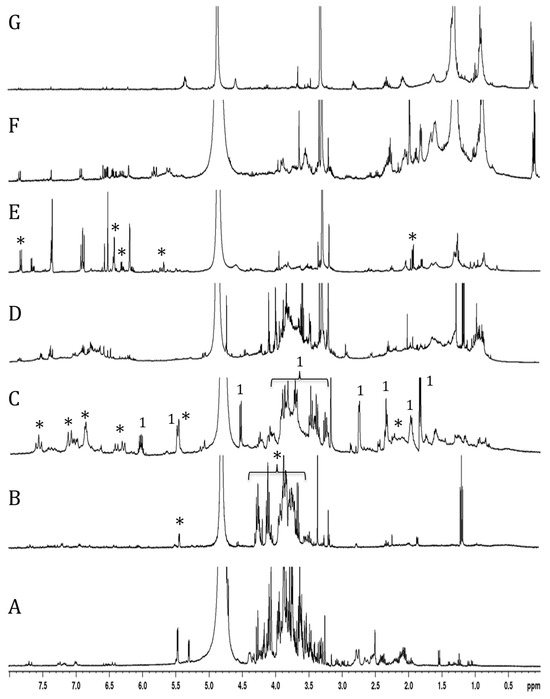
Figure 4.
H1 NMR spectra of T. maritimum extract fractions eluted with water (A), 20% MeOH (B), 40% MeOH (C), 60% MeOH (D), 80% MeOH (E), 100% MeOH (F), and ethyl acetate (G). Stars in spectra (B,C,E) indicate signals of fructo-oligosaccharide, 3,5-dicaffeoylquinic acid, and matricaria lactone, respectively. Specific signals (1) of tripleurospermine are indicated on spectrum (C).
The 1H-NMR spectrum of the MeOH40% fraction exhibited numerous signals in the aliphatic (1.8–2.8 ppm), anomeric (3.2–4.6 ppm), and unsaturated (5.5–7.6 ppm) regions (Figure 4C). Further examination of this fraction, following sub-fractionation and subsequent 13C- and 2D NMR analyses, allowed us to identify a major compound in the most polar sub-fraction, eluted with 20% MeOH. This compound was characterized by signals between 3.2 and 4.2 ppm associated with a doublet in the anomeric region (4.5 ppm), suggesting a sugar residue. Other signals in the unsaturated (5.5 and 6.1 ppm) and aliphatic (from 1.8 to 2.8 ppm) regions were also found, and 2D-NMR COSY and TOCSY experiments showed two spin systems (Supplementary Data S3). The first one was characterized by signals at 4.56 (H1, d), 3.29 (H2, dd), 3.47 (H3, m), 3.39 (H4, m), 3.44 (H5, m), 3.71 (H6a, m), and 3.90 (H6b, m) ppm. Corresponding carbons were identified by means of an HMQC experiment at 105.4, 76.1, 78.6, 72.5, 78.5, and 63.8 ppm, respectively (Supplementary Data S2). The second spin system was characterized by signals at 1.85 (H1′, dd), 6.06 (H2a′, m), 5.49 (H3′, dd), 2.76 (H6′, d), 3.92 (H7′), 1.98 (H8′, m), and 2.32 (H9′, t) ppm. These protons could be correlated by the HMQC experiment to carbons at 18.5, 142.5, 111.9, 28.4, 82.1, 32.9, and 36.1 ppm, respectively. Examination of the J-MOD spectrum suggested that the first spin system belongs to a sugar residue, and HMBC experiments allowed us to correlate this residue to the second spin system (Supplementary Data S3). Finally, acid hydrolysis of this subfraction provided a glucose residue, and 2D NMR experiments showed that the lateral chain corresponded to dec-8-ene-6-ynoic acid (Table 2). This observation was confirmed by mass spectrometry analysis, where the detected molecular ion [M+Na] corresponded well to the theoretical m/z for a C6H12O6Na2C10H12O2 structure (367.1663 versus 367.1354, respectively). Finally, the structure was identified as 4-O-β-D glucose derivative of dec-8-ene-6-ynoic acid (Figure 5A). This newly reported compound was named tripleurospermine. Moreover, MeOH25% subfraction showed signals in the aliphatic (2.1–2.3 ppm), anomeric (3.6, 4.1 ppm), and aromatic (5.6, 6.3, 6.8–7.1, and 7.6 ppm) regions (already seen in Figure 4C). The latter group of signals may be assigned to the dicaffeoyl moiety. Following 13C- and 2D NMR experiments on this fraction (Supplementary Data S1), we identified unequivocally 3,5-di-O-caffeoylquinic acid as the major constituent (Figure 5B).

Table 2.
1D- and 2D-NMR data (in ppm) of tripleurospermine identified in the MeOH40% fraction of T. maritimum leaf extract.
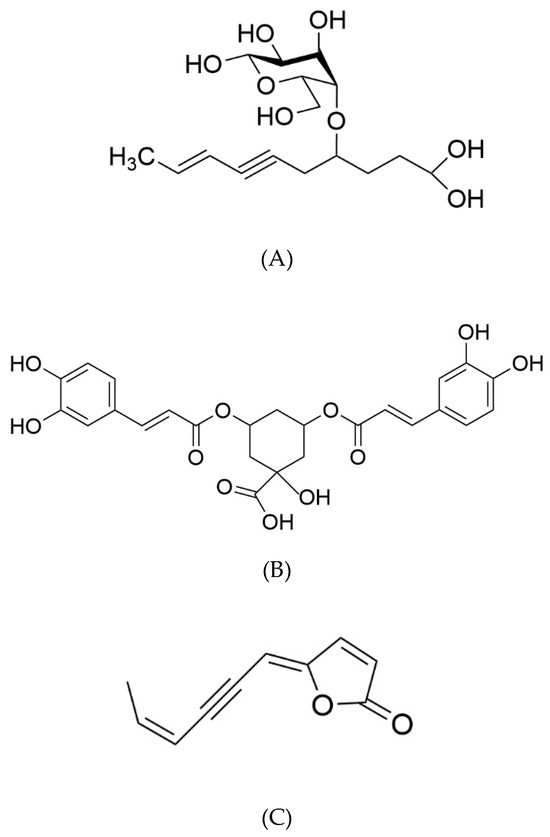
Figure 5.
