Impact of Marine Chemical Ecology Research on the Discovery and Development of New Pharmaceuticals
Abstract
1. Introduction
2. Chemical Ecology-Driven Discovery of Marine Medicines
2.1. Marine Macrobiota–Microbial Interactions
2.1.1. Macroalgal Chemical Defenses against Microbial Attacks
2.1.2. Chemical Defenses Mediated by Marine Invertebrate-Associated Microbial Symbionts
2.1.3. Chemical-Mediated Defenses in Marine Holobionts
2.2. Activated/Induced Chemical Defenses
2.3. Allelochemicals in Competition for Space and Resources
2.4. Allelochemicals in Phycosphere of Phytoplanktons
2.5. Phylogeny-Based and Concerted Discovery Strategies
2.6. Spatial and Temporal Chemical Variation of Natural Products
3. Application of Innovative Techniques for Marine Chemical Ecology Research
4. Impacts of Climate Change and Human Activity on Marine Chemical Ecology
5. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hay, M.E. Marine chemical ecology: Chemical signals and cues structure marine populations, communities, and ecosystems. Ann. Rev. Mar. Sci. 2009, 1, 193–212. [Google Scholar] [CrossRef]
- Puglisi, M.P.; Sneed, J.M.; Ritson-Williams, R.; Young, R. Marine chemical ecology in benthic environments. Nat. Prod. Rep. 2019, 36, 410–429. [Google Scholar]
- Brown, E.R.; Cepeda, M.R.; Mascuch, S.J.; Poulson-Ellestad, K.L.; Kubanek, J. Chemical ecology of the marine plankton. Nat. Prod. Rep. 2019, 36, 1093–1116. [Google Scholar]
- Chen, Z.H.; Guo, Y.W.; Li, X.W. Recent advances on marine mollusk-derived natural products: Chemistry, chemical ecology and therapeutical potential. Nat. Prod. Rep. 2022. [Google Scholar] [CrossRef]
- Meinwald, J.; Leal, W.S.; Kubanek, J. Molecules as biotic messengers. ACS Omega 2018, 3, 4048–4053. [Google Scholar]
- Wichard, T.; Beemelmanns, C. Role of chemical mediators in aquatic interactions across the prokaryote-eukaryote boundary. J. Chem. Ecol. 2018, 44, 1008–1021. [Google Scholar]
- Brunetti, A.E.; Carnevale Neto, F.; Vera, M.C.; Taboada, C.; Pavarini, D.P.; Bauermeister, A.; Lopes, N.P. An integrative omics perspective for the analysis of chemical signals in ecological interactions. Chem. Soc. Rev. 2018, 47, 1574–1591. [Google Scholar]
- Caporale, L.H. Chemical ecology: A view from the pharmaceutical industry. Proc. Natl. Acad. Sci. USA 1995, 92, 75–82. [Google Scholar] [CrossRef]
- Singh, A.; Thakur, N.L. Significance of investigating allelopathic interactions of marine organisms in the discovery and development of cytotoxic compounds. Chem. Biol. Interact. 2016, 243, 135–147. [Google Scholar]
- de Nys, R.; Steinberg, P.D. Linking marine biology and biotechnology. Curr. Opin. Biotechnol. 2002, 13, 244–248. [Google Scholar]
- Folmer, F.; Jaspars, M.; Dicato, M.; Diederich, M. Marine natural products as targeted modulators of the transcription factor NF-kappaB. Biochem. Pharmacol. 2008, 75, 603–617. [Google Scholar]
- Park, M.H.; Hong, J.T. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells 2016, 5, 15. [Google Scholar] [CrossRef]
- Oliver, E.; Nandakumar, N.; Faibish, H.; Gopas, J.; Kushmaro, A. Coral-associated bacterial extracts inhibit cellular NF-κB pathway. Cogent Environ. Sci. 2017, 3, 1292865. [Google Scholar]
- Samples, R.M.; Balunas, M.J. Bridging the gap: Plant-endophyte interactions as a roadmap to understanding small-molecule communication in marine microbiomes. Chembiochem 2020, 21, 2708–2721. [Google Scholar]
- Saha, M.; Berdalet, E.; Carotenuto, Y.; Fink, P.; Harder, T.; John, U.; Not, F.; Pohnert, G.; Potin, P.; Selander, E.; et al. Using chemical language to shape future marine health. Front. Ecol. Environ. 2019, 17, 530–537. [Google Scholar]
- Kamio, M.; Yambe, H.; Fusetani, N. Chemical cues for intraspecific chemical communication and interspecific interactions in aquatic environments: Applications for fisheries and aquaculture. Fish. Sci. 2022, 88, 203–239. [Google Scholar]
- Dixson, D.L.; Hay, M.E. Corals chemically cue mutualistic fishes to remove competing seaweeds. Science 2012, 338, 804–807. [Google Scholar]
- Hastie, L.C.; Wallace, C.; Birkett, M.A.; Douglas, A.; Jones, O.; Mordue (Luntz), A.J.; Ritchie, G.; Pickett, J.A.; Webster, J.L.; Bowman, A.S. Prevalence and infection intensity of sea lice (Lepeophtheirus salmonis) on Atlantic salmon (Salmo salar) host is reduced by the non-host compound 2-aminoacetophenone. Aquaculture 2013, 410, 179–183. [Google Scholar]
- Sánchez-Lozano, I.; Hernández-Guerrero, C.J.; Muñoz-Ochoa, M.; Hellio, C. Biomimetic approaches for the development of new antifouling solutions: Study of incorporation of macroalgae and sponge extracts for the development of new environmentally-friendly coatings. Int. J. Mol. Sci. 2019, 20, 4863. [Google Scholar]
- Jorissen, H.; Galand, P.E.; Bonnard, I.; Meiling, S.; Raviglione, D.; Meistertzheim, A.L.; Hédouin, L.; Banaigs, B.; Payri, C.E.; Nugues, M.M. Coral larval settlement preferences linked to crustose coralline algae with distinct chemical and microbial signatures. Sci. Rep. 2021, 11, 14610. [Google Scholar]
- Harder, T.; Campbell, A.H.; Egan, S.; Steinberg, P.D. Chemical mediation of ternary interactions between marine holobionts and their environment as exemplified by the red alga Delisea pulchra. J. Chem. Ecol. 2012, 38, 442–450. [Google Scholar]
- Dworjanyn, S.; De Nys, R.; Steinberg, P. Localisation and surface quantification of secondary metabolites in the red alga Delisea pulchra. Mar. Biol. 1999, 133, 727–736. [Google Scholar] [CrossRef]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar]
- Manefield, M.; de Nys, R.; Naresh, K.; Roger, R.; Givskov, M.; Steinberg, P.; Kjelleberg, S. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology 1999, 145, 283–291. [Google Scholar]
- Hentzer, M.; Riedel, K.; Rasmussen, T.B.; Heydorn, A.; Andersen, J.B.; Parsek, M.R.; Rice, S.A.; Eberl, L.; Molin, S.; Høiby, N.; et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 2002, 148, 87–102. [Google Scholar]
- Muñoz-Cázares, N.; Castillo-Juárez, I.; García-Contreras, R.; Castro-Torres, V.A.; Díaz-Guerrero, M.; Rodríguez-Zavala, J.S.; Quezada, H.; González-Pedrajo, B.; Martínez-Vázquez, M. A brominated furanone inhibits Pseudomonas aeruginosa quorum sensing and type III secretion, attenuating its virulence in a murine cutaneous abscess model. Biomedicines 2022, 10, 1847. [Google Scholar]
- Lane, A.L.; Nyadong, L.; Galhena, A.S.; Shearer, T.L.; Stout, E.P.; Parry, R.M.; Kwasnik, M.; Wang, M.D.; Hay, M.E.; Fernandez, F.M.; et al. Desorption electrospray ionization mass spectrometry reveals surface-mediated antifungal chemical defense of a tropical seaweed. Proc. Natl. Acad. Sci. USA 2009, 106, 7314–7319. [Google Scholar]
- Parrot, D.; Papazian, S.; Foil, D.; Tasdemir, D. Imaging the unimaginable: Desorption electrospray ionization-imaging mass spectrometry (DESI-IMS) in natural product research. Planta Med. 2018, 84, 584–593. [Google Scholar]
- Teasdale, M.E.; Prudhomme, J.; Torres, M.; Braley, M.; Cervantes, S.; Bhatia, S.C.; La Clair, J.J.; Le Roch, K.; Kubanek, J. Pharmacokinetics, metabolism, and in vivo efficacy of the antimalarial natural product bromophycolide A. ACS Med. Chem. Lett. 2013, 4, 989–993. [Google Scholar]
- Chhetri, B.K.; Tedbury, P.R.; Sweeney-Jones, A.M.; Mani, L.; Soapi, K.; Manfredi, C.; Sorscher, E.; Sarafianos, S.G.; Kubanek, J. Marine natural products as leads against SARS-CoV-2 infection. J. Nat. Prod. 2022, 85, 657–665. [Google Scholar]
- Morita, M.; Schmidt, E.W. Parallel lives of symbionts and hosts: Chemical mutualism in marine animals. Nat. Prod. Rep. 2018, 35, 357–378. [Google Scholar]
- Raghuvanshi, R.; Bharate, S.B. Preclinical and clinical studies on bryostatins, a class of marine-derived protein kinase C modulators: A mini-review. Curr. Top. Med. Chem. 2020, 20, 1124–1135. [Google Scholar]
- Trindade-Silva, A.E.; Lim-Fong, G.E.; Sharp, K.H.; Haygood, M.G. Bryostatins: Biological context and biotechnological prospects. Curr. Opin. Biotechnol. 2010, 21, 834–842. [Google Scholar]
- Lopanik, N.B. Chemical defensive symbioses in the marine environment. Funct. Ecol. 2014, 28, 328–340. [Google Scholar]
- Mathew, M.; Lopanik, N.B. Host differentially expressed genes during association with its defensive endosymbiont. Biol. Bull. 2014, 226, 152–163. [Google Scholar]
- Ramesh, C.; Tulasi, B.R.; Raju, M.; Thakur, N.; Dufossé, L. Marine natural products from tunicates and their associated microbes. Mar. Drugs 2021, 19, 308. [Google Scholar]
- Tsukimoto, M.; Nagaoka, M.; Shishido, Y.; Fujimoto, J.; Nishisaka, F.; Matsumoto, S.; Harunari, E.; Imada, C.; Matsuzaki, T. Bacterial production of the tunicate-derived antitumor cyclic depsipeptide didemnin B. J. Nat. Prod. 2011, 74, 2329–2331. [Google Scholar] [CrossRef]
- Xu, Y.; Kersten, R.D.; Nam, S.J.; Lu, L.; Al-Suwailem, A.M.; Zheng, H.J.; Fenical, W.; Dorrestein, P.C.; Moore, B.S.; Qian, P.Y. Bacterial biosynthesis and maturation of the didemnin anticancer agents. J. Am. Chem. Soc. 2012, 134, 8625–8632. [Google Scholar]
- Lindquist, N.; Hay, M.E.; Fenical, W. Defense of ascidians and their conspicuous larvae: Adult vs. larval chemical defenses. Ecol. Monogr. 1992, 62, 547–568. [Google Scholar] [CrossRef]
- Lindquist, N.; Hay, M.E. Can small rare prey be chemically defended? The case for marine larvae. Ecology 1995, 76, 1347–1358. [Google Scholar] [CrossRef]
- Gu, W.; Schmidt, E.W. Three principles of diversity-generating biosynthesis. Acc. Chem. Res. 2017, 50, 2569–2576. [Google Scholar] [CrossRef]
- Lin, Z.; Torres, J.P.; Tianero, M.D.; Kwan, J.C.; Schmidt, E.W. Origin of chemical diversity in Prochloron-tunicate symbiosis. Appl. Environ. Microbiol. 2016, 82, 3450–3460. [Google Scholar] [CrossRef]
- Baur, P.; Kühl, M.; Comba, P.; Behrendt, L. Possible functional roles of patellamides in the ascidian-Prochloron symbiosis. Mar. Drugs 2022, 20, 119. [Google Scholar] [CrossRef]
- Schmidt, E.W. The secret to a successful relationship: Lasting chemistry between ascidians and their symbiotic bacteria. Invertebr. Biol. 2015, 134, 88–102. [Google Scholar] [CrossRef]
- Zan, J.; Li, Z.; Tianero, M.D.; Davis, J.; Hill, R.T.; Donia, M.S. A microbial factory for defensive kahalalides in a tripartite marine symbiosis. Science 2019, 364, eaaw6732. [Google Scholar] [CrossRef]
- Becerro, M.A.; Goetz, G.; Paul, V.J.; Scheuer, P.J. Chemical defenses of the Sacoglossan mollusk Elysia rufescens and its host alga Bryopsis sp. J. Chem. Ecol. 2001, 27, 2287–2299. [Google Scholar] [CrossRef]
- Torres, J.P.; Lin, Z.; Winter, J.M.; Krug, P.J.; Schmidt, E.W. Animal biosynthesis of complex polyketides in a photosynthetic partnership. Nat. Commun. 2020, 11, 2882. [Google Scholar] [CrossRef]
- Wasserman, H.H.; Friedland, D.J.; Morrison, D.A. A novel dipyrrolyldipyrromethene prodigiosin analog from Serratia marcescens. Tetrahedron Lett. 1968, 9, 641–644. [Google Scholar] [CrossRef]
- Franks, A.; Haywood, P.; Holmstrom, C.; Egan, S.; Kjