Effect of Storage Time and Temperature on the Bioactivity of a Chitosan-Derived Epigenetic Modulation Scaffold
Abstract
1. Introduction
2. Results
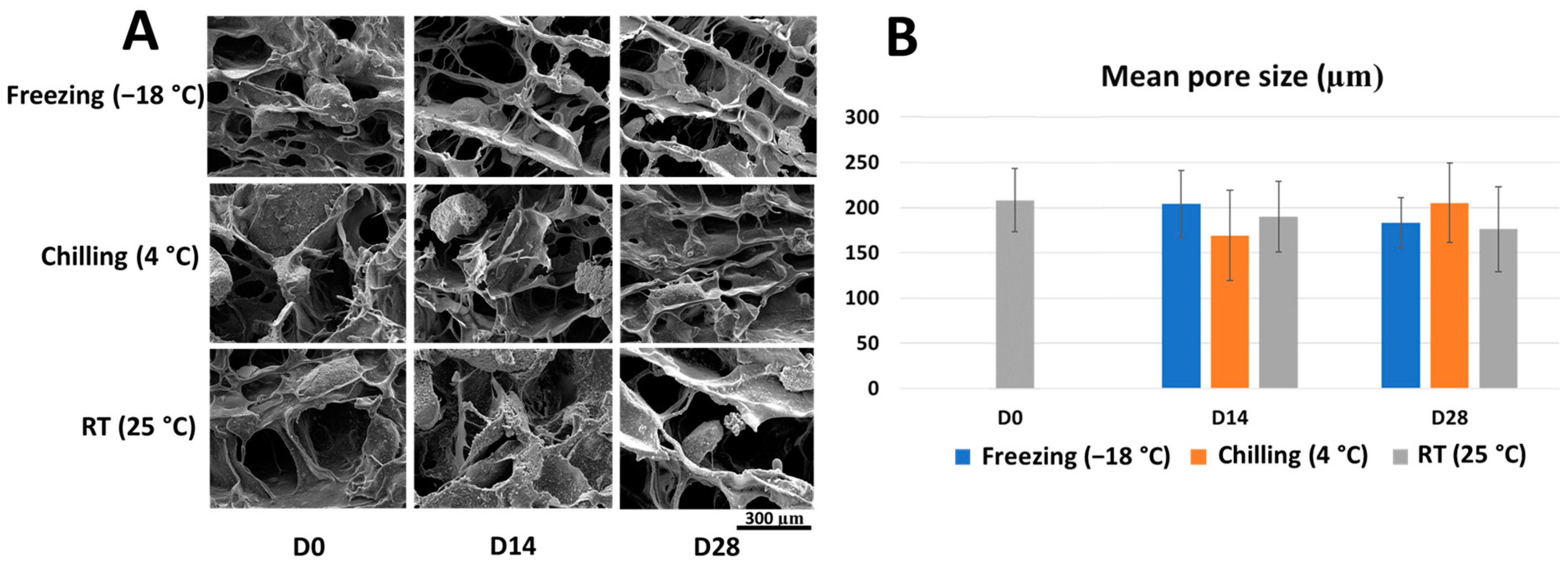
2.1. Microstructure and Porosity of the Stored Scaffold
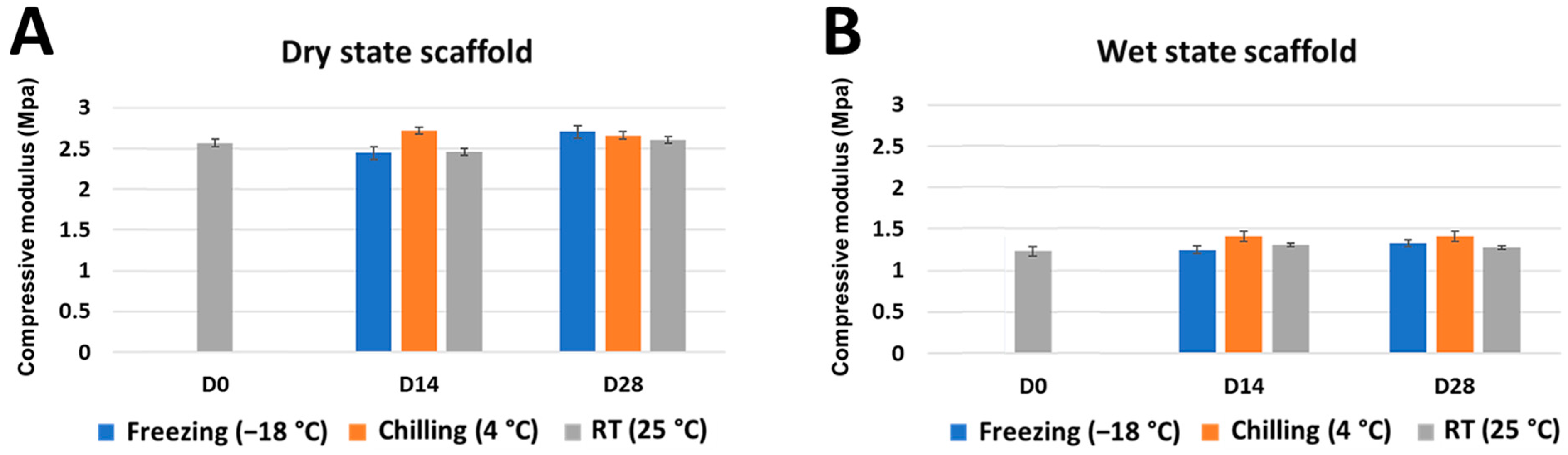
2.2. Mechanical Strength and Shape Memory Ability of the Stored Scaffold
2.3. Release and Bioactivity of TSA from the Stored Scaffold
3. Discussion
3.1. An Appropriate Storage Protocol Is an Important Key for Commercialized Clinical Applications of the CS/BCP/TSA Scaffold
3.2. Different Storage Times and Temperatures Did Not Affect the Scaffolds’ Mechanical Properties
3.3. Released TSA Lost Its Activity When Scaffolds Were Stored at RT or Chilling Temperature
4. Materials and Methods
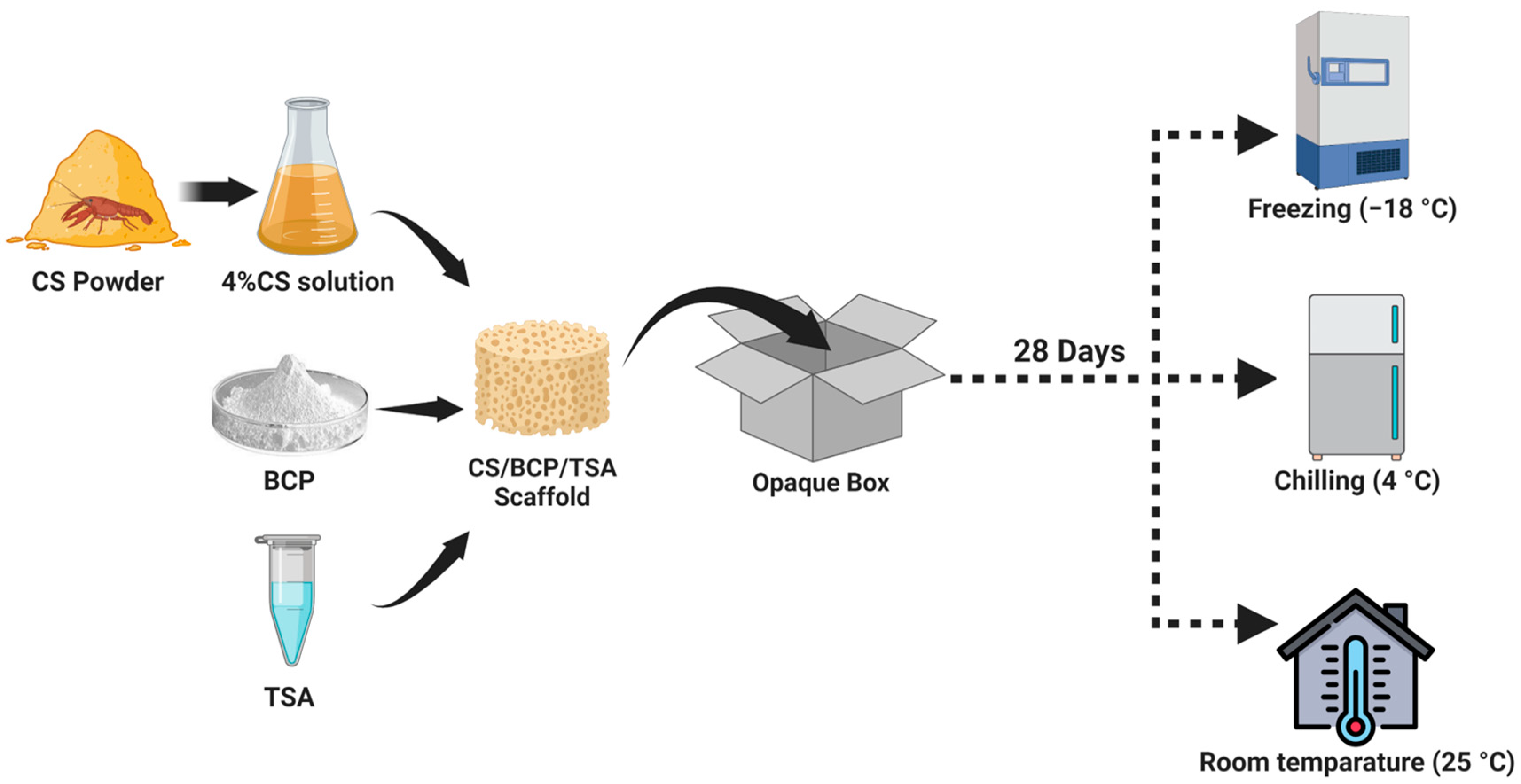
4.1. Fabrication of CS/BCP/TSA Scaffold
4.2. Storage Protocol
4.3. Surface Morphologies
4.4. Compressive Mechanical Properties
4.5. Shape Memory Testing
4.6. Culture of Primary Human Periodontal Ligament Cells
4.7. In Vitro Release and Bioactivity of TSA
4.8. Statistical Test Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, G.; Sultana, A.; Abdullah, K.M.; Pothuraju, R.; Nasser, M.W.; Batra, S.K.; Siddiqui, J.A. Epigenetic regulation of bone remodeling and bone metastasis. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar] [CrossRef]
- Jain, G.; Blaauw, D.; Chang, S. A Comparative Study of Two Bone Graft Substitutes—InterOss® Collagen and OCS-B Collagen®. J. Funct. Biomater. 2022, 13, 28. [Google Scholar] [CrossRef]
- Mahardawi, B.; Rochanavibhata, S.; Jiaranuchart, S.; Arunjaroensuk, S.; Mattheos, N.; Pimkhaokham, A. Autogenous tooth bone graft material prepared chairside and its clinical applications: A systematic review. Int. J. Oral Maxillofac. Surg. 2022, 52, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Fernández, R.F.; Bucchi, C.; Navarro, P.; Beltrán, V.; Borie, E. Bone grafts utilized in dentistry: An analysis of patients’ preferences. BMC Med. Ethic 2015, 16, 71. [Google Scholar] [CrossRef]
- Wickramasinghe, M.L.; Dias, G.J.; Premadasa, K.M.G.P. A novel classification of bone graft materials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 1724–1749. [Google Scholar] [CrossRef] [PubMed]