Chemical structures of major compounds identified in T. maritimum extract fractions: tripleurospermine (A), 3,5-di-O-caffeoylquinic acid (B), and matricaria lactone (C).
The MeOH60% fraction showed several signals in the 6–8 ppm region, corresponding to aromatic compounds (Figure 4D). Some of them are likely glycosylated, as revealed by the signals in the ose and anomeric proton regions.
The NMR spectrum of the MeOH80% fraction showed numerous signals between 5.6 and 8 ppm, and pronounced ones between 1 and 2 ppm, corresponding to aromatic and aliphatic protons, respectively (Figure 4E). Further fractioning of this fraction allowed us to isolate a pure compound eluted with 65% MeOH. This constituent was characterized by signals at 1.9, 5.7, 6.1, 6.3, and 7.9 ppm on the 1H-NMR spectrum, and 2D-NMR (COSY, HMQC, and HMBC) experiments allowed us to identify this compound as 5-(4-hexen-2-ynylidene) furan-2-one, also known as matricaria lactone (Figure 5C).
The last two fractions, eluted with MeOH100% and ethyl acetate, contained apolar compounds showing aliphatic protons and minor signals in the 1–2 ppm and 6–7 ppm regions, respectively (Figure 4F,G).
3. Discussion
Over the past decades, the search for natural products in plants has led to the discovery of a number of biologically active substances, particularly secondary metabolites. These compounds are widely represented in most medicinal or halophytic species, where they exhibit a number of biological activities [24]. Within Asteraceae, the chemical composition and biological properties of extracts from Aster or Matricaria genera are widely documented [10,15,19,25] and mainly reveal antioxidant properties. However, research on Tripleurospermum has received little attention [20,26]. Therefore, with the aim of characterizing the chemical composition and biological activities of the halophyte sea mayweed (T. maritimum), we describe the partial purification of hydroalcoholic leaf extract and the identification of some of its major components, along with several biological properties.
Crude extract of T. maritimum leaves showed a medium content of total phenolic compounds, compared to other halophytic species. It was lower than well-known rich plants like Limonium vulgare, Mesembryanthemum edule, and Arthrocnemum macrostachyum [24,27,28]. However, its content was similar to that in Crithmum maritimum [29] and higher than in Juncus acutus, Halimione portulacoides, Limoniastrum monopetalum, or Halocnemum strobilaceum [28,30].
Solid–liquid partition chromatography of the crude extract of sea mayweed leaves on C18-bound silica gel yielded fractions enriched in some major constituents. The fraction eluted with 20% methanol (MeOH20% fraction) appeared to be composed mainly of an oligofructan, α-D-Glcp-(1 → 2)-[β-D-Fruf-(2 → 1)-β-D-Frucf]n-(2 → 1)-β-D-Fruf, which is already described in wild Matricaria maritima leaf extract [23]. Along with well-known prebiotic uses, this kind of fructo-oligosaccharide (FOS) may have promising pharmacological applications or be used for reinforcement of polymer matrix composites thanks to its mechanical properties [31]. Here, this fraction exhibited a mild antioxidant capacity, probably related to its low phenolic content. However, this fraction did not show any other biological activity with the bioassays used here.
Particular attention was given to the MeOH40% fraction, where 1D and 2D NMR studies led to the identification of 3,5-dicaffeoylquinic acid and a never-before-reported carbohydrate, called tripleurospermine, as major constituents. Interestingly, this fraction exhibited the highest phenolic content and the strongest antioxidant activities (with FRAP and ABTS bioassays). Phenolic compounds are well known to have a number of biological properties, including antioxidant activity [32]. Consequently, halophytes, which are rich in phenolics, have been reported to have a strong antioxidant potential [24,33,34]. Among phenolic compounds, caffeoyl derivatives are known to contribute significantly to the antioxidant activity of plants such as Asteraceae [25]. These authors reported the presence of 3,5-dicaffeoylquinic acid in several members of this family, including a species closely related to T. maritima, Matricaria perforata. The antioxidant capacity of the two main compounds identified in the MeOH40% fraction has been evaluated in their respective enriched subfractions. Interestingly, our results suggested for the first time that the newly identified molecule, tripleurospermine, would exert a significant activity, though lower than that of the caffeoyl derivative. Importantly, the MeOH40% fraction also exhibited a marked capacity to inhibit tyrosinase activity, a well-known oxidizing enzyme responsible for melanin formation in the skin. The activity level, reported here for the first time in this species, was close to that of the standard tyrosinase inhibitor kojic acid. Moreover, it appears higher than that recently reported in other halophytic species like Citrullus colocynthis, Crithmum maritimum, Daemia cordata, Plantago coronopus, or the Asteraceae Inula crithmoides [29,35,36]. In this family, Duke [27] reported such anti-tyrosinase property in the closely related species Matricaria recutita, thanks to its phenolic compounds kaempferol, p-coumaric acid, and quercetins. We finally confirmed these observations by measuring a significant anti-tyrosinase activity in the subfractions enriched with tripleurospermine or 3,5-dicaffeoylquinic acid. Therefore, these two compounds are likely responsible for the tyrosinase inhibition found in the MeOH40% fraction. This result confirms that a positive correlation could be made between antioxidant and anti-tyrosinase activities, as reported previously by Choi et al. [37]. However, this fraction exhibited no activities against melanogenesis or other ageing-related enzymes like elastase and collagenase, confirming a previous observation made in Matricaria recutita [38]. In addition, the MeOH40% fraction exhibited a marked capacity to inhibit α-glucosidase activity, a well-known hypoglycemic enzyme involved in carbohydrate digestion. This observation is in agreement with recent work by Spínola and Castilho [39], showing a strong hypoglycemic effect of caffeoylquinic acids from Asteraceae plants. Therefore, this fraction could be used as an adjuvant to prevent Type 2 diabetes.
The MeOH60% fraction showed the widest range of biological activities, as seen by its antioxidant, anti-tyrosinase, anti-inflammatory, neuroprotective, and anti-diabetic capacities. Its strong capacity to inhibit NO production and α-glucosidase was noteworthy, making this fraction a potential source of bioactive drugs, particularly against inflammation or diabetes syndromes. Since the NMR spectrum of the MeOH60% fraction showed the prevalence of phenolic compounds, it could be that these constituents are responsible for its wide range of activities. Accordingly, the anti-inflammatory effect of phenolics was reported [40,41], as well as their anti-diabetic properties in other Asteraceae [39,42,43]. In addition, phenolics have been shown to inhibit tyrosinase [44] and ameliorate the neurodegenerative process [45,46]. Further investigation aimed at purifying major constituents in this fraction is needed to ascribe the contribution of the different compounds in the exhibited activities.
