Therapeutic Potential of Neopyropia yezoensis: An Updated Review
Abstract
1. Introduction
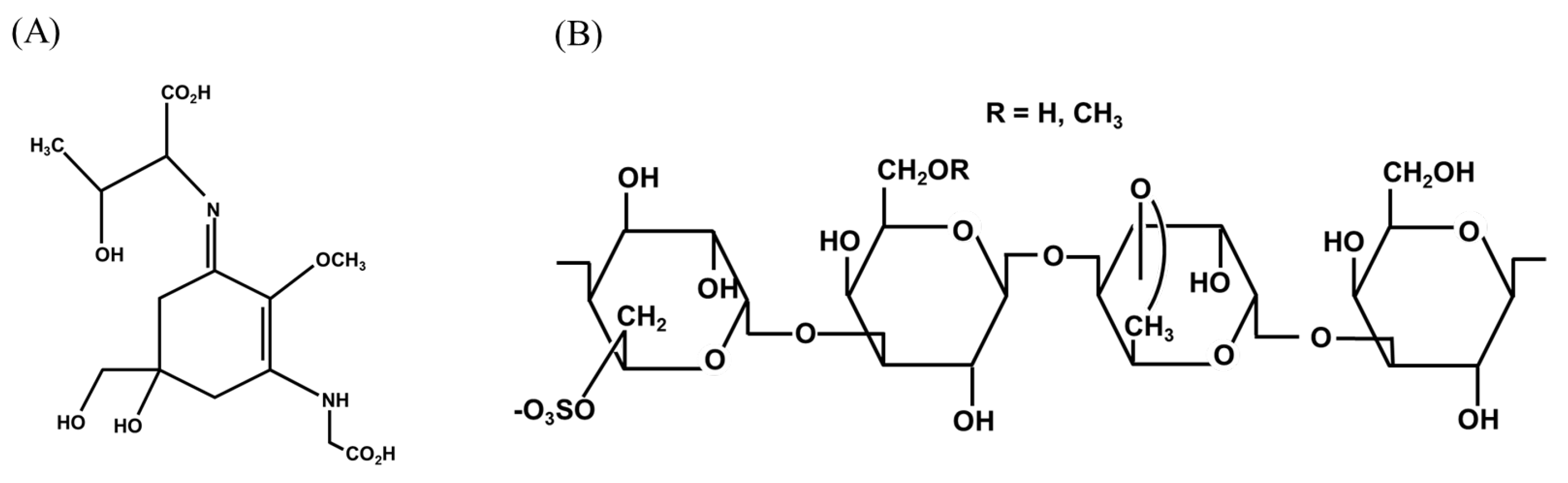
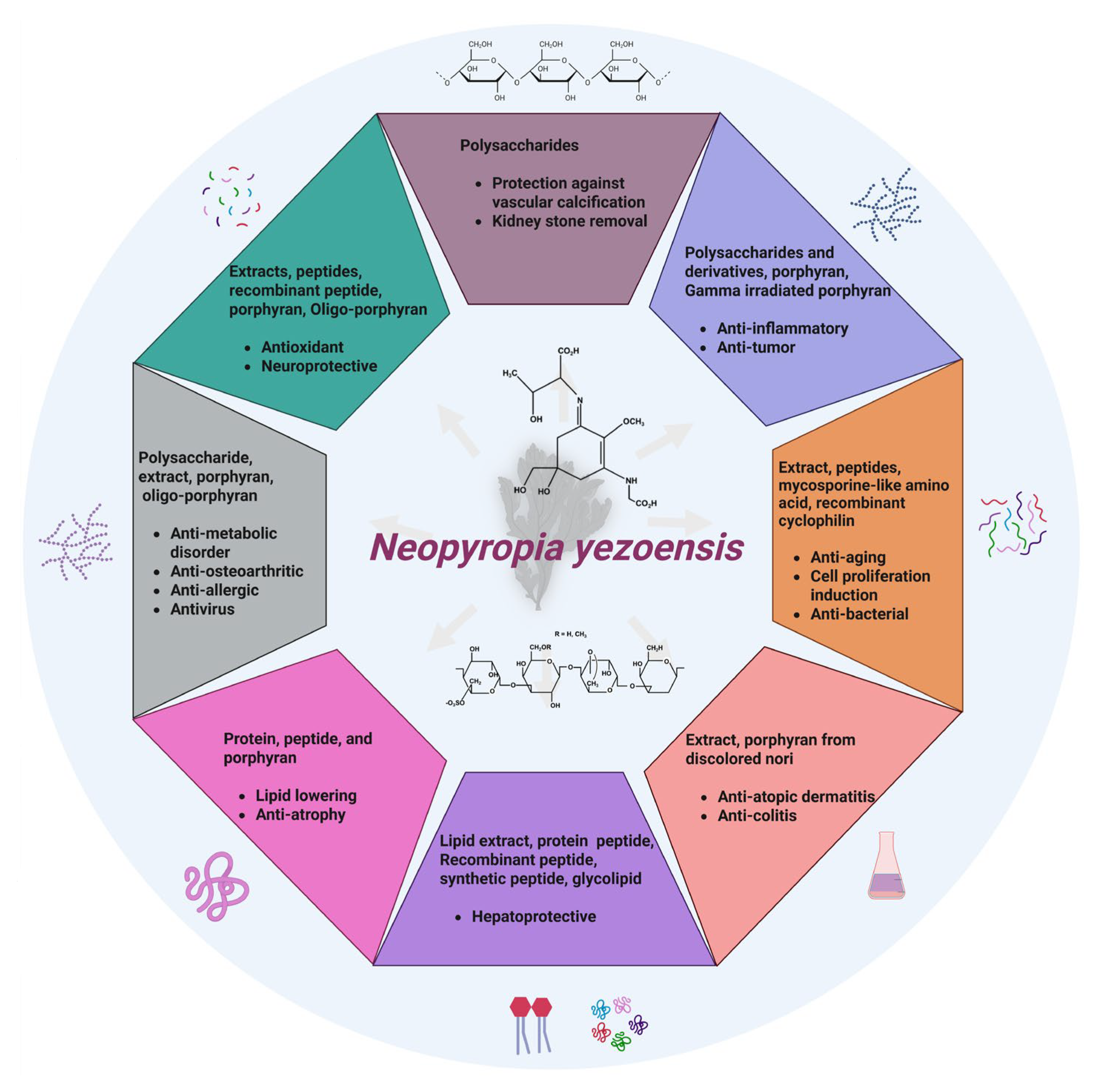
2. Important Metabolites
3. Literature Search Strategy
4. Therapeutic Effects
4.1. Antioxidant
4.2. Anti-Inflammatory
4.3. Neuroprotective
4.4. Anticancer
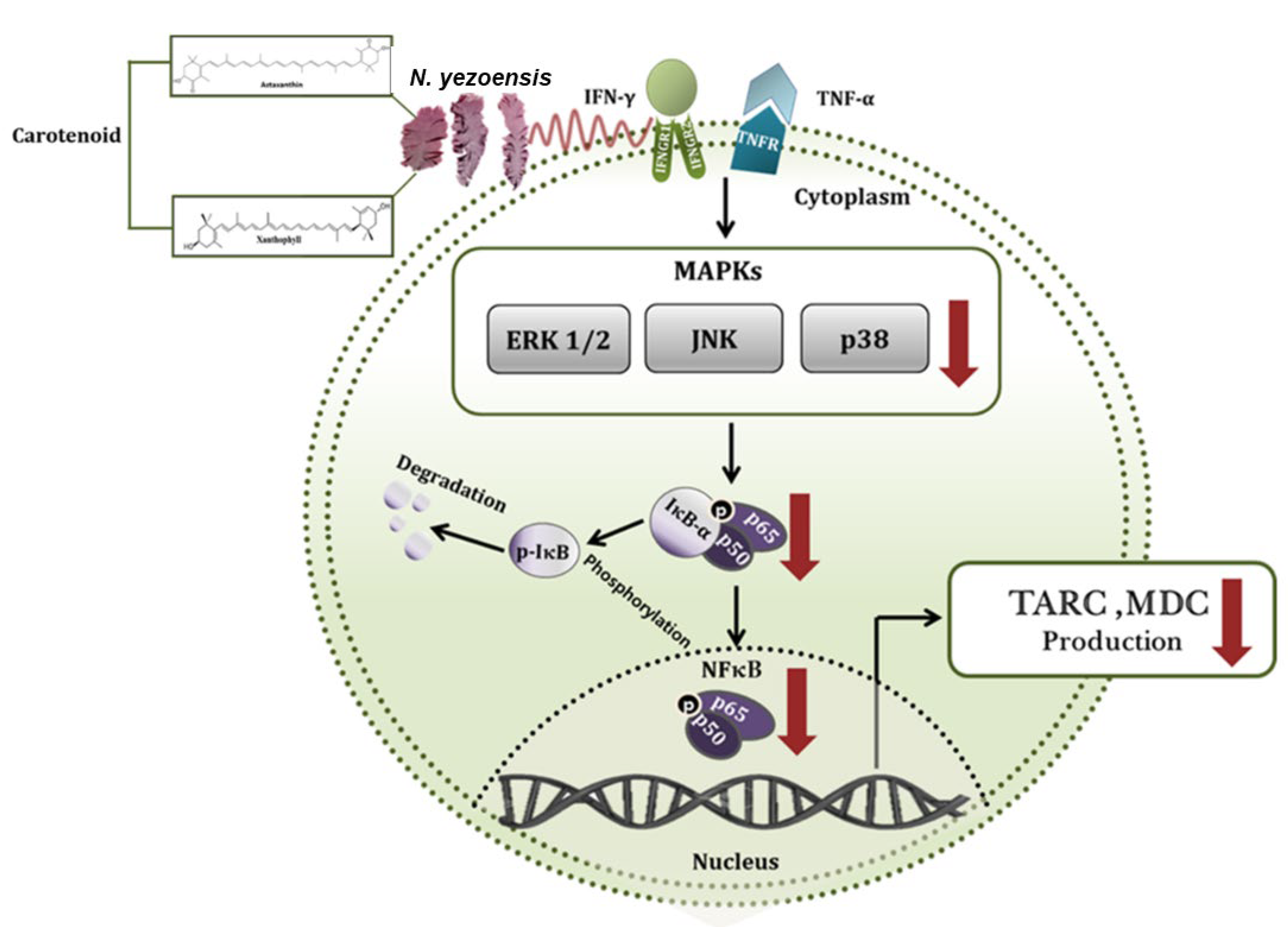
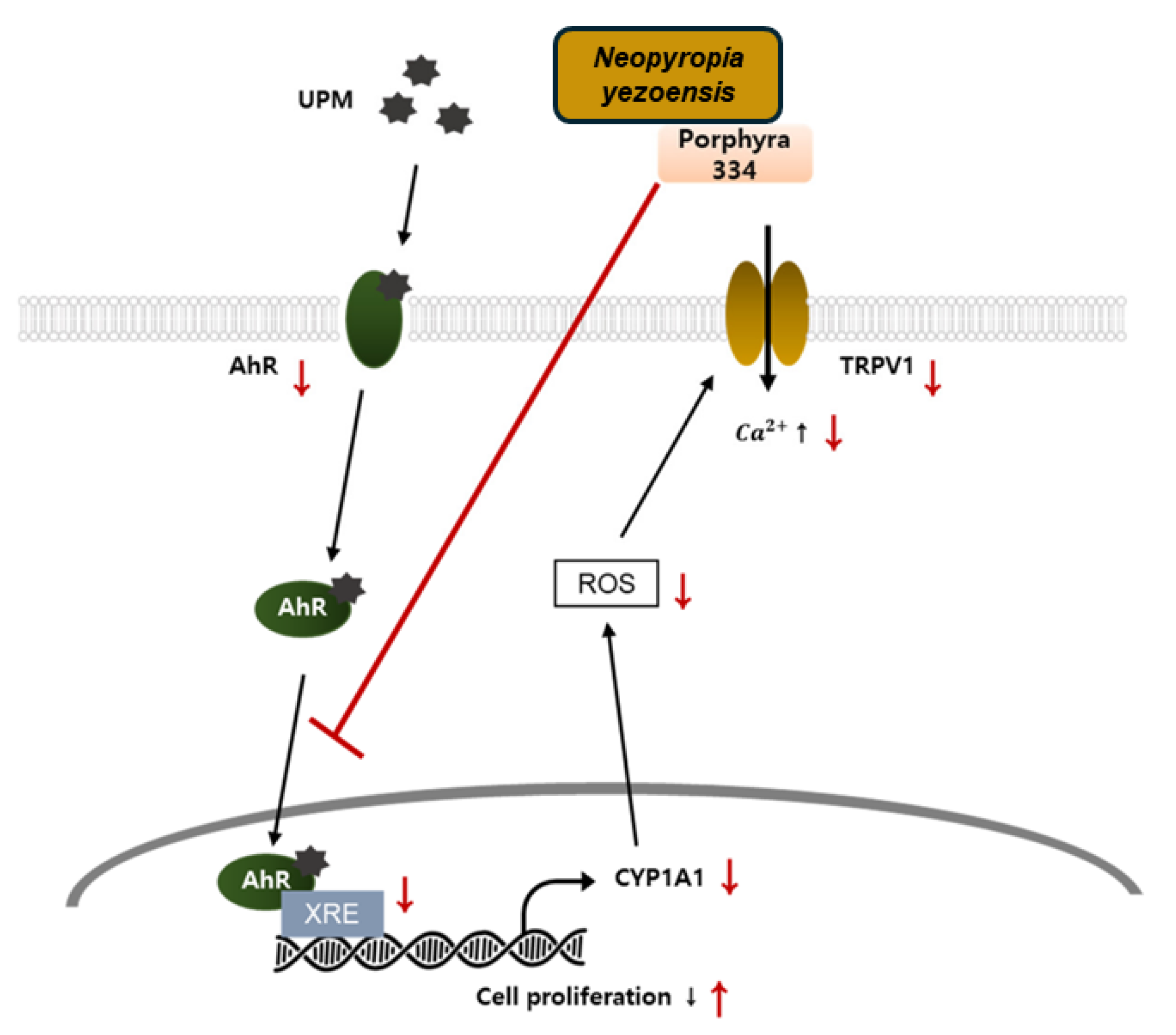
4.5. Anti-Atopic Dermatitis
4.6. Anti-Colitis
| Test Material | Experimental Model | Outcomes/Mechanisms | Ref. |
|---|---|---|---|
| Anti-atopic dermatitis | |||
| N. yezoensis extract (PYE) | HaCaT Cells+ PYE (40, 200, and 1000 µg/mL), TNF-α or IFN-γ (10 ng/mL), 24 h | ↓ TARC, ↓ MDC, ↓ ERK, ↓ JNK, ↓ p38, ↓ NF-κB | [51] |
| Anti-colitis | |||
| Porphyran from decolored N. yezoensis | C57BL/6 mice + DSS (4%, 8 days), replaced every two days + porphyran (50 mg/kg, 100 μL) orally or intraperitoneal, 7 days | Reversed BW reduction, ↓ DAI score, ↓ colon length, ↓ inflammatory cell infiltration. In colon: ↓ CD11c, ↓ DCs, ↓ TCR-β+, ↓ NK, ↓ neutrophils. ↓ CD11c (macrophages). In mesenteric lymph nodes CD4 T cells: ↓ IFN-γ, ↓ IL-17 CD8 T cells: ↓ IFN-γ, ↓ IL-17, ↓ T-bet, ↓ RORγt Serum: ↓ IFN-γ, ↓ IL-17. Colonic DCs and macrophages: ↓ CD40, ↓ CD80, ↓ CD86. ↓ IL-1β, ↓ IL-12, ↓ IL-6, ↓ IL-23 | [53] |
4.7. Anti-Aging
4.8. Induction of Cell Proliferation and Related Signaling Pathway
4.9. Anti-Atrophy
| Test Material | Experimental Model | Outcomes/Mechanisms | Ref. |
|---|---|---|---|
| Peptide (PYP1-5) | C2C12 myotubes + DEX (100 μM) + PYP1–5 (500 ng/mL), 24 h | ↓ MAFbx, ↓ MuRF1 | [66] |
| Peptide (PYP15) | C2C12 myotubes + DEX group (100 μM), DEX + PYP15 group (100 μM + 500 ng/mL), and PYP15 group (500 ng/mL) | ↑ p-mTOR, ↑ p-IGF-IR, ↑ Raptor, ↑ p-Akt, ↑ p-IRS-1, ↑ REDD1, ↑ KLF-15, ↑ p-p70S6K, ↑ p-S6, ↑ p-4E-BP1, ↑ eIF4E, ↑ p-FoxO1, ↑ p-FoxO3a, ↓ 20S proteasome activity. Downregulation of autophagy lysosomal system | [67] |
| Crude protein (PYCP) | C2C12 myotubes + DEX exposure (100 μM) + PYCP (20 and 40 µg/mL), 24 h | Dose-dependent increase myotube diameter, ↑ myogenin, ↑ p-FoxO1, ↑ p-FoxO3a, ↓ MAFbx, ↓ MuRF1, ↓ 20S proteasome activity, ↓ cathepsin-L, ↓ LC-I to LC-II | [68] |
| Protein (PYCP) | C57BL/6 mice + DEX (3 mg/kg BW, I.P.) + PYCP (oral administration, 150 and 300 mg/kg BW) | Prevented reduction in BW, calf thickness, ↑ gastrocnemius, ↑ tibialis anterior muscle weight, ↓ glucose levels, ↓ CK, ↓ LDH, In gastrocnemius muscle: ↑ p-Akt, ↑ p-IGF-IR, ↑ p-mTOR, ↑ Raptor, ↑ Rheb protein,↑ p-IRS-1, ↑ p-p70S6K, ↑ p-S6, ↑ p-4E-BP1, ↑ eIF4E | [69] |
| Protein (PYCP) | C2C12 myotubes + TNF-α (20 ng/mL) + PYCP (25, 50, and 100 µg/mL), 48 h | ↑ myotube diameter, ↓ ROS, ↓ TNF-R1. Cytosolic: ↓ p-IκBα, ↑ NF-κB, Nuclear: ↓ NF-κB, ↓ atrogin-1/MAFbx, ↓ MuRF1, ↓ 20S proteasome activity, ↓ IL-6, ↑ MyoD, ↑ myogenin | [70] |
4.10. Metabolic Health-Promoting Effects
4.11. Anti-Osteoarthritis
4.12. Kidney Stone Treatment
4.13. Anti-Allergic
4.14. Protection Against Vascular Calcification
4.15. Antivirus
4.16. Antibacterial
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, L.E.; Deng, Y.Y.; Xu, G.P.; Russell, S.; Lu, Q.Q.; Brodie, J. Redefining Pyropia (Bangiales, Rhodophyta): Four new genera, resurrection of Porphyrella and description of Calidia pseudolobata sp. nov. from China. J. Phycol. 2020, 56, 862–879. [Google Scholar] [CrossRef]
- Sutherland, J.E.; Lindstrom, S.C.; Nelson, W.A.; Brodie, J.; Lynch, M.D.; Hwang, M.S.; Choi, H.G.; Miyata, M.; Kikuchi, N.; Oliveira, M.C. A new look at an ancient order: Generic revision of the Bangiales (Rhodophyta) 1. J. Phycol. 2011, 47, 1131–1151. [Google Scholar] [CrossRef]
- Wang, D.; Yu, X.; Xu, K.; Bi, G.; Cao, M.; Zelzion, E.; Fu, C.; Sun, P.; Liu, Y.; Kong, F. Pyropia yezoensis genome reveals diverse mechanisms of carbon acquisition in the intertidal environment. Nat. Comm. 2020, 11, 4028. [Google Scholar] [CrossRef]
- Yang, X.-Q.; Huang, H.-C.; Pan, C.; Wang, J.-X.; Zhao, Y.-Q.; Qi, B. Advances on nutrient components, biological activities and comprehensive utilization of Porphyra. Food Ferment. Ind. 2020, 46, 306–313. [Google Scholar]
- Aoki, Y.; Kamei, Y. Preparation of recombinant polysaccharide-degrading enzymes from the marine bacterium, Pseudomonas sp. ND137 for the production of protoplasts of Porphyra yezoensis. Eur. J. Phycol. 2006, 41, 321–328. [Google Scholar] [CrossRef]
- Nisizawa, K.; Noda, H.; Kikuchi, R.; Watanabe, T. The main seaweed foods in Japan. Hydrobiologia 1987, 151, 5–29. [Google Scholar] [CrossRef]
- Nakamura, Y.; Sasaki, N.; Kobayashi, M.; Ojima, N.; Yasuike, M.; Shigenobu, Y.; Satomi, M.; Fukuma, Y.; Shiwaku, K.; Tsujimoto, A. The first symbiont-free genome sequence of marine red alga, Susabi-nori (Pyropia yezoensis). PLoS ONE 2013, 8, e57122. [Google Scholar] [CrossRef]
- Venkatraman, K.L.; Mehta, A. Health benefits and pharmacological effects of porphyra species. Plant Foods Hum. Nutr. 2019, 74, 10–17. [Google Scholar] [CrossRef]
- Hwang, M.-S.; Kim, S.-M.; Ha, D.-S.; Baek, J.-M.; Kim, H.-S.; Choi, H.-G. DNA sequences and identification of Porphyra cultivated by natural seeding on the southwest coast of Korea. Algae 2005, 20, 183–196. [Google Scholar] [CrossRef]
- Lee, E.-J.; Kim, G.-R.; Lee, H.-J.; Kwon, J.-H. Monitoring microbiological contamination, pre-decontamination, and irradiation status of commercial dried laver (Porphyra sp.) products. Korean J. Food Sci. Technol. 2017, 49, 20–27. [Google Scholar] [CrossRef]
- Ministry of Oceans and Fisheries (MOF). Statics News. 2017. Available online: https://www.mof.go.kr/statPortal/stp/cts/anr/statsAnlrpt.do (accessed on 18 April 2025).
- Admassu, H.; Abera, T.; Abraha, B.; Yang, R.; Zhao, W. Proximate, mineral and amino acid composition of dried laver (Porphyra spp.) seaweed. J. Acad. Ind. Res. 2018, 6, 149. [Google Scholar]
- Cho, T.J.; Rhee, M.S. Health functionality and quality control of laver (Porphyra, Pyropia): Current issues and future perspectives as an edible seaweed. Mar. Drugs 2020, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Kang, T.; Kim, K. The nutritional and functional constituents of laver. Bull. Fish Sic. Inst. Yosu Nat’l Univ. 2000, 9, 133–137. [Google Scholar]
- Park, J.M. Who will be the winner of the heated ‘land-based gim (laver) farming war? World Aquacult. 2025, 34–36. Available online: https://www.hdhy.co.kr/news/articleView.html?idxno=31117 (accessed on 18 April 2025).
- Allur Subramaniyan, S.; Begum, N.; Kim, S.J.; Choi, Y.H.; Nam, T.-J. Biopeptides of Pyropia yezoensis and their potential health benefits: A review. Asian Pac. J. Trop. Biomed. 2021, 11, 375–384. [Google Scholar] [CrossRef]
- Wang, H.; Luan, F.; Shi, Y.; Yan, S.; Xin, B.; Zhang, X.; Guo, D.; Sun, J.; Zou, J. Extraction, structural features, and pharmacological effects of the polysaccharides from Porphyra yezoensis: A review. Int. J. Biol. Macromol. 2024, 279, 134745. [Google Scholar] [CrossRef]
- Kim, K.-W.; Hwang, J.-H.; Oh, M.-J.; Kim, M.-Y.; Choi, M.-R.; Park, W.-M. Studies on the major nutritional components of commercial dried lavers (Porphyra yezoensis) cultivated in Korea. Korean J. Food Preser. 2014, 21, 702–709. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, B.; Yao, Z. Porphyran, porphyran oligosaccharides and porphyranase: Source, structure, preparation methods and applications. Algal Res. 2023, 73, 103167. [Google Scholar] [CrossRef]
- Kim, B.-S.; Im, J.-H.; Yoon, Y.-S.; Kim, H.; Cho, J.-Y.; Ham, J.-R.; Heo, Y.-J.; Lee, H.-I. Analysis of Nutritional Composition and Flavor Patterns by Variety (Porphyra dentata and Porphyra yezoensis) in Dried Laver from Jeonnam, Korea. Foods 2025, 14, 335. [Google Scholar] [CrossRef]
- Lee, H.-A.; Kim, I.-H.; Nam, T.-J. Bioactive peptide from Pyropia yezoensis and its anti-inflammatory activities. Int. J. Mol. Med. 2015, 36, 1701–1706. [Google Scholar] [CrossRef]
- Ulagesan, S.; Choi, J.-W.; Nam, T.-J.; Choi, Y.-H. Peptidyl-prolyl isomerase and the biological activities of recombinant protein cyclophilin from Pyropia yezoensis (PyCyp). Protein Expr. Purif. 2020, 172, 105636. [Google Scholar] [CrossRef] [PubMed]
- Ulagesan, S.; Nam, T.-J.; Choi, Y.-H. Extraction and purification of R-phycoerythrin alpha subunit from the marine red algae Pyropia yezoensis and its biological activities. Molecules 2021, 26, 6479. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, J.; Wang, S.; Xu, X. Porphyra species: A mini-review of its pharmacological and nutritional properties. J. Med. Food 2016, 19, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.-L.; Kim, G.H.; Kang, M.-C.; Jeon, Y.-J. Protective effects of extracts from six local strains of Pyropia yezoensis against oxidative damage in vitro and in zebrafish model. Algae 2020, 35, 189–200. [Google Scholar] [CrossRef]
- Guan, B.; Sun, Y.; Liu, X.; Zhong, C.; Li, D.; Shan, X.; Hui, X.; Lu, C.; Huo, Y.; Sun, R.; et al. Comparative evaluation of amino acid profiles, fatty acid compositions, and nutritional value of two varieties of head water Porphyra yezoensis: “Jianghaida No. 1” and “Sutong No.1”. Food Chem. X 2024, 22, 101375. [Google Scholar] [CrossRef]
- Lee, J.-H.; Ahn, G.; Ko, J.-Y.; Kang, N.; Jung, K.; Han, E.-J.; Kim, G.-H.; Kim, H.J.; Choi, C.S.; Jeon, Y.-J. Liposoluble portion of the red alga Pyropia yezoensis protects alcohol induced liver injury in mice. Algae 2021, 36, 219–229. [Google Scholar] [CrossRef]
- Uji, T.; Gondaira, Y.; Fukuda, S.; Mizuta, H.; Saga, N. Characterization and expression profiles of small heat shock proteins in the marine red alga Pyropia yezoensis. Cell Stress Chaperones 2019, 24, 223–233. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, B.; Yan, X. Isolation and characterization of a heat-resistant strain with high yield of Pyropia yezoensis Ueda (Bangiales, Rhodophyta). Aquac. Fish. 2016, 1, 24–33. [Google Scholar] [CrossRef][Green Version]
- Khan, S.; Mao, Y.; Gao, D.; Riaz, S.; Niaz, Z.; Tang, L.; Khan, S.; Wang, D. Identification of proteins responding to pathogen-infection in the red alga Pyropia yezoensis using iTRAQ quantitative proteomics. BMC Genom. 2018, 19, 842. [Google Scholar] [CrossRef]
- Kim, E.-Y.; Choi, Y.H.; Nam, T.-J. Identification and antioxidant activity of synthetic peptides from phycobiliproteins of Pyropia yezoensis. Int. J. Mol. Med. 2018, 42, 789–798. [Google Scholar] [CrossRef]
- Ulagesan, S.; Eom, T.; Nam, T.-J.; Choi, Y.-H. Antioxidant and chemoprotective peptides from simulated gastrointestinal digested (SGID) protein hydrolysate of Pyropia yezoensis against acetaminophen-induced HepG2 cells. Bioprocess Biosyst. Eng. 2022, 45, 1645–1660. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, P.; Wang, Q.; Xu, F.; Bai, Y.; Pan, S.; Wang, W.; Tang, D.Y.Y.; Show, P.L. Peptidomics-inspired discovery and activity evaluation of antioxidant peptides in multiple strains mixed fermentation of Porphyra yezoensis. Food Chem. 2024, 455, 139779. [Google Scholar] [CrossRef]
- Hyun, J.; Lee, S.-W.; Jeong, H.H.; Kim, J.-I. Comparative study on antioxidant activity of Gold 1, a new strain of Pyropia yezoensis. Fish. Aquat. Sci. 2023, 26, 158–168. [Google Scholar] [CrossRef]
- Kim, E.-Y.; Choi, Y.H.; Choi, C.G.; Nam, T.-J. Effects of the cyclophilin-type peptidylprolyl cis-trans isomerase from Pyropia yezoensis against hydrogen peroxide-induced oxidative stress in HepG2 cells. Mol. Med. Rep. 2017, 15, 4132–4138. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Long, X.; Wang, J.; Qi, B.; Cao, Y.; Hu, X. Rheological Behavior, Textural Properties, and Antioxidant Activity of Porphyra yezoensis Polysaccharide. Molecules 2025, 30, 882. [Google Scholar] [CrossRef]
- Harikishore, A.; Sup Yoon, H. Immunophilins: Structures, mechanisms and ligands. Curr. Mol. Pharmacol. 2016, 9, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Hama, Y.; Yamaguchi, K.; Oda, T. Inhibitory effect of sulphated polysaccharide porphyran on nitric oxide production in lipopolysaccharide-stimulated RAW264.7 macrophages. J. Biochem. 2011, 151, 65–74. [Google Scholar] [CrossRef]
- Yanagido, A.; Ueno, M.; Jiang, Z.; Cho, K.; Yamaguchi, K.; Kim, D.; Oda, T. Increase in anti-inflammatory activities of radical-degraded porphyrans isolated from discolored nori (Pyropia yezoensis). Int. J. Biol. Macromol. 2018, 117, 78–86. [Google Scholar] [CrossRef]
- Wang, Y.; Hwang, J.; Yadav, D.; Oda, T.; Lee, P.C.; Jin, J.O. Inhibitory effect of porphyran on lipopolysaccharide-induced activation of human immune cells. Carbohydr. Polym. 2020, 232, 115811. [Google Scholar] [CrossRef]
- Wang, Y.; Hwang, J.-Y.; Park, H.-B.; Yadav, D.; Oda, T.; Jin, J.-O. Porphyran isolated from Pyropia yezoensis inhibits lipopolysaccharide-induced activation of dendritic cells in mice. Carbohydr. Polym. 2020, 229, 115457. [Google Scholar] [CrossRef]
- Isaka, S.; Cho, K.; Nakazono, S.; Abu, R.; Ueno, M.; Kim, D.; Oda, T. Antioxidant and anti-inflammatory activities of porphyran isolated from discolored nori (Porphyra yezoensis). Int. J. Biol. Macromol. 2015, 74, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Kim, E.Y.; Nam, T.J. Phycoerythrin-derived tryptic peptide of a red alga Pyropia yezoensis attenuates glutamate-induced ER stress and neuronal senescence in primary rat hippocampal neurons. Mol. Nutr. Food Res. 2018, 62, 1700469. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Kim, E.-Y.; Nam, T.-J. Phycoerythrin peptide from Pyropia yezoensis alleviates endoplasmic reticulum stress caused by perfluorooctane sulfonate-induced calcium dysregulation. Mar. Drugs 2018, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Deng, Z.; Geng, L.; Wang, J.; Zhang, Q. In vitro evaluation of the neuroprotective effect of oligo-porphyran from Porphyra yezoensis in PC12 cells. J. Appl. Phycol. 2019, 31, 2559–2571. [Google Scholar] [CrossRef]
- Gutiérrez-Rodríguez, A.G.; Juarez-Portilla, C.; Olivares-Banuelos, T.; Zepeda, R.C. Anticancer activity of seaweeds. Drug Discov. Today 2018, 23, 434–447. [Google Scholar] [CrossRef]
- He, D.; Wu, S.; Yan, L.; Zuo, J.; Cheng, Y.; Wang, H.; Liu, J.; Zhang, X.; Wu, M.; Choi, J.I. Antitumor bioactivity of porphyran extracted from Pyropia yezoensis Chonsoo2 on human cancer cell lines. J. Sci. Food Agric. 2019, 99, 6722–6730. [Google Scholar] [CrossRef]
- He, D.; Yan, L.; Ma, X.; Cheng, Y.; Wu, S.; Zuo, J.; Park, E.-J.; Liu, J.; Wu, M.; Choi, J.-I. Gamma-irradiation degraded sulfated polysaccharide from a new red algal strain Pyropia yezoensis Sookwawon 104 with in vitro antiproliferative activity. Oncol. Lett. 2020, 20, 91. [Google Scholar] [CrossRef]
- Pham, T.N.A.; Le, B.; Yang, S.H. Anticancer activity of the potential Pyropia yezoensis galactan fractionated in human prostate cancer cells. Biotechnol. Bioprocess Eng. 2021, 26, 63–70. [Google Scholar] [CrossRef]
- Park, H.Y.; Han, M.H.; Park, C.; Jin, C.Y.; Kim, G.Y.; Choi, I.W.; Kim, N.D.; Nam, T.J.; Kwon, T.K.; Choi, Y.H. Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem. Toxicol. 2011, 49, 1745–1752. [Google Scholar] [CrossRef]
- Ha, Y.; Lee, W.H.; Jeong, J.; Park, M.; Ko, J.Y.; Kwon, O.W.; Lee, J.; Kim, Y.J. Pyropia yezoensis extract suppresses IFN-gamma- and TNF-alpha-induced proinflammatory chemokine production in HaCaT cells via the downregulation of NF-κB. Nutrients 2020, 12, 1238. [Google Scholar] [CrossRef]
- Kaur, A.; Goggolidou, P. Ulcerative colitis: Understanding its cellular pathology could provide insights into novel therapies. J. Inflamm. 2020, 17, 15. [Google Scholar] [CrossRef]
- Park, H.-B.; Kim, S.-J.; Yadav, D.; An, E.-K.; Zhang, W.; Eom, H.-Y.; Kwak, M.; Oda, T.; Lee, P.C.-W.; Jin, J.-O. Pyropia yezoensis-derived porphyran attenuates acute and chronic colitis by suppressing dendritic cells. Int. J. Biol. Macromol. 2023, 231, 123148. [Google Scholar] [CrossRef]
- Ryu, J.; Park, S.-J.; Kim, I.-H.; Choi, Y.H.; Nam, T.-J. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int. J. Mol. Med. 2014, 34, 796–803. [Google Scholar] [CrossRef]
- Kim, C.-R.; Kim, Y.-M.; Lee, M.-K.; Kim, I.-H.; Choi, Y.-H.; Nam, T.-J. Pyropia yezoensis peptide promotes collagen synthesis by activating the TGF-β/Smad signaling pathway in the human dermal fibroblast cell line Hs27. Int. J. Mol. Med. 2017, 39, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, H.; Choi, S.; Pandey, L.K.; Depuydt, S.; De Saeger, J.; Park, J.-T.; Han, T. Extracts of red seaweed, Pyropia yezoensis, inhibit melanogenesis but stimulate collagen synthesis. J. Appl. Phycol. 2021, 33, 653–662. [Google Scholar] [CrossRef]
- Choi, S.; Lee, J.H.; Oh, S.W.; Yu, E.; Kwon, K.; Jang, S.J.; Shin, D.S.; Moh, S.H.; Lee, J. Anti-pollutant activity of Porphyra yezoensis water extract and its active compound, porphyra 334, against urban particulate matter-induced keratinocyte cell damage. Mar. Drugs 2023, 21, 121. [Google Scholar] [CrossRef] [PubMed]
- Gândara, R.M.; Mahida, Y.R.; Potten, C.S. Regional differences in stem and transit cell proliferation and apoptosis in the terminal ileum and colon of mice after 12 Gy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e521–e528. [Google Scholar] [CrossRef]
- Galvan, V.; Logvinova, A.; Sperandio, S.; Ichijo, H.; Bredesen, D.E. Type 1 insulin-like growth factor receptor (IGF-IR) signaling inhibits apoptosis signal-regulating kinase 1 (ASK1). J. Biol. Chem. 2003, 278, 13325–13332. [Google Scholar] [CrossRef]
- Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2000, 103, 211–225. [Google Scholar] [CrossRef]
- Lee, M.K.; Kim, I.H.; Choi, Y.H.; Nam, T.J. A peptide from Porphyra yezoensis stimulates the proliferation of IEC-6 cells by activating the insulin-like growth factor I receptor signaling pathway. Int. J. Mol. Med. 2015, 35, 533–538. [Google Scholar] [CrossRef]
- Lee, M.K.; Kim, I.H.; Choi, Y.H.; Choi, J.W.; Kim, Y.M.; Nam, T.J. The proliferative effects of Pyropia yezoensis peptide on IEC-6 cells are mediated through the epidermal growth factor receptor signaling pathway. Int. J. Mol. Med. 2015, 35, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-H.; Choi, J.-W.; Lee, M.-K.; Choi, Y.-H.; Nam, T.-J. Effect of cyclophilin from Pyropia yezoensis on the proliferation of intestinal epithelial cells by epidermal growth factor receptor/Ras signaling pathway. Mar. Drugs 2019, 17, 297. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Leeuwenburgh, C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp. Gerontol. 2006, 41, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.A.V.; Al-Khalaf, M.; Megeney, L.A. The beneficial role of proteolysis in skeletal muscle growth and stress adaptation. Skelet. Muscle 2016, 6, 16. [Google Scholar] [CrossRef]
- Lee, M.K.; Kim, Y.M.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Pyropia yezoensis peptide PYP1-5 protects against dexamethasone-induced muscle atrophy through the downregulation of atrogin1/MAFbx and MuRF1 in mouse C2C12 myotubes. Mol. Med. Rep. 2017, 15, 3507–3514. [Google Scholar] [CrossRef]
- Lee, M.-K.; Choi, J.-W.; Choi, Y.H.; Nam, T.-J. Protective effect of Pyropia yezoensis peptide on dexamethasone-induced myotube atrophy in C2C12 myotubes. Mar. Drugs 2019, 17, 284. [Google Scholar] [CrossRef]
- Lee, M.-K.; Choi, J.-W.; Choi, Y.H.; Nam, T.-J. Pyropia yezoensis protein prevents dexamethasone-induced myotube atrophy in C2C12 myotubes. Mar. Drugs 2018, 16, 497. [Google Scholar] [CrossRef]
- Lee, M.-K.; Choi, J.-W.; Choi, Y.H.; Nam, T.-J. Pyropia yezoensis protein supplementation prevents dexamethasone-induced muscle atrophy in c57bl/6 mice. Mar. Drugs 2018, 16, 328. [Google Scholar] [CrossRef]
- Lee, M.-K.; Choi, Y.H.; Nam, T.-J. Pyropia yezoensis protein protects against TNF-α-induced myotube atrophy in C2C12 myotubes via the NF-κB signaling pathway. Mol. Med. Rep. 2021, 24, 486. [Google Scholar] [CrossRef]
- He, D.; Yan, L.; Hu, Y.; Wu, Q.; Wu, M.; Choi, J.-I.; Tong, H. Optimization of porphyran extraction from Pyropia yezoensis by response surface methodology and its lipid-lowering effects. Mar. Drugs 2021, 19, 53. [Google Scholar] [CrossRef]
- Cao, J.; Wang, S.; Yao, C.; Xu, Z.; Xu, X. Hypolipidemic effect of porphyran extracted from Pyropia yezoensis in ICR mice with high fatty diet. J. Appl. Phycol. 2016, 28, 1315–1322. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kwon, M.J.; Kim, I.H.; Nam, T.J. Chemoprotective effects of a protein from the red algae Porphyra yezoensis on acetaminophen-induced liver injury in rats. Phytother. Res. 2008, 22, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Yamaguchi, K.; Oda, T.; Nam, T.J. Chemical and mass spectrometry characterization of the red alga Pyropia yezoensis chemoprotective protein (PYP): Protective activity of the N-terminal fragment of PYP1 against acetaminophen-induced cell death in Chang liver cells. Int. J. Mol. Med. 2015, 35, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kim, E.-Y.; Mikami, K.; Nam, T.J. Chemoprotective effects of a recombinant protein from Pyropia yezoensis and synthetic peptide against acetaminophen-induced Chang liver cell death. Int. J. Mol. Med. 2015, 36, 369–376. [Google Scholar] [CrossRef]
- Kim, I.-H.; Choi, J.-W.; Nam, T.-J. PYP1-4 peptide from Pyropia yezoensis protects against acetaminophen-induced hepatotoxicity in HepG2 cells. Exp. Ther. Med. 2020, 19, 849–860. [Google Scholar] [CrossRef]
- Yanagita, T.; Tsuge, K.; Koga, M.; Inoue, N.; Nagao, K. Eicosapentaenoic acid-containing polar lipids from seaweed Susabinori (Pyropia yezoensis) alleviate hepatic steatosis in obese db/db mice. Arch. Biochem. Biophys. 2020, 691, 108486. [Google Scholar] [CrossRef]
- Iizasa, S.; Nagao, K.; Tsuge, K.; Nagano, Y.; Yanagita, T. Identification of genes regulated by lipids from seaweed Susabinori (Pyropia yezoensis) involved in the improvement of hepatic steatosis: Insights from RNA-Seq analysis in obese db/db mice. PLoS ONE 2023, 18, e0295591. [Google Scholar] [CrossRef]
- Choi, J.W.; Kim, I.H.; Kim, Y.M.; Lee, M.K.; Choi, Y.H.; Nam, T.J. Protective effect of Pyropia yezoensis glycoprotein on chronic ethanol consumption-induced hepatotoxicity in rats. Mol. Med. Rep. 2016, 14, 4881–4886. [Google Scholar] [CrossRef]
- Choi, J.W.; Kim, I.H.; Kim, Y.M.; Lee, M.K.; Nam, T.J. Pyropia yezoensis glycoprotein regulates antioxidant status and prevents hepatotoxicity in a rat model of D-galactosamine/lipopolysaccharide-induced acute liver failure. Mol. Med. Rep. 2016, 13, 3110–3114. [Google Scholar] [CrossRef]
- He, D.; Wu, Q.; Lu, C.; Wu, J.; Chen, P.; Wu, M.; Choi, J.i.; Tong, H. Pyropia yezoensis porphyran alleviates metabolic disorders via modulating gut microbiota in high-sucrose-fed Drosophila melanogaster. J. Sci. Food Agric. 2022, 102, 4802–4812. [Google Scholar] [CrossRef]
- Hodgman, M.J.; Garrard, A.R. A review of acetaminophen poisoning. Crit. Care Clin. 2012, 28, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Lee, S.B.; Kim, D.K.; Lee, S.Y.; Kim, C.S. Pyropia yezoensis extract attenuates osteoarthritis progression in vitro and in vivo. Prev. Nutr. Food Sci. 2025, 30, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.-W.; Li, C.-Y.; Huang, Y.-P.; Liu, W.-F.; Zhang, Q.; Long, J.; Wu, W.-Q.; Huang, L.-H.; Zeng, G.-H.; Sun, X.-Y. Mechanism of Porphyra Yezoensis Polysaccharides in Inhibiting Hyperoxalate-Induced Renal Injury and Crystal Deposition. J. Agric. Food Chem. 2024, 72, 6372–6388. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, Y.; Ueno, H.; Minato, K.-I.; Mizuno, M. Polysaccharides from Pyropia yezoensis f. narawaensis ameliorate type I hypersensitivity through the secretion of interleukin 10. Food Sci. Technol. Res. 2020, 26, 847–854. [Google Scholar]
- Nakamura, S.; Minato, K.-i.; Mizuno, M. Interleukin 10 and hydrogen peroxide mediate anti-allergic activity of polysaccharide from Pyropia yezoensis f. narawaensis. Int. J. Biol. Macromol. 2025, 308, 142547. [Google Scholar] [CrossRef]
- Huang, L.H.; Liu, H.; Chen, J.Y.; Sun, X.Y.; Yao, Z.H.; Han, J.; Ouyang, J.M. Seaweed Porphyra yezoensis polysaccharides with different molecular weights inhibit hydroxyapatite damage and osteoblast differentiation of A7R5 cells. Food Funct. 2020, 11, 3393–3409. [Google Scholar] [CrossRef]
- Geng, L.; Zhang, Q.; Suo, Q.; Wang, J.; Wang, Y.; Wang, C.; Wu, N. Inhibitory activity of a sulfated oligo-porphyran from Pyropia yezoensis against SARS-CoV-2. Carbohydr. Polym. 2023, 299, 120173. [Google Scholar] [CrossRef]
- Dispenza, M.C. Classification of hypersensitivity reactions. Allergy Asthma Proc. 2019, 40, 470–473. [Google Scholar] [CrossRef]
| Compound Name | Composition (%) | Jianghaida No. 1 (%) * | Sutong No. 1 (%) * |
|---|---|---|---|
| Octanoic acid (C8:0) | 0.01 | -- | -- |
| Lauric acid (C12:0) | -- | 0.03 | 0.06 |
| Myristic acid (C14:0) | 1.26 | 0.20 | 0.25 |
| Pentadecanoic acid (C15:0) | 1.44 | 0.22 | 0.24 |
| Palmitic acid (C16:0) | 21.07 | 41.74 | 43.01 |
| Palmitoleic acid (C16:1) | 1.63 | 0.32 | 0.32 |
| Heptadecanoic acid (C17:0) | -- | 0.08 | 0.10 |
| Stearic acid (C18:0) | 0.48 | 1.11 | 1.14 |
| Oleic acid (C18:1(n-9), cis) | 1.10 | 2.44 | 2.46 |
| Alpha-linoleic acid (C18:2(n-6), cis) | 4.99 | 1.88 | 1.86 |
| Linolenic acid (C18:3(n-3)) | 0.34 | 0.28 | 0.3 |
| Gamma-linolenic acid (C18:3(n-6)) | 0.61 | 0.22 | 0.2 |
| Arachidic acid (C20:0) | -- | 0.06 | 0.06 |
| Cis-11-eicosenoic acid (C20:1) | 0.33 | 5.77 | 5.73 |
| Cis-11,14-eicosadienoic acid (C20:2) | 0.40 | 1.35 | 1.30 |
| Cis-11,14,17-eicosatrienoic acid (C20:3 (n-3)) | 0.03 | 0.47 | 0.15 |
| Cis-8,11,14-eicosatrienoic acid (C20:3 (n-6)) | 2.75 | 2.14 | 1.99 |
| Cis-5,8,11,14-eicosatetraenoic acid (C20:4 (n-6)) | 0.67 | 1.85 | 1.75 |
| Cis-5,8,11,14,17-eicosapentaenoic acid (C20:5 (n-3)) | 54.12 | 36.52 | 36.00 |
| Heneicosanoic acid (C21:0) | -- | 0.02 | 0.03 |
| Behenic acid (C22:0) | -- | 0.05 | 0.04 |
| Erucic acid (C22:1(n-9)) | 0.32 | 1.01 | 0.93 |
| Cis-13,16-docosadienoic acid (C22:2) | 1.70 | -- | -- |
| Docosahexaenoic acid (C22:6n-3) | -- | 0.08 | 0.13 |
| Tricosanoic acid (C23:0) | 6.43 | 1.85 | 1.75 |
| Lignoceric acid (C24:0) | 0.35 | -- | -- |
| Nervonic Acid (C24:1) | -- | 0.33 | 0.21 |
| Test Material | Experimental Model | Outcomes/Mechanisms | Ref. |
|---|---|---|---|
| Polyphenols and protein-rich extracts (PP) | Vero cells + PP-rich extract (12.5, 25, and 50 μg/mL) 1 h + post AAPH (10 mM) treatment, 24 h | ↓ ROS levels, Dose-dependent reduction in apoptotic bodies | [25] |
| Zebrafish embryos PP-rich extract Sinan (12.5, 25, and 50 μg/mL) 1 h + post AAPH (15 mM, 25 μL) treatment, 24 h | ↓ ROS levels, ↓ lipid peroxidation | ||
| 13 synthetic peptides (PBP 1–13) | HepG2 cells + PBP 1–13 (1 µg/mL), + H2O2 (5 mM), 1 h | ↓ ROS levels, ↑ p-Nrf2, ↑ SOD, ↑ cells’ survival, ↓ apoptosis | [31] |
| SGID protein hydrolysate | ORAC | SGID = 432.763 mM TE/mg, Unhydrolyzed protein (UP) = 106.03 mM TE/mg | [32] |
| ABTS | SGID= 64.42 mM TE/mg UP =12.44 mM TE/mg | ||
| DPPH | SGID = 58.99% UP = 10.86% | ||
| Superoxide radical | SGID = 47.65%, UP= 7.8% | ||
| Hydroxyl radical | SCID=61.63%, UP = 8.3% | ||
| NO assay: HepG2 + (5, 25, 50, and 100 µg/mL), APAP (15 mM) 37 °C, 18 h | ↓ NO concentration-dependent | ||
| N. yezoensis fermented using B. amyloliquefaciens MMB-02, L. plantarum L13, S. cerevisiae A8 | DPPH | F = 54.87%, control (C) = 14.68% | [33] |
| ABTS | F = 57.39%, C = 21.82% | ||
| FRAP value | F (Fe3+ equivalents) = 1.43, C = 0.61 | ||
| SOD, CAT, and GSH levels | SOD (U/mL): F= 17.08, C = 4.28, CAT (U/mL): F = 0.072, C = 0.046, GSH (μmol/L) F = 28.15, C = 14.69 | ||
| Resistant strain (G1), commercial strain (CP) | 293T cells + CP or G1 (250, 500, and 1000 µg/mL), 3 h + H2O2 (600 µM, 600 µL), 1 h DCFH-DA, apoptosis (H2O2, 600 µM) | ↓ ROS levels, Pro-apoptotic genes: ↓ P53, ↓ Bax, ↓ caspase-3, ↑ Bcl-2, G1 more effective than CP | [34] |
| Recombinant PPI protein | HepG2 cells + PPI protein (0.001, 0.01, 0.1 and 1 µg/mL) + H2O2 (1 mM, 1 h) | ↓ ROS, ↑ CAT, ↑ GPx, ↑ SOD, ↑ TRR activities and expressions | [35] |
| N. yezoensis polysaccharides (PYPS) | DPPH (2, 4, 6, 8, and 10 mg/mL) | Dose-dependent effect 28.60 % to 49.40 % | [36] |
| ABTS (2, 4, 6, 8, and 10 mg/mL) | 76.19% (10 mg/mL) | ||
| Hydroxyl (2, 4, 6, 8, and 10 mg/mL) | 50.05 % (2 mg/mL) and 59.52 % (4 mg/mL) |
| Test Material | Experimental Model | Outcomes/Mechanisms | Ref. |
|---|---|---|---|
| Porphyran | RAW264.7 cells + porphyran (250 and 500 µg/mL), 1 h + LPS (2 ng/mL) | No cytotoxicity, ↓ NO, ↓ iNOS, ↓ NF-κB | [38] |
| Porphyran (D1, D2, D3, D4) | RAW264.7 cells + porphyrans (250, 500, and 1000 µg/mL), 1 h + LPS (20 ng/mL) 24 h | No cytotoxicity, D2 porphyran = ↓ NO, ↓ iNOS, ↓ TNF-α ↓ Osteoclastogenesis | [39] |
| Porphyran | PBMCs + porphyran pre-treatment (10, 25, 50, and 100 µg/mL), 1 h + LPS (20 ng/mL), 24 h | ↓ IL-1ꞵ, ↓ IL-6, ↓ IL-12p70, ↓ TNF-α, ↓ IFN-ϒ | [40] |
| Differentiated mature MODCs, porphyran pre-treatment (10, 25, 50, and 100 µg/mL), 1 h + LPS (20 ng/mL), 24 h | ↓ IL-12p70, ↓ TNF-α, ↓ IL-6, ↓ CD40, ↓ CD80, ↓ Cd86, ↓ CCR7 | ||
| PBDCs + porphyran pre-treatment (50 µg/mL), 1 h + LPS (20 ng/mL), 24 h | ↓ CD40, ↓ CD80, ↓ Cd86, ↓ MHCI, ↓ MHCII, suppressed proliferation/activation of CD4 T cells | ||
| Porphyran | Bone marrow-derived dendritic (BMDC) from C57BL/6 mice, porphyran pre-treatment (0, 10, 25, 50, and 100 μg/mL), 1 h + LPS (20 ng/mL) | Inhibited BMDCs activation ↓ CD40, ↓ CD80, ↓ Cd86, ↓ CCR7, ↓ IL-6, ↓ TNF-α, ↓ IL-12p70 (dose-dependent) | [41] |
| C57BL/6 mice + porphyran pre-treatment (i.p., 12.5, 25, 50, and 100 mg/kg), 1 h + LPS (i.p., 100 μg/kg) | ↓ spleen dendritic cells ↓ CD40, ↓ CD80, ↓ Cd86, ↓ CCR7 ↓ IL-6, ↓ TNF-α, ↓ IL-12p70 (dose-dependent) ↓ CD4T, ↓ CD8T, ↓ IFN-ϒ, ↓ T-bet |
| Test Material | Experimental Model | Outcomes/Mechanism | Ref. |
|---|---|---|---|
| Phycoerythrin-derived tryptic peptide (PYP) | Primary rat hippocampal neurons+ glutamate (50–200 μM) or PYP (0.25–2 μg/mL), 24 h | ↓ GRP78, ↓ ER stress, ↓ SA-β-gal, ↓ neurite dysregulation, ↓ JNK, Activation of TrkB-PI3K-ERK1/2 signaling | [43] |
| PYP | Rat prefrontal cortex + PFOS (25–400 µM) + PYP (0.25–2 µg/mL), 24 h | ↓ GRP78, ↓ JNK,↓ ER stress, Activated TrkB-PI3K-ERK1/2 signaling, ↓ calcium levels | [44] |
| Oligo-porphyran (OP) | PC12 cells + OP (50, 100, 200 μg/mL), 24 h + 6-OHDA (100 μM), 24 h + 30 min DCFH-DA assay | ↓ apoptosis, ↑ MMP, ↓ ROS levels, ↑ SOD, ↑ GSH, ↓ Bax/Bcl-2, ↓ cytochrome c, ↑ TH, ↑ DAT, ↓ TNF-α, ↓ IL-1β, ↓ IL-6 | [45] |
| Test Material | Experimental Model | Outcomes/Mechanisms | Ref. |
|---|---|---|---|
| Porphyran (PYP) and its derivatives | Hep3B, HeLa, and MDA-MB-231 cells + PYP, PYP-20, PYP-50 (200 μg/mL), 48 h | ↓ HeLa viability- PYP: 75%, PYP-50: 50%, PYP-20: 50%. ↓ Cyclin B1, ↓ CDK1, ↑ p53, ↑ p21. Blocking of G2/M phase of HeLa cell cycle | [47] |
| ↓ MDA-MB-231 viability- PYP-50: 42%, PYP-20: 42% | |||
| Hep3B viability- PYP: 80%, PYP-50: 25%, PYP-20: 40% | |||
| N. yezoensis Sookwawon 104 polysaccharides (PYSP) and derivatives | HeLa, Hep3B and MDA-MB-231 cells + PYSP, PYSP-20 or PYSP-100 (200, 500 µg/mL), 48 h | Antiproliferative activity. ↓ Cyclin B1, ↓ Cdk1, ↑ P53, ↑ P21 | [48] |
| Sulfate polysaccharide, Galactan (GPY) | DU145 and PC-3 cells + GPYcrude, GPY300, or GPY10, 50 µL of 10 µmol/L DCFH-DA, 24 h | ↓ DU145 cell viability: GPYcrude: 64%, GPY10: 80%, GPY300: 68%. ↑ ROS in DU145-GPY10: ↓ SOD2, ↑ apoptosis in DU145- GPY10: ↑ Bax, ↑ caspase 9, ↑ caspase 8, ↑ caspase 3, ↓ PI3K, ↓ Akt, ↓ mTOR | [49] |
| ↓ PC-3 cell viability: GPY10: 73%. DU145 was more sensitive to GPY10 |
| Test Material | Experimental Model | Outcomes/Mechanisms | Ref. |
|---|---|---|---|
| Mycosporine-like amino acid (Porphyra-334) | Human skin fibroblasts (CCD-986sk) + UVA light irradiation (10 J/cm2) + porphyra-334 (10, 20, and 40 μM), 24 h | ↓ intracellular ROS, ↓ SA-β-gal, ↓ MMP-1, ↑ procollagen secretion, ↑ COL1A1, ↑ elastin | [54] |
| Peptide (PYP1–5) | Human dermal fibroblast cells (Hs27) + PYP1–5 (250, 500 and 1000 ng/mL), 24 h | ↓ MMP-1, ↑ TIMP-1, ↑ TIMP-2, ↑ elastin, ↑ COL1A1, ↑ COL1A2, ↑ TGF-β1, ↑ p-Smad3, ↑ p-Smad2, ↓ Smad7 (inhibitor), ↑ Sp1 | [55] |
| N. yezoensis extract | Mouse melanocytes (Melan-A), human dermal keratinocytes (HaCaT cells), human dermal fibroblasts (1064 SK) + extract (100, 200, 400, and 800 μg/mL) | ↓ melanin content: 400 μg/mL (44.1%), 800 μg/mL (53.8%), ↓ tyrosinase activity at 800 μg/mL (35.5%). ↓ melanogenic enzymes: MITF, TRP-1, TRP-2, ↑ type I procollagen, ↓ MMP-2, ↓ MMP-9. | [56] |
| Clinical Application | |||
| Lotion with N. yezoensis extract (0.1% w/w) | 23 subjects, 4 weeks and 8 weeks | ↑ Skin brightness, 8 weeks, extract = 1.32% control 0.46% (p < 0.05). ↓ melanin content = 2.4% (4 weeks) and 3.0% (8 weeks), control no change (p < 0.05). ↑ skin-lightening effect = (p < 0.05). | |
| Test Material | Experimental Model | Outcomes/Mechanisms | Ref. |
|---|---|---|---|
| N. yezoensis peptide (PY-PE) | IEC-6 cells + PY-PE (1000, 500, 250, and 125 pg/mL), 24 h | ↑ IGF-IR, ↑ IRS-1, ↑ Shc, ↑ py99, ↓ JNK, ↓ p38, ↑ ERK1/2, ↑ p85, ↑ p110, ↑ PDK1, ↑ c-Jun, ↑ p-Akt, ↑ c-fos c | [61] |
| N. yezoensis peptide [PYP1 (1–20)] | IEC-6 cells + PYP1 (1–20) (1000, 500, 250, and 125 pg/mL) | ↑ p-EGFR, ↑ Shc, ↑ Grb2, ↑ Sos, ↑ Ras, ↑ Raf, ↑ MEK, ↑ p-ERK, ↑ cell cycle progression, ↑ cyclin D1 and E, ↓ p21, ↑ Cdk4, ↑ Cdk2, ↑ Cdk6, ↑ pRb, ↓ p27 | [62] |
| Recombinant cyclophilin (pyCyp) | IEC-6 cells + pyCyp (50, 25, and 5 pg/mL), 48 h | ↑ p-EGFR, ↑ Sos1,↑ Grb2,↑ Ras, ↑ p-Raf1, ↑ p-MEK, ↑ p-ERK, ↑ cyclin A, ↑ cyclin E, ↑ Cdk2, ↑ Cdc25a, ↑ pRb, ↑ p-pRb, ↓ p27, ↓ p21 | [63] |
| Test Material | Experimental Model | Outcomes/Mechanisms | Ref. |
|---|---|---|---|
| Porphyran (PPYP) | HepG2 cells+ palmitic acid (25, 50, 100, 200 μM) + PPYP (200 μM), 48 h | ↓ TG, ↓ SREBP, ↓ ACC, ↓ FAS, ↑ CPT1, ↑ PPARα | [71] |
| D. melanogaster larvae + high sucrose (1 M sucrose) + PPYP (25 mg/mL) | ↓ TG, ↓ SREBP, ↓ FAS, ↑ Acox57D-d, ↑ FABP | ||
| Porphyran | Male ICR mice+ regular diet group, HFD group, treatment groups: HFD, + porphyran (50, 100, and 200 mg/kg daily), Zhibituo group: HFD + Zhibituo (42 mg/kg daily), 4 weeks | ↓ BW gain, Serum: ↑ HFD, ↓ TG, ↓ TC, ↓ LDL-C Fecal: ↑ TC, ↑ TG, effective dose 200 mg/kg. Liver: ↓ liver weight reduction, ↓ TC, ↓ TG, ↓ ALP, ↓ AST, ↓ ALT | [72] |
| N. yezoensis protein (PYP) | SD rats + PYP (100 mg/kg), 2 weeks + AAP (700 mg/kg BW) intraperitoneal injection, 24 h | ↓ GOT, ↓ GPT, ↑ GSH, ↓ caspase-3 activity and DNA fragmentation in liver tissue | [73] |
| Synthetic peptide PYP1 (1–20) | Chang liver cell line (CCL-13) + PYP1 (1–20) (250 or 500 ng/mL) + acetaminophen (15 mM), 24 h | No cytotoxicity, Recovered viability of acetaminophen-triggered cells | [74] |
| Recombinant peptides (PYP1-AC, PYP1, PYP1-B), and synthetic peptide (SP) | Chang liver cell line (HPV-18) + PYP1-AC, PYP1, PYP1-B, and SP (125, 250, 500, and 1000 pg/mL) + acetaminophen (15 mM), 24 h | No cytotoxicity, ↑ cell viability | [75] |
| Peptide PYP1–4 | HepG2 cells + acetaminophen (15 mM) + PYP1–4 (125, 250 and 500 ng/mL) | ↓ NO, ↓ ROS, ↑ HO1, ↑ CAT, ↑ SOD2, ↑ NQO1, ↓ p-JNK ↓ p-p38. ↑ nuclear translocation of Nrf2, ↑ p-GSK3β, ↑ p-Akt, ↑ p-AMPK. ↓ apoptosis, ↓ Bad, ↑ Bcl-2, ↑ Bid, ↑ caspase-3, ↑ caspase-9, ↑ IGF-IR, ↑ EGFR, ↑ IRS-1, ↑ PI3Kp85, ↑ PTEN, ↑ p70S6K, ↑ eIF4E, ↑ GRB2, ↑ p-Akt/Akt, ↑ SHC, ↑ SOS, ↑ p-mTOR/mTOR, ↑ p-MEK/MEK, ↑ p-ERK/ERK | [76] |
| Susabinori lipid | C57BL/6J mice (normal group), db/db mice, Control diet group, Susabinori lipids diet, 2%, 4 weeks | ↓ Liver TG, ↑ adiponectin, ↓ FAS, ↓ malic enzyme, ↓ MCP1, ↑ PPARδ, ↓ ACC1, ↓ SCD1, ↓ SREBP1c | [77] |
| Lipid extraction from Susabinori powder using chloroform: methanol (V/V = 2:1) | C57BL/6J mice (normal group), male BKS.Cg- +Leprdb/+Leprdb/Jcl (db/db) mice control diet group and Susabinori lipids diet, 2%, 4 weeks | Hepatic fatty acid content: ↓ LA, ↓ DGLA, ↓ GLA, ↓ AA, ↑ EPA. 15 genes upregulated. AA and LA metabolism-related genes. ↓ Magl, ↓Fabp4 | [78] |
| Polyunsaturated fatty acids -rich extract (PYLP) (using 70% ethanol) | Male BALB/c mice, normal group, alcohol group (alcohol 3 g/kg + saline, 100 μL each), PYLP + alcohol group (PYLP 25 mg/kg + alcohol 3 g/kg mice), silymarin + alcohol group (silymarin 50 mg/kg + alcohol 3 g/kg) | ↓ hepatic damage and degeneration. In serum: ↓ GOT, ↓ GPT, ↓ total cholesterol, ↓ MDH In liver: ↑ SOD, ↑ GPx, ↑ CAT, ↓ TBARS Anti-apoptosis: ↓ p53, ↓ Bax, ↑ Bcl-xL | [27] |
| N. yezoensis glycoprotein (PYGP) | SD rats, CON group; ethanol group, ethanol (20%) 3.7 g/kg/BW; ethanol+ PYGP groups, ethanol (20%) + PYGP (150 and 300 mg/kg/BW), 30 days | ↓ GOT, ↓ GPT, ↑ GSH, ↑ GSH-px, ↑ CAT, ↓ CYP2E1, ↓ iNOS, ↓ p-p38, ↓ COX-2, ↓ p-JNK, ↓ p-ERK | [79] |
| PYGP | SD rats, control group; D-GalN (500 mg/kg/BW) + LPS (10 µg/kg/BW) group. D-GalN + LPS + PYGP (150 and 300 mg/kg/BW) groups. PYGP once a day, 7 days | ↓ GOT, ↓ GPT, ↓ TBARS, ↑ GSH, ↑ GST, ↑ CAT, ↓ p-JNK, ↓ p-ERK, ↓ p-p38, ↓ COX-2, ↓ iNOS | [80] |