- Fillingham, Y.; Jacobs, J. Bone grafts and their substitutes. Bone Jt. J. 2016, 98-B, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.-S.; Oh, J.-K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef]
- Roden, R.D. Principles of Bone Grafting. Oral Maxillofac. Surg. Clin. North Am. 2010, 22, 295–300. [Google Scholar] [CrossRef]
- Iijima, K.; Otsuka, H. Cell Scaffolds for Bone Tissue Engineering. Bioengineering 2020, 7, 119. [Google Scholar] [CrossRef]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2016, 25, 854–866. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Pimkhaokham, A.; Ampornaramveth, R.S. Chitosan-Based Scaffold for Mineralized Tissues Regeneration. Mar. Drugs 2021, 19, 551. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; El Omari, N.; Bakha, M.; Aanniz, T.; El Menyiy, N.; El Hachlafi, N.; El Baaboua, A.; El-Shazly, M.; Alshahrani, M.M.; Al Awadh, A.A.; et al. Pharmacological Properties of Trichostatin A, Focusing on the Anticancer Potential: A Comprehensive Review. Pharmaceuticals 2022, 15, 1235. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.C.; Everts, V.; Pavasant, P.; Ampornaramveth, R.S. Inhibition of Histone Deacetylases Enhances the Osteogenic Differentiation of Human Periodontal Ligament Cells. J. Cell. Biochem. 2015, 117, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.C.-N.; Everts, V.; Ampornaramveth, R.S. Histone deacetylases and their roles in mineralized tissue regeneration. Bone Rep. 2017, 7, 33–40. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Chanamuangkon, T.; Nampuksa, K.; Monmaturapoj, N.; Sumrejkanchanakij, P.; Pimkhaokham, A.; Ampornaramveth, R.S. Novel Epigenetic Modulation Chitosan-Based Scaffold as a Promising Bone Regenerative Material. Cells 2022, 11, 3217. [Google Scholar] [CrossRef]
- Govoni, M.; Vivarelli, L.; Mazzotta, A.; Stagni, C.; Maso, A.; Dallari, D. Commercial Bone Grafts Claimed as an Alternative to Autografts: Current Trends for Clinical Applications in Orthopaedics. Materials 2021, 14, 3290. [Google Scholar] [CrossRef]
- Nover, A.B.; Stefani, R.M.; Lee, S.L.; Ateshian, G.A.; Stoker, A.M.; Cook, J.L.; Hung, C.T. Long-term storage and preservation of tissue engineered articular cartilage. J. Orthop. Res. 2015, 34, 141–148. [Google Scholar] [CrossRef]
- Leal-Marin, S.; Kern, T.; Hofmann, N.; Pogozhykh, O.; Framme, C.; Börgel, M.; Figueiredo, C.; Glasmacher, B.; Gryshkov, O. Human Amniotic Membrane: A review on tissue engineering, application, and storage. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 109, 1198–1215. [Google Scholar] [CrossRef]
- Jang, T.H.; Park, S.C.; Yang, J.H.; Kim, J.Y.; Seok, J.H.; Park, U.S.; Choi, C.W.; Lee, S.R.; Han, J. Cryopreservation and its clinical applications. Integr. Med. Res. 2017, 6, 12–18. [Google Scholar] [CrossRef] [PubMed]
- James, S.J.; James, C. Chapter 20—Chilling and Freezing. In Food Safety Management; Motarjemi, Y., Lelieveld, H., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 481–510. [Google Scholar]
- Bouin, A.-S.; Wierer, M. Quality standards of the European Pharmacopoeia. J. Ethnopharmacol. 2014, 158, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Poh, P.S.P.; Valainis, D.; Bhattacharya, K.; van Griensven, M.; Dondl, P. Optimization of Bone Scaffold Porosity Distributions. Sci. Rep. 2019, 9, 9170. [Google Scholar] [CrossRef]
- Cheng, A.; Schwartz, Z.; Kahn, A.; Li, X.; Shao, Z.; Sun, M.; Ao, Y.; Boyan, B.D.; Chen, H. Advances in Porous Scaffold Design for Bone and Cartilage Tissue Engineering and Regeneration. Tissue Eng. Part B Rev. 2019, 25, 14–29. [Google Scholar] [CrossRef]
- Maruyama, M.; Pan, C.-C.; Moeinzadeh, S.; Storaci, H.W.; Guzman, R.A.; Lui, E.; Ueno, M.; Utsunomiya, T.; Zhang, N.; Rhee, C.; et al. Effect of porosity of a functionally-graded scaffold for the treatment of corticosteroid-associated osteonecrosis of the femoral head in rabbits. J. Orthop. Transl. 2021, 28, 90–99. [Google Scholar] [CrossRef]
- Khan, M.A.; Razak, S.A.; Al Arjan, W.; Nazir, S.; Anand, T.S.; Mehboob, H.; Amin, R. Recent Advances in Biopolymeric Composite Materials for Tissue Engineering and Regenerative Medicines: A Review. Molecules 2021, 26, 619. [Google Scholar] [CrossRef]
- Huang, K.; Yang, M.-S.; Tang, Y.-J.; Ling, S.-Y.; Pan, F.; Liu, X.-D.; Chen, J. Porous shape memory scaffold of dextran and hydroxyapatite for minimum invasive implantation for bone tissue engineering applications. J. Biomater. Appl. 2020, 35, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; He, J.; Yong, X.; Lu, J.; Xiao, J.; Liao, Y.; Li, Q.; Xiong, C. Biodegradable poly (lactic acid-co-trimethylene carbonate)/chitosan microsphere scaffold with shape-memory effect for bone tissue engineering. Colloids Surf. B Biointerfaces 2020, 195, 111218. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Yang, J.-M.; Chang, Y.-H. Water-induced shape memory behavior of poly (vinyl alcohol) and p-coumaric acid-modified water-soluble chitosan blended membrane. Carbohydr. Polym. 2021, 257, 117633. [Google Scholar] [CrossRef]
- Gupta, A.; Kim, B.S. Shape Memory Polyurethane Biocomposites Based on Toughened Polycaprolactone Promoted by Nano-Chitosan. Nanomaterials 2019, 9, 225. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Suwattanachai, P.; Everts, V.; Pimkhaokham, A.; Ampornaramveth, R.S. In Vivo Bone Regeneration Induced by a Scaffold of Chitosan/Dicarboxylic Acid Seeded with Human Periodontal Ligament Cells. Int. J. Mol. Sci. 2019, 20, 4883. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Winnicka, K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef] [PubMed]
- Park, D.S.; Kim, H.K.; Park, K.S.; Yoon, T.R. Trichostatin A enhances osteogenic differentiation through activation of ERK pathways in mouse bone marrow multipotent stromal cells. Tissue Eng. Regen. Med. 2014, 11, 131–136. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, T.; Peng, L.; Sun, Q.; Wei, Y.; Han, B. Advancements in Hydrogel-Based Drug Sustained Release Systems for Bone Tissue Engineering. Front. Pharmacol. 2020, 11, 622. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, A.T.; Wei, Y.; Wan, J.; Zhu, J.; Peng, Y.; Kadir, S.Y.A.; Zainol, J.; Oglah, Z.; Cheng, L.; Shi, Z. Bone Tissue Engineering through 3D Bioprinting of Bioceramic Scaffolds: A Review and Update. Life 2022, 12, 903. [Google Scholar] [CrossRef]
- Bozorgi, A.; Khazaei, M.; Soleimani, M.; Jamalpoor, Z. Application of nanoparticles in bone tissue engineering; a review on the molecular mechanisms driving osteogenesis. Biomater. Sci. 2021, 9, 4541–4567. [Google Scholar] [CrossRef]