The MeOH80% fraction exhibited a strong antioxidant capacity. Following sub-fractioning, a major constituent was identified as 2(5H)-furanone, 5-(4-hexen-2-yn-1-ylidene), also known as matricaria lactone. This monoterpene lactone was previously identified in other members of Asteraceae, including Conyza (=Erygeron) and Chamomilla (=Matricaria) genera [14,47], where it was reported to exert antifungal properties [48,49]. Since matricaria lactone appeared as the main component in the MeOH80% fraction, it likely explains its strong antioxidant and anti-tyrosinase properties. However, to the best of our knowledge, neither antioxidant nor anti-tyrosinase activity had been reported for this compound hitherto. Interestingly, the MeOH80% fraction also showed a strong anti-inflammatory capacity (three-fold higher than the standard L-NAME). Since terpene lactones have been reported to have anti-inflammatory effects [42], it could be that the NO inhibitory effect of the MeOH80% fraction is due to matricaria lactone. In addition, this fraction strongly inhibited α-glucosidase. A potential anti-diabetic action of lactones has not been reported hitherto, and further work is needed to assess the potential effect of pure matricaria lactone on this enzyme.
The last two fractions, eluted with MeOH100% and ethyl acetate, mainly exhibited apolar compounds, as evidenced by their NMR analysis showing major signals of aliphatic protons. These signals suggest the presence of terpenoids and/or steroids as major constituents in these fractions, since such chemicals are commonly found in Asteraceae [49,50,51]. Interestingly, steroids and terpenoids have been reported to inhibit tyrosinase [52,53,54], and the last two fractions of sea mayweed extract exhibited the most powerful anti-tyrosinase activity, being as active as the whitening standard agent (kojic acid) used in the cosmetic industry. However, these fractions had low antioxidant activities, though tyrosinase catalyzed two oxidative reactions. Hence, the contribution of non-antioxidant mechanisms to tyrosinase inhibition could not be excluded. Moreover, the last fraction of sea mayweed extract was the only one to exhibit anti-melanogenesis properties and showed a remarkable α-glucosidase inhibitory action. These activities could be related to some low-polar constituents like terpenoids, as reported by Kanlayavattanakul and Lourith [44] and Patel et al. [55], respectively. In addition, no other skin anti-ageing activities (e.g., anti-elastase or anti-collagenase activities) were observed in sea mayweed raw extract or fractions at the tested concentrations. Our results confirm those reported in Matricaria recutita, a T. maritima-related species, where neither anti-elastase nor anti-collagenase activity could be found [38].
4. Materials and Methods
4.1. Chemicals, Culture Media, and Supplements
Lipase (EC 3.1.1.3), acetylcholinesterase (EC 3.1.1.7), α-glucosidase (EC 3.2.1.20), α-amylase (EC 3.2.1.1), tyrosinase (EC 1.14.18.1), and the B16 A45 murine melanoma cell line were purchased from Sigma-Aldrich (Hamburg, Germany). Folin-Ciocalteau phenol reagent, all chemicals (ABTS, L-DOPA, DPPH, DTNB, EGCG, and HEPES), standards (acarbose, arbutine, galantamine, and L-NAME), solvents (ethanol and methanol) used for chemical analyses, and bioassays were supplied by Sigma Aldrich (St. Louis, MO, USA).
4.2. Plant Material
Seeds of T. maritimum were collected on a sand beach at Le Conquet (48°21′37″ North, 4°46′15″ West, France). Seeds were disinfected with a saturated solution of calcium hypochlorite (30%) for 3 min and rinsed in distilled water. They were germinated for 10 days at 20 °C in Petri dishes on filter paper moistened with 0.1 mM CaSO4. Then, germinated seeds were transferred to 250 mL pots filled with a mixture of sand and sterile loam (1:1 v/v) and watered daily with Hewitt nutrient solution [56]. Seedlings were grown in a greenhouse under controlled conditions: 14–23 °C and 50–70% relative humidity, 8–16 h night–day photoperiod. The aerial parts of 3-month-old T. maritimum plants were harvested, immediately frozen, and freeze-dried. Dried samples were then powdered and stored at room temperature until extraction.
4.3. Extraction and Fractionation
About 1 g of leaf powder was homogenized with 25 mL of water/ethanol (1:2) under magnetic stirring at 4 °C for 20 min. This solvent is commonly considered an efficient means for the extraction of secondary metabolites, including phenolics, and it is suitable to provide extracts for food, cosmetic, or medicinal applications. After centrifugation of the mixture (15 min at 4 °C, 4.000× g), the resulting pellet was extracted twice following the same protocol. The supernatants were collected, pooled, and filtrated over glass wool. The obtained extract was concentrated by rotary evaporation at 40 °C and resuspended in 50% ethanol.
Fractionation of the raw extract (approx. 0.3 g) was performed by solid–liquid partition chromatography on C18-bound silica gel (GRACE Davisil RP18, Düren, Germany). The elution of polar compounds proceeded by increasing methanol concentration in water (successively 0, 20, 40, 60, 80, and 100%) and, finally, ethyl acetate. The fractions were then concentrated by rotary evaporation at 30 °C and resuspended in the corresponding solvent. Further fractionation of the MeOH40 fraction was performed on the same column, using the same method but with smaller MeOH increments (i.e., MeOH 20%, 25%, 30%, 35%, and 40%). When necessary, a subfraction was submitted to acid hydrolysis treatment (1 N HCl, 110 °C for 1 h) before structural elucidation with NMR experiments. All these fractions were used for the following bioassays.
4.4. Evaluation of Antioxidant Activities
4.4.1. DPPH Scavenging Activity
The scavenging activity of the stable 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical was determined by the method of Marwah et al. [57]. Briefly, the reaction medium contained 100 μL of 100 μM DPPH solution in ethanol and 100 μL of plant extract at different concentrations (or water for the control). The reaction mixture was incubated in the dark for 15 min, and the absorbance was recorded at 517 nm with a Multiskan FC microplate reader (Thermo Scientific Technologies, Beijing, China). The assay was carried out in triplicate. The decrease in absorbance upon the addition of test samples was used to calculate the inhibition percentage (%IP) of DPPH radical, following the equation as follows:
where Ac and As are the absorbances of the control and the test sample, respectively. From a plot of concentration against %IP, a linear regression analysis was performed to determine the antiradical activity, as expressed by the IC50 (extract concentration resulting in a 50% inhibition) value for each sample.