| Porphyran (PYP) | D. melanogaster w1118 embryos + Control group, HS group: Sucrose (350 g/kg), PYP group: Sucrose (350 g/kg) + PYP (15, 25, and 50 g/kg) | ↓ TG, ↓ circulating sugars (25 or 50 g/kg), ↑ Dilp2, ↑ Dilp3, ↑ Dilp5, ↓ Upd3. Modulated gut microbiota | [81] |
| Test Material | Experimental Model | Outcomes/Mechanisms | Ref. |
|---|---|---|---|
| Anti-osteoarthritic | |||
| Fermented ethanolic extract of N. yezoensis (FEPY) | Primary chondrocytes + pretreatment (30% FEPY: 0.25, 0.5, 1, and 2), 1 h + IL-1β (10 ng/mL), 2 or 24 h. | ↓ Nitrite, ↓ PGE2, ↓ iNOS and ↓ COX-2, ↓ MMP13, ↓ MMP3, ↓ MMP1, ↓ ADAMTS5 and ↓ ADAMTS4 | [83] |
| Sprague-Dawley rats ex vivo: the knees of postnatal rats (5-day-old), post 3 days + pretreatment FEPY (30%), 1 h + IL-1β (10 ng/mL), 48 h | Articular cartilage stain was restored to a darker color. ↑ collagen type II,↑ aggrecan, ↓ p-ERK, ↓ p-JNK, and ↓ p-P38, ↑ NF-κB p65,↓ p-IκB-α | ||
| Group 1 (normal), group 2 (sham), group 3 (DMM, + normal saline), and groups 4 to 6 (DMM + FEPY 30% (50, 100, and 200 mg/kg BW), 8 weeks | Proteoglycans content OARSI score = DMM surgery group (12); FEPY 50 (7); FEPY 100 (6); FEPY 200 (5), ↑ collagen type II | ||
| Kidney stone treatment | |||
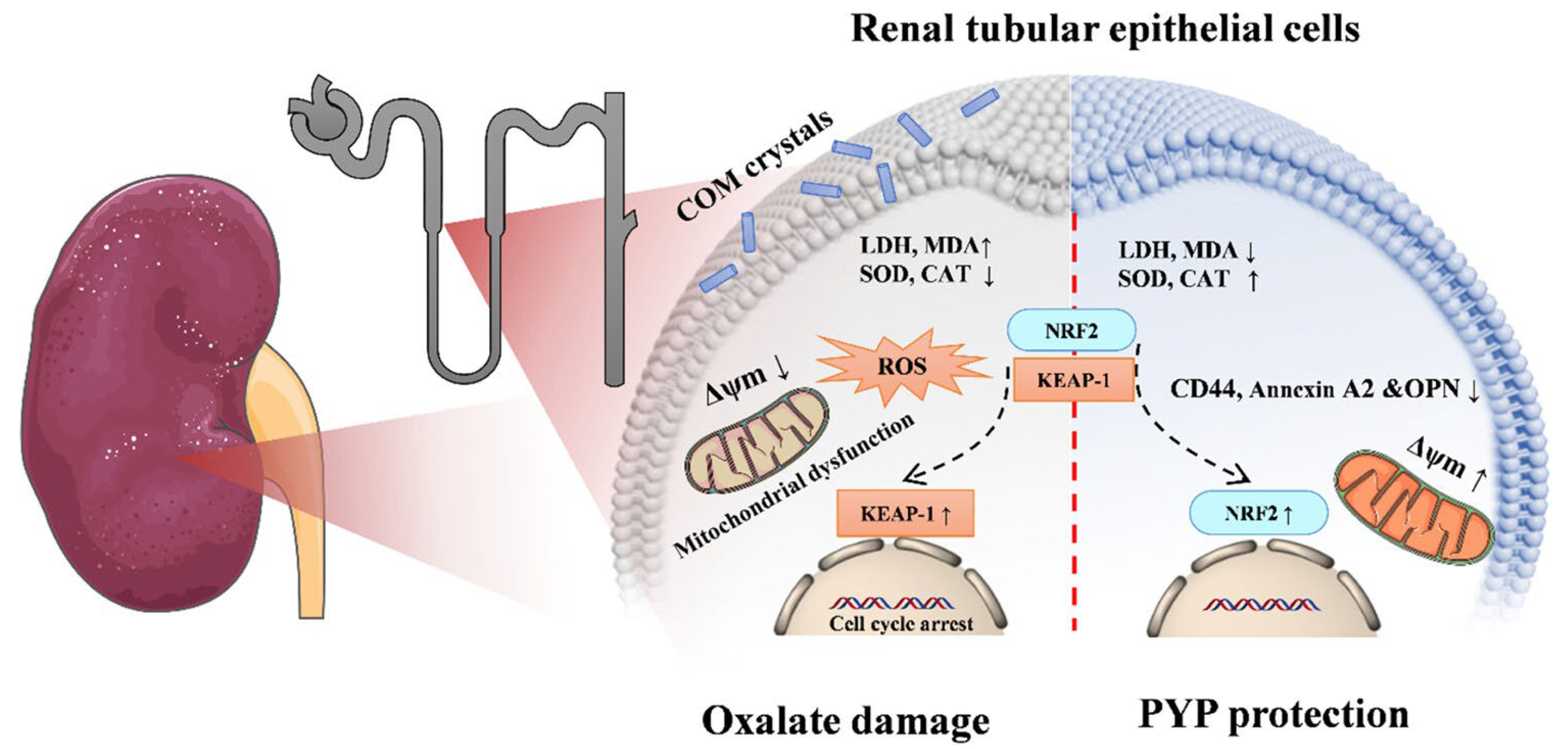
| Polysaccharide from N. yezoensis (PYP) | Viability assay: HK-2, NRK-52E, NK-49F, MDCK + PYP (10, 20, 30, 40, 60, 80, 120, 160, 240 μg/mL), 24 h HK-2 cells + oxalate solution (1.0 mM) + PYP (10, 30, and 60 μg/mL), 24 h | PYP > 120 μg/mL was safe ↑ HK-2 cells viability, ↓ ROS, ↓ MDH, ↑ SOD, ↑ CAT, ↑ Nrf2, ↓ keap1, ↓ lactate dehydrogenase release, restored mitochondrial membrane depolarization, protected membrane integrity, ↓ CD44, ↓ OPN, ↓ Annexin A2 | [84] |
| In vivo distribution assay: C57BL/6 mice Normal group: saline 200 μL daily, 1 week (I.P.) PYP-ICG (I.P.) 200 mg/kg/BW Glyoxylic acid stone modeling group: GA 70 mg/kg/day daily, 1 week (I.P.), PYP-ICG (I.P.) 200 mg/kg/BW. Fluorescence expression was monitored at 2, 4, 8, 24, and 48 h using a small animal imaging system. | fluorescent signal = abdomen mainly disappeared after 48 h | ||
| Therapeutic evaluation: SD rats Normal group: standard diet Stone group: standard diet + ethylene glycol (1%), 28 days PYP group: ethylene glycol + 50, 200, and 400 mg/kg wt, 28 days, | Prevented CaOx crystal formation, deposition and adhesion risk: ↓ CD44 and ↓ OPN Inhibited renal injury ↓ Kim-1 (renal injury factor), ↑ SOD, ↑ CAT, ↑ Nrf2, ↓ keap1 ↓ IL-6, ↓ MCP-1 | ||
| Anti-allergic | |||
| Polysaccharide from N. yezoensis f. narawaensis (PPY) | Female BALB/c mice, PPY (1 mg/mL), oral dose, 4 days + anti-TNP IgE antibody (IV injection 30 min. 2,4,6-trinitrochlorobenzene 1.6 % (w/v) in acetone/olive oil (1:1) + anti-IL-10 (100 µg/mL) antibody or irrelevant IgG (100 µg/mL), every 2 days | PCA reaction: inhibited ear edema = 39% Dose-dependent inhibition by PPY ↑ IL-10 | [85] |
| RBL-2H3 cells or Co-culture system (Caco-2 and RBL-2H3 cells): + PPY (1 or 10 mg/mL) 2 h + anti-DNP IgE (1 μg/mL),1 h + DNP-BSA (100 ng/mL), 1 h | No release of β-hexosaminidase | ||
| Polysaccharide from N. yezoensis f. narawaensis (PPY) | PCA reaction: BALB/c mice + PPY (500, 750, 1000 μg, oral dose), 4 days + anti-TNP IgE antibody (2 μg/100 μL, IV), 30 min + 1.6 % (w/v) picryl chloride in acetone/olive oil (1:1) after 2 h. Active cutaneous anaphylaxis (ACA): PPY (oral dose, 7 days) + adjuvant 300 μL OVA (10 μg) and Al (OH)3 (1 mg), I.P., 4 times, 5 days + OVA (5 μg) I.V. | Inhibited ear edema dose-dependent ↑ IL-10 ↓ rectal temperature | [86] |
| RBL-2H3 + IL-10 (10 ng/mL) or H2O2 (0.01, 0.1, 1, 10 μM), 2 h + anti-TNP IgE (200 ng/mL), 2 h + TNP-BSA, 30 min HT-29/RBL-2H3 co-culture system + PPY (10 mg/mL), 2 h on apical side of HT-29 + IL-10 (10 mg/mL) | ↓ β-hexosaminidase release together by IL-10 and H2O2 treatment PPY increased H2O2 production from HT-29 cells, pretreatment with an NADPH oxidase inhibitor suppressed H2O2 production, inhibited mast cell degranulation | ||
| Protection against vascular calcification | |||
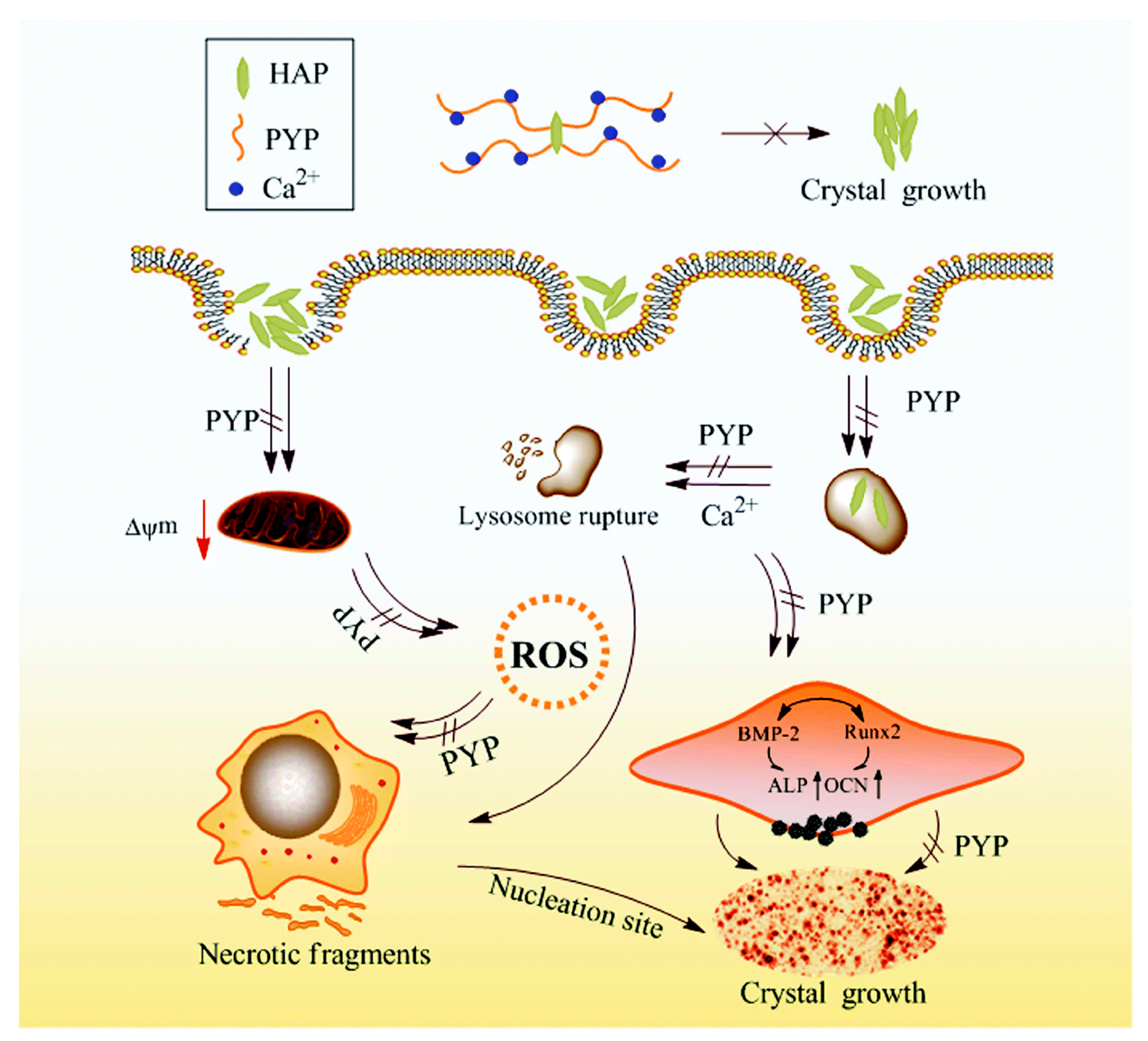
| Polysaccharides from N. yezoensis (PYP1-PYP4) | A7R5 cells + HAP (200 μg/mL) + PYP1-PYP4 (50, 100, 200, and 400 μg/mL), 24 h | Inhibited HAP damage, ↑ cell viability, Protecting effects 200 > 400 > 100 > 50 μg/mL. ↓ LDH release, ↓ ROS. Inhibited decline in mitochondrial membrane potential, Inhibited: intracellular calcium level, Ca deposition, ALP, HAP adhesion and endocytosis. ↓ cell necrosis, Inhibited osteogenic transformation: ↓ BMP-2, ↓ Runx2, ↓ OCN | [87] |
| C57BL/6 mice normal group; calcification model group; PYP4 treated groups: 50, 100, and 200 mg kg/day) + adenine 0.2% (w/w). | ↓ aortic calcium level, ↓ creatinine, ↓ phosphate, ↓ BUN | ||
| Antivirus | |||
| Sulfated oligo-porphyran OP145 | Pseudovirus packaged by lentivirus vector with the S-protein (25 μL) + OP145 (0.391, 1.563, 6.25, 25, 100 and 400 μg/mL), + ACE2 overexpressed target cells, Opti-HEK293/ACE2, 24 h | Competitively inhibited attachment of S-protein to ACE2 | [88] |
| Antibacterial | |||
| Recombinant cyclophilin (PyCyP) | E. coli (KCTC 2571), Staphylococcus aureus (KCTC 1621), Pseudomonas aeruginosa (KCTC 1750), and Bacillus subtilis (KCTC 1028) + PyCyP (100, 50, 25, 12.5, 6.25, and 3.125, μg/mL) | MIC (μg/mL): PyCyP = E. coli (6.25), S. aureus (6.25), B. subtilis (12.5), and P. aeruginosa (3.12), positive control Chloramphenicol (c) = (3.12) | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Yoon, N.Y.; Lee, H.-J. Therapeutic Potential of Neopyropia yezoensis: An Updated Review. Mar. Drugs 2025, 23, 415. https://doi.org/10.3390/md23110415
Sharma A, Yoon NY, Lee H-J. Therapeutic Potential of Neopyropia yezoensis: An Updated Review. Marine Drugs. 2025; 23(11):415. https://doi.org/10.3390/md23110415
Chicago/Turabian StyleSharma, Anshul, Na Young Yoon, and Hae-Jeung Lee. 2025. "Therapeutic Potential of Neopyropia yezoensis: An Updated Review" Marine Drugs 23, no. 11: 415. https://doi.org/10.3390/md23110415
APA StyleSharma, A., Yoon, N. Y., & Lee, H.-J. (2025). Therapeutic Potential of Neopyropia yezoensis: An Updated Review. Marine Drugs, 23(11), 415. https://doi.org/10.3390/md23110415