- Raza, A.; Sime, F.B.; Cabot, P.J.; Maqbool, F.; Roberts, J.A.; Falconer, J.R. Solid nanoparticles for oral antimicrobial drug delivery: A review. Drug Discov. Today 2019, 24, 858–866. [Google Scholar] [CrossRef]
- Suwattanachai, P.; Pimkhaokham, A.; Chirachanchai, S. Multi-functional carboxylic acids for chitosan scaffold. Int. J. Biol. Macromol. 2019, 134, 156–164. [Google Scholar] [CrossRef]



| Material or Commercial Name | Osteoinductive Molecule | Scaffold Carrier | Performance and Applications |
|---|---|---|---|
| CS/BCP/TSA scaffold | TSA | Chitosan/Biphasic Calcium Phosphate | Maxillo-facial bone augmentations Alveolar ridge preservation |
| INFUSE® | Recombinant human bone morphogenetic protein-2 (rhBMP-2) | Collagen sponge | Maxillo-facial bone augmentations Spinal fusion procedures |
| OsteoAMP® | Allogeneic morphogenetic protein (AMP) | Allograft | Spinal fusion procedures |
| Augment® | Recombinant human platelet-derived growth factor-BB (rhPDGF-BB) | β-Tricalcium phosphate | Foot or ankle fusion procedures |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukpaita, T.; Chirachanchai, S.; Pimkhaokham, A.; Ampornaramveth, R.S. Effect of Storage Time and Temperature on the Bioactivity of a Chitosan-Derived Epigenetic Modulation Scaffold. Mar. Drugs 2023, 21, 175. https://doi.org/10.3390/md21030175
Sukpaita T, Chirachanchai S, Pimkhaokham A, Ampornaramveth RS. Effect of Storage Time and Temperature on the Bioactivity of a Chitosan-Derived Epigenetic Modulation Scaffold. Marine Drugs. 2023; 21(3):175. https://doi.org/10.3390/md21030175
Chicago/Turabian StyleSukpaita, Teerawat, Suwabun Chirachanchai, Atiphan Pimkhaokham, and Ruchanee Salingcarnboriboon Ampornaramveth. 2023. "Effect of Storage Time and Temperature on the Bioactivity of a Chitosan-Derived Epigenetic Modulation Scaffold" Marine Drugs 21, no. 3: 175. https://doi.org/10.3390/md21030175
APA StyleSukpaita, T., Chirachanchai, S., Pimkhaokham, A., & Ampornaramveth, R. S. (2023). Effect of Storage Time and Temperature on the Bioactivity of a Chitosan-Derived Epigenetic Modulation Scaffold. Marine Drugs, 21(3), 175. https://doi.org/10.3390/md21030175