%IP = [(Ac − As)/Ac] × 100
4.4.2. Ferric Reducing Ability (FRAP)
The assay is based on the reaction of Fe2+ with 2,4,6-tri(pyridyl)-s-triazine (TPTZ) to form a violet-blue colour with maximal absorbance at 593 nm [58,59]. The FRAP solution was prepared by mixing 10 volumes of acetate buffer (300 mM, pH 3.6) with 1 volume of TPTZ (40 mM in HCl) and 1 volume of ferric chloride (20 mM in water). The solution was prepared daily and warmed at 37 °C for 10 min before use. A 280 μL aliquot of this solution was mixed with 20 μL of samples (extract, fractions, or water for the blank) in a 96-well microplate. The mixture was incubated at 37 °C in the dark for 30 min and then read at 593 nm. The increase in absorbance upon addition of test samples was used to calculate the reducing capacity, as expressed by the efficacy percentage (%EP):
where As and Ac are the absorbances of the control and the test sample, respectively. From a plot of concentration against %EP, a linear regression analysis was performed to determine the EC50 (extract concentration resulting in 50% efficacy) value for each sample.
%EP = [(As − Ac)/Ac] × 100
4.4.3. ABTS Scavenging Activity
The ABTS radical scavenging assay was based on the method described by Re et al. [60] with a slight modification. Briefly, a 7 mM ABTS stock solution was prepared by dissolving ABTS in ethanol:water (5:1 v/v). Then, an aliquot of this solution was reacted with 2.45 mM potassium persulfate in ethanol:water (1:3 v/v) and allowed to stand in the dark at room temperature for 16–20 h to prepare the ABTS radical cation (ABTS+). This ABTS radical solution was diluted to an absorbance at 734 nm of 0.70 ± 0.02. Finally, the absorbance of a mixture consisting of the sample (or water for the blank) and ABTS reagent was measured at 734 nm. The antiradical capacity of the samples was expressed as Trolox equivalents (mg TE/g DW).
4.5. Evaluation of Anti-Ageing Activity
Anti-tyrosinase assay was performed using L-DOPA as substrate, according to the method described by Masuda et al. [61]. The samples (plant extract or fractions) were dissolved in 50% dimethyl sulfoxide (DMSO). Then, 40 μL of each sample was mixed with 80 μL of phosphate buffer (0.1 M, pH 6.8), 40 μL of tyrosinase (31 units/mL in phosphate buffer, pH 6.5), and 40 μL of 2.5 mM L-DOPA in a 96-well microplate. The absorbance of the mixture was measured at 475 nm and compared to that of a positive control containing kojic acid and a blank containing all the components except L-DOPA. The anti-tyrosinase activity was expressed as kojic acid equivalents (mg KAE/g DW).
Anti-elastase activity was assayed according to the method described by Kim et al. [62], slightly modified to perform the assay in 96-well microplates. The substrate N-Succinyl-Ala-Ala- Ala-p-nitroanilide (SANA) was dissolved in Tris-HCL buffer (0.2 M, pH 8.0) at 1.6 mM. Plant extract or fractions were incubated with porcine pancreatic elastase (E.C. 3.4.21.36) at 10 μg/mL for 20 min at 37 °C before adding substrate. Epigallocatechin gallate (EGCG) was used as a positive control. Absorbance at 410 nm was measured 20 min after substrate addition. The percentage of enzyme inhibition was calculated as follows:
Enzyme inhibition (%) = [(Acontrol − Asample)/Acontrol] × 100
Anti-collagenase activity was assayed using the ENZO MMP-1 colorimetric kit (Enzo Life Sciences, Lyon, France), adapted to 96-well microplates. The assay was performed in 50 mM HEPES buffer (pH 7.5) containing 0.05% Brij-35, 1 mM DTNB, and 10 mM CaCl2. MMP-1 (Matrix MetalloProteinase-1) was dissolved in this buffer at an initial concentration of 0.76 units/mL. The synthetic substrate (chromogenic peptidic substrate) was dissolved in HEPES buffer to 1 mM. Plant extracts were incubated with the enzyme in buffer for 15 min before adding the substrate. The specific inhibitor [N-Isobutyl-N-(4-methoxyphenylsulfonyl) glycyl hydroxamic acid] was used as a positive control. Absorbance at 412 nm was measured every minute from 10 to 20 min after substrate addition. The percentage of enzyme inhibition was calculated as follows:
where Vsample and Vcontrol are the linear regression slopes of the inhibition due to sample and control, respectively.
Enzyme inhibition (%) = (Vsample/Vcontrol) × 100
Moreover, the anti-melanogenic activity of sea mayweed extract and fractions was assessed in vitro on B16 4A5 melanoma cells according to Bouzaiene et al. [63]. Cells were seeded at 3.5 × 104 cells/well into 12-well plates, allowed to adhere for 24 h, and then treated with sea mayweed extract concentrations that allowed cellular viability higher than 80% for 72 h. Thereafter, adherent cells were trypsinized and solubilized in 1 mL of 1% sodium dodecyl sulphate (SDS). The absorbance of the samples was measured at 475 nm, and the melanin content was estimated using a standard curve of synthetic melanin (0–25 μg/mL).
4.6. Evaluation of Neuroprotective Activity
The neuroprotective property of sea mayweed extract was evaluated through the in vitro inhibition of acetylcholinesterase (AChE) according to Custódio et al. [64]. Samples (20 µL at concentrations of 1, 5, and 10 mg/mL) were mixed with 140 µL of sodium phosphate buffer (0.1 mM, pH 8.0) and 20 µL of AChE solution (0.28 U/mL) in 96-well microplates. The mixture was incubated for 15 min at room temperature, and the reaction was initiated by the addition of 10 µL of 4 mg/mL ATChI and 20 µL of 1.2 mg/mL DTNB. The absorbance was read at 405 nm, and the results were expressed as IC50 relative to a control containing water instead of extract. Galantamine was used as a positive control.
4.7. Evaluation of Anti-Diabetic Activity
Sea mayweed extract and fractions, at concentrations ranging from 1 to 5 mg/mL, were evaluated for their capacity to inhibit α-amylase and α-glucosidase according to Zengin [65]. Moreover, extracts and fractions were tested against porcine lipase according to McDougall et al. [66]. Acarbose was used as a positive control for α-amylase and α-glucosidase, and orlistat was used as a positive control for lipase inhibition. Results were expressed as IC50 relative to a control containing DMSO.
4.8. Evaluation of Anti-Inflammatory Activity
Nitric oxide (NO) production by LPS-stimulated RAW 264.7 macrophages was assessed as described by Rodrigues et al. [28]. RAW 264.7 cells were cultured in RPMI 1640 culture medium, enriched with 10% heat-inactivated FBS, 1% L-glutamine (2 mM), and 1% penicillin (50 U/mL)/streptomycin (50 μg/mL), and kept at 37 °C in a 5% CO2 humidified atmosphere. Murine cells were seeded in a 96-well plate at 2.5 × 105 cells per well and allowed to adhere overnight. Then, they were co-treated with 100 ng/mL of LPS and sea mayweed raw extract (at concentrations that allowed cellular viability higher than 80%) for 24 h. NO production was assessed using the Griess assay. Results were expressed as a percentage of inhibition of NO production, relative to a control containing DMSO (0.5%, v/v), and compared to the positive control L-nitroarginine methyl ester (L-NAME).
4.9. NMR Analyses
Dry fractions were dissolved in 700 μL of 99.5% D2O and placed in 5 mm NMR tubes. 1H and 13C NMR spectra were obtained at 298°K on a Bruker DRX-400 spectrometer (400 MHz), equipped with a 5 mm dual 1H/13C probe head, using standard pulse sequences. A typical 1D 1H NMR spectrum consisted of 32 scans, and 2,2,3,3-tetradeuterio-3-(trimethylsilyl)-propanoic acid sodium salt was used as an internal standard. For 13C (J-mod) and 2D 1H NMR (COSY, HMBC, HMQC, and TOCSY) analyses, experiments were carried out at 298°K on a Bruker Avance III HD500 spectrometer equipped with an inverse 5 mm TCI cryoprobe with z gradient. Data were processed using TopSpin® 4.0 software (Bruker, Wissembourg, France).
4.10. Mass Spectrometry Analysis
When needed, characterization of a purified compound was performed on an Autoflex (Bruker, France) ion trap mass spectrometer equipped with a Matrix-Assisted Laser Desorption/Ionization (MALDI) source and a quadrupole time-of-flight (QTOF) analyzer. Spectra were acquired in negative-ion mode over a mass range from m/z 100 to 1500 with 5 Hz frequency. Operating parameters of the MALDI ion source were as follows: capillary voltage 3 kV, dry gas flow 6 L/min, dry gas temperature 200 °C, nebulizer pressure 0.7 bar, collision radio frequency 700.0 V, transfer time 100.0 μs, and pre-pulse storage 7.0 μs. Ultrapure nitrogen was used as drying and nebulizer gas, and argon was used as collision gas. Collision energy was set automatically from 15 to 75 eV depending on the m/z of the fragmented ion. Acquired data were calibrated internally with sodium formate introduced to the ion source at the beginning and ending of each separation via a 20 μL loop. Control and data acquisition were carried out with the Bruker DataAnalysis 4.3 software.
4.11. Statistical Analyses
All extractions and assays were conducted in triplicate. Results were expressed as mean ± standard deviation (SD), and the means were compared by using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range tests performed by the “Statistica v. 5.1” software (Statsoft, 2008). Differences between individual means were deemed to be significant at p < 0.05. The IC50 values were calculated by the sigmoidal fitting of the data using the GraphPad Prism v. 5.0 programme.
5. Conclusions
Biological activities and chemical composition of Tripleurospermum maritimum were thoroughly investigated here for the first time. Solid–liquid partition chromatography of the crude hydroalcoholic extract and NMR analyses allowed us to identify fructo-oligosaccharides (FOS) in the MeOH20% fraction, a new carbohydrate tripleurospermine (1), 3-5-dicaffeoylquinic acid (2) in MeOH40% fraction, and matricaria lactone (3) in the MeOH80% fraction as the major components. Though the crude extract of T. maritimum leaves did not show strong biological activities in vitro, some of its fractions revealed highly promising potential. Thus, MeOH40% fraction exhibited strong antioxidant, anti-tyrosinase (thus skin-whitening potential), and anti-glycosidase activities, but no other anti-ageing properties. The MeOH60% fraction exhibited the highest diversity of activities, with antioxidant, anti-tyrosinase, and remarkable anti-inflammatory, neuroprotective, and anti-diabetic properties. The MeOH80% fraction showed anti-inflammatory and anti-diabetic potential. The less-polar fractions were particularly promising with skin-whitening potential. Overall, this work shows that sea mayweed could find dietary or medicinal uses, as an interesting source of prebiotics (FOS) and of phyto-pharmaceutical preparations for skin-ageing, inflammation or diabetes control, and neuroprotection. Further work should be addressed (i) to characterize the strongly bioactive MeOH60% fraction, (ii) to confirm the biological activities of pure tripleurospermine and of matricaria lactone, and (iii) to validate in vivo the results observed here. Alternatively, investigations on seasonal and organ variability of biochemical contents and bioactivities in T. maritimum plants grown in an open field should also be addressed.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/md23110420/s1; Supplementary data S1: NMR spectroscopic data (500 MHz, CDCl3) for 3,5-di-O-caffeoylquinic acid identified in MeOH40% fraction of T. maritimum leaf extract. (s: singlet, d: doublet, t: triplet, dd: doublet of doublets, m: multiplet, brs: broad singlet). Supplementary data S2: NMR spectroscopic data (500 MHz, CDCl3) for tripleurospermine identified in MeOH40% fraction of T. maritimum leaf extract. (s: singlet, d: doublet, t: triplet, dd: doublet of doublets, m: multiplet). Supplementary data S3: 2D-NMR spectra of MeOH20% sub-fraction of the MeOH40% fraction of T. maritimum leaf extract, showing spin systems of tripleurospermine in COSY (A), HMQC (B) and HMBC (C) experiments.
Author Contributions
The experimental design was carried out by X.D. and C.M. The laboratory experiments were performed by C.L. and M.J.R. The manuscript was written by C.L. and M.J.R. and supervised by S.C., C.M. and L.M.B.C. Finally, the revised version was written by C.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge ANRT for funding the Ph.D. work of C. Lemoine (convention No. 2014/1093). This study benefited from equipment funded by the French Contrat de Plan Etat-Région (CPER) Bioalternatives, 2021–2027. The Portuguese team acknowledges the Foundation for Science and Technology (FCT) and the Portuguese National Budget (CCMAR/Multi/04326/2019 project). Luísa Custódio was supported by the FCT Investigator Programme (CEECIND/00425/2017).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Abbreviations
ABTS: 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid); L-DOPA: L-dihydroxyphenylalanine; DPPH: 2,2-diphenyl-1-picrylhydrazyl; DTNB: 5,5′-dithiobis-(2-nitrobenzoic acid); EGCG: Epigallocatechin gallate; FRAP: Ferric reducing antioxidant power; HEPES: N-2-Hydroxyethylpiperazine-N′-2-ethanesulfonic acid; HMBC: heteronuclear multiple bond coherence; HMQC: heteronuclear multiple quantum coherence; MALDI: Matrix-Assisted Laser Desorption/Ionization; MeOD: deuterated methanol; MeOH: methanol; NMR: nuclear magnetic resonance.
References
- Applequist, W.L. A Reassessment of the Nomenclature of Matricaria L. and Tripleurospermum Sch. Bip. (Asteraceae). Taxon 2002, 51, 757. [Google Scholar] [CrossRef]
- Oberprieler, C.; Himmelreich, S.; Vogt, R. A new subtribal classification of the tribe Anthemideae (Compositae). Willdenowia 2007, 37, 89–114. [Google Scholar] [CrossRef]
- Kim, S.; Jung, E.; Kim, J.H.; Park, Y.H.; Lee, J.; Park, D. Inhibitory effects of (-)-α-bisabolol on LPS-induced inflammatory response in RAW264.7 macrophages. Food Chem. Toxicol. 2011, 49, 2580–2585. [Google Scholar] [CrossRef]
- Bulgari, M.; Sangiovanni, E.; Colombo, E.; Maschi, O.; Caruso, D.; Bosisio, E.; Dell’Agli, M. Inhibition of Neutrophil Elastase and Metalloprotease-9 of Human Adenocarcinoma Gastric Cells by Chamomile (Matricaria recutita L.) Infusion. Phytother. Res. 2012, 26, 1817–1822. [Google Scholar] [CrossRef] [PubMed]
- Ranpariya, V.L.; Parmar, S.K.; Sheth, N.R.; Chandrashekhar, V.M. Neuroprotective activity of Matricaria recutita against fluoride-induced stress in rats. Pharm. Biol. 2011, 49, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekhar, V.M.; Halagali, K.S.; Nidavani, R.B.; Shalavadi, M.H.; Biradar, B.S.; Biswas, D.; Muchchandi, I.S. Anti-allergic activity of German chamomile (Matricaria recutita L.) in mast cell mediated allergy model. J. Ethnopharmacol. 2011, 137, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.; Barbosa, L.; Seito, L.; Fernandes Junior, A. Antimicrobial activity and phytochemical analysis of crude extracts and essential oils from medicinal plants. Nat. Prod. Res. 2012, 26, 1510–1514. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Nazaruk, J.; Polito, L.; Bezerra Morais-Braga, M.F.; Rocha, J.E.; Melo Coutinho, H.D.; Salehi, B.; Tabanelli, G.; Montanari, C.; del Mar Contreras, M.; et al. Matricaria genus as a source of antimicrobial agents: From farm to pharmacy and food applications. Microbiol. Res. 2018, 215, 76–88. [Google Scholar] [CrossRef]
- Matić, I.Z.; Juranić, Z.; Savikin, K.; Zdunić, G.; Nađvinski, N.; Gođevac, D. Chamomile and marigold tea: Chemical characterization and evaluation of anticancer activity. Phytother. Res. 2013, 27, 852–858. [Google Scholar] [CrossRef]
- Sebai, H.; Jabri, M.A.; Souli, A.; Rtibi, K.; Selmi, S.; Tebourbi, O.; El-Benna, J.; Sakly, M. Antidiarrheal and antioxidant activities of chamomile (Matricaria recutita L.) decoction extract in rats. J. Ethnopharmacol. 2014, 152, 327–332. [Google Scholar] [CrossRef]
- Zekovic, Z.; Pekic, B.; Lepojevic, Z.; Petrovic, L. Study of the extraction of chamomile flowers with supercritical carbon dioxide. Chromatographia 1994, 39, 587–590. [Google Scholar]
- Öztürk, E.; Ozer, H.; Cakir, A.; Mete, E.; Kandemir, A.; Polat, T. Chemical Composition of the Essential Oil of Tripleurospermum corymbosum E. Hossain, an Endemic Species from Turkey. J. Essent. Oil Bear. Pl. 2013, 13, 148–153. [Google Scholar] [CrossRef]
- Mulinacci, N.; Romani, A.; Pinelli, P.; Vincieri, F.; Prucher, D. Characterization of Matricaria recutita L.-flower extracts by HPLC-MS and HPLC-DAD analysis. Chromatographia 2000, 51, 301–307. [Google Scholar] [CrossRef]
- Raal, A.; Püssa, T.; Sepp, J.; Malmiste, B.; Arak, E. Content of phenolic compounds in aerial parts of Chamomilla suaveolens from Estonia. Nat. Prod. Commun. 2011, 6, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.Y.; Wang, R.; Shi, Y.P. Flavonoids from the Flowers of Matricaria chamomilla. Chem. Nat. Compd. 2014, 50, 910–911. [Google Scholar] [CrossRef]
- Kovacik, J.; Repcak, M. Accumulation of coumarin-related compounds in leaves of Matricaria chamomilla related to sample processing. Food Chem. 2008, 111, 755–757. [Google Scholar] [CrossRef]
- Kovalikova Ducaiova, Z.; Sajko, M.; Mihaličová, S.; Repčák, M. Dynamics of accumulation of coumarin-related compounds in leaves of Matricaria chamomilla after methyl jasmonate elicitation. Plant Growth Regul. 2015, 79, 81–94. [Google Scholar] [CrossRef]
- Petronilho, S.; Maraschin, M.; Coimbra, M.A.; Rocha, S.M. In vitro and in vivo studies of natural products: A challenge for their valuation. The case study of chamomile (Matricaria recutita L.). Ind. Crops Prod. 2012, 40, 1–12. [Google Scholar] [CrossRef]
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2011, 5, 82–95. [Google Scholar] [CrossRef]
- Parvini, S.; Hosseini, M.J.; Bakhtiarian, A. The Study of Analgesic Effects and Acute Toxicity of Tripleurospermum disciforme in Rats by Formalin Test. Toxicol. Mech. Methods 2007, 17, 575–580. [Google Scholar] [CrossRef]
- Tofighi, Z.; Molazem, M.; Doostdar, B.; Taban, P.; Shahverdi, A.R.; Samadi, N.; Yassa, N. Antimicrobial Activities of Three Medicinal Plants and Investigation of Flavonoids of Tripleurospermum disciforme. Iran J. Pharm. Res. 2015, 14, 225–231. [Google Scholar] [PubMed]
- Al-Saleem, M.S.; Awaad, A.S.; Alothman, M.R.; Alqasoumi, S.I. Phytochemical standardization and biological activities of certain desert plants growing in Saudi Arabia. Saudi Pharm. J. 2018, 26, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Cérantola, S.; Kervarec, N.; Pichon, R.; Magné, C.; Bessières, M.A.; Deslandes, E. NMR characterisation of inulin-type fructooligosaccharides as the major water-soluble carbohydrates from Matricaria maritima L. Carbohydr. Res. 2004, 339, 2445–2449. [Google Scholar] [CrossRef] [PubMed]
- Ksouri, R.; Megdiche, W.; Jallali, I.; Debez, H.; Magné, C.; Isoda, H.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef]
- Fraisse, D.; Felgines, C.; Texier, O.; Lamaison, J. Caffeoyl Derivatives: Major Antioxidant Compounds of Some Wild Herbs of the Asteraceae Family. Food Nutr. Sci. 2011, 2, 181–192. [Google Scholar] [CrossRef]
- Ćavar Zeljković, S.; Ayaz, F.A.; Inceer, H.; Hayirlioglu-Ayaz, S.; Colak, N. Evaluation of chemical profile and antioxidant activity of Tripleurospermum insularum, a new species from Turkey. Nat. Prod. Res. 2015, 29, 293–296. [Google Scholar] [CrossRef]
- Duke, J.A. Handbook of Phytochemical Constituents of GRAS Herbs and Other Economic Plants; CRC Press: Boca Raton, FL, USA, 1992; p. 654. [Google Scholar]
- Rodrigues, M.J.; Gangadhar, K.N.; Vizetto-Duarte, C.; Wubshet, S.G.; Nyberg, N.T.; Barreira, L.; Varela, J.; Custódio, L. Maritime halophyte species from southern Portugal as sources of bioactive molecules. Mar. Drugs 2014, 12, 2228–2244. [Google Scholar] [CrossRef]
- Lemoine, C.; Rodrigues, M.J.; Dauvergne, X.; Cerantola, S.; Custodio, L.; Magné, C. Characterization of biological activities and bioactive phenolics from the nonvolatile fraction of the edible and medicinal halophyte sea fennel (Crithmum maritimum L.). Foods 2024, 13, 1294. [Google Scholar] [CrossRef]
- El-Amier, Y.A.; Soufan, W.; Almutairi, K.F.; Zaghloul, N.S.; Abd-ElGawad, A.M. Proximate Composition, Bioactive Compounds, and Antioxidant Potential of Wild Halophytes Grown in Coastal Salt Marsh Habitats. Molecules 2022, 27, 28. [Google Scholar] [CrossRef]
- Davies, P.; Bourmaud, A.; Pajot, A.; Baley, C. A preliminary evaluation of Matricaria maritimum fibres for polymer reinforcement. Ind. Crops Prod. 2011, 34, 1652–1654. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure–antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [PubMed]
- Méot-Duros, L.; Magné, C. Antioxidant activity and phenol content of Crithmum maritimum (L.) leaves. Plant Physiol. Biochem. 2009, 47, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Falleh, H.; Oueslati, S.; Guyot, S.; Ben Dali, A.; Magné, C.; Abdelly, C.; Ksouri, R. LC/ESI-MS/MS characterisation of procyanidins and propelargonidins responsible for the strong antioxidant activity of the edible halophyte Mesembryanthemum edule L. Food Chem. 2011, 127, 1732–1738. [Google Scholar]
- Jdey, A.; Falleh, H.; Ben Jannet, S.; Mkadmini Hammi, K.; Dauvergne, X.; Ksouri, R.; Magné, C. Phytochemical investigation and antioxidant, antibacterial and anti-tyrosinase performances of six medicinal halophytes. S. Afr. J. Bot. 2017, 112, 508–514. [Google Scholar] [CrossRef]
- Jdey, A.; Falleh, H.; Ben Jannet, S.; Hammi, K.M.; Dauvergne, X.; Magné, C.; Ksouri, R. Anti-aging activities of extracts from Tunisian medicinal halophytes and their aromatic constituents. EXCLI J. 2017, 16, 755–769. [Google Scholar]
- Choi, H.K.; Lim, Y.S.; Kim, Y.S.; Park, S.Y.; Lee, C.H.; Hwang, K.W. Free-radical-scavenging and tyrosinase-inhibition activities of Cheonggukjang samples fermented for various times. Food Chem. 2008, 106, 564–568. [Google Scholar] [CrossRef]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef]
- Spínola, V.; Castilho, P.C. Evaluation of Asteraceae herbal extracts in the management of diabetes and obesity. Contribution of caffeoylquinic acids on the inhibition of digestive enzymes activity and formation of advanced glycation end-products (in vitro). Phytochemistry 2017, 143, 29–35. [Google Scholar] [CrossRef]
- Jiang, F.; Dusting, G.J. Natural phenolic compounds as cardiovascular therapeutics: Potential role of their antiinflammatory effects. Curr. Vasc. Pharmacol. 2003, 1, 135–156. [Google Scholar] [CrossRef]
- Chen, L.; Teng, H.; Jia, Z.; Battino, M.; Miron, A.; Yu, Z.; Cao, H.; Xiao, J. Intracellular signaling pathways of inflammation modulated by dietary flavonoids: The most recent evidence. Crit. Rev. Food Sci. Nutr. 2018, 58, 2908–2924. [Google Scholar] [CrossRef]
- Song, J.L.; Song, R.K.; Gao, Y.Y. Anti-inflammatory effect of methanolic extract of Conyza canadensis in lipopolysaccharide (LPS)-stimulated RAW264.7 murine macrophage cells. Pak. J. Pharm. Sci. 2016, 29, 935–940. [Google Scholar]
- Idres, A.Y.; Tousch, D.; Dhuyque-Mayer, C.; Hammad, I.; Lambert, K.; Cazals, G.; Portet, K.; Ferrare, K.; Bidel, L.P.R.; Poucheret, P. An Original Asteraceae Based Infused Drink Prevents Metabolic Syndrome in Fructose-Rat Model. Antioxidants 2023, 12, 340. [Google Scholar] [CrossRef] [PubMed]
- Kanlayavattanakul, M.; Lourith, N. Plants and natural products for the treatment of skin pigmentation—A review. Planta Medica 2018, 84, 988–1006. [Google Scholar] [PubMed]
- Shahidi, F.; Yeo, J.D. Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases: A Review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Shimazawa, M.; Mishima, S.; Hara, H. Water extract of propolis and its main constituents, caffeoyl quinic acid derivatives, exert neuroprotective effect via antioxidant action. Life Sci. 2007, 80, 370–377. [Google Scholar] [CrossRef]
- Vidari, G.; Abdo, S.; Gilardoni, G.; Ciapessoni, A.; Gusmeroli, M.; Zanoni, G. Fungitoxic metabolites from Erigeron apiculatus. Fitoterapia 2006, 77, 318–320. [Google Scholar] [CrossRef]
- Queiroz, S.C.; Cantrell, C.L.; Duke, S.O.; Wedge, D.E.; Nandula, V.K.; Moraes, R.M.; Cerdeira, A.L. Bioassay-directed isolation and identification of phytotoxic and fungitoxic acetylenes from Conyza canadensis. J. Agric. Food Chem. 2012, 60, 5893–5898. [Google Scholar] [CrossRef]
- Ekundayo, O.; Laasko, I.; Hiltunen, R. Essential oil of Ageratum conyzoides. Planta Medica 1988, 54, 55–57. [Google Scholar] [CrossRef]
- Wiedenfeld, H.; Roder, E. Pyrrolizidine Alkaloids from Ageratum conyzoides. Planta Medica 1991, 57, 578–579. [Google Scholar] [CrossRef]
- Okunade, A.L. Ageratum conyzoides L. (Asteraceae). Fitoterapia 2002, 73, 1–16. [Google Scholar] [CrossRef]
- Magid, A.A.; Voutquenne-Nazabadioko, L.; Bontemps, G.; Litaudon, M.; Lavaud, C. Tyrosinase inhibitors and sesquiterpene diglycosides from Guioa villosa. Planta Medica 2008, 74, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Baek, N.; Nam, T.G. Natural, semisynthetic and synthetic tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2016, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stefanis, I.; Hadjipavlou-Litina, D.; Bilia, A.R.; Karioti, A. LC-MS- and NMR-guided isolation of monoterpene dimers from cultivated Thymus vulgaris Varico 3 hybrid and their antityrosinase activity. Planta Medica 2019, 85, 941–946. [Google Scholar] [PubMed]
- Patel, D.K.; Prasad, S.K.; Kumar, R.; Hemalatha, S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed. 2012, 2, 320–330. [Google Scholar] [CrossRef]
- Hewitt, E.J. Sand and Water Culture Methods Used in the Study of Plant Nutrition, 2nd ed.; Technical Communication No. 22; Commonwealth Bureau: London, UK, 1966. [Google Scholar]
- Marwah, R.G.; Fatope, M.O.; Mahrooqi, R.A.; Varma, G.B.; Abadi, H.A.; Al-Burtamani, S.K.S. Antioxidant capacity of some edible and wound healing plants in Oman. Food Chem. 2007, 101, 465–470. [Google Scholar] [CrossRef]
- Jimenez-Alvarez, D.; Giuffrida, F.; Vanrobaeys, F.; Golay, P.A.; Cotting, C.; Lardeau, A.; Keely, B.J. High-throughput methods to assess lipophilic and hydrophilic antioxidant capacity of food extracts in vitro. J. Agric. Food Chem. 2008, 56, 3470–3477. [Google Scholar] [CrossRef]
- Bolanos de la Torre, A.A.S.; Henderson, T.; Nigam, P.S.; Owusu-Apenten, R.K. A universally calibrated microplate ferric reducing antioxidant power (FRAP) assay for foods and applications to Manuka honey. Food Chem. 2015, 174, 119–123. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef]
- Kim, Y.; Uyama, H.; Kobayashi, S. Inhibition effects of (+)-catechinaldehyd polycondensates on proteinases causing proteolytic degradation of extracellular matrix. Biochem. Biophys. Res. Commun. 2004, 320, 256–261. [Google Scholar] [CrossRef]
- Bouzaiene, N.N.; Chaabane, F.; Sassi, A.; Chekir-Ghedira, L.; Ghedira, K. Effect of apigenin-7-glucoside, genkwanin and naringenin on tyrosinase activity and melanin synthesis in B16F10 melanoma cells. Life Sci. 2016, 144, 80–85. [Google Scholar] [CrossRef]
- Custódio, L.; Soares, F.; Pereira, H.; Rodrigues, M.J.; Barreira, L.; Rauter, A.P.; Alberício, F.; Varela, J. Botryococcus braunii and Nanochloropsis oculata extracts inhibit cholinesterases and protect human dopaminergic SH-SY5Y cells from H2O2-induced cytotoxicity. J. Appl. Phycol. 2015, 27, 839–848. [Google Scholar] [CrossRef]
- Zengin, G. A study on in vitro enzyme inhibitory properties of Asphodeline anatolica: New sources of natural inhibitors for public health problems. Ind. Crops Prod. 2016, 83, 39–43. [Google Scholar] [CrossRef]
- McDougall, G.J.; Kulkarni, N.N.; Stewart, D. Berry polyphenols inhibit pancreatic lipase activity in vitro. Food Chem. 2009, 115, 193–199. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).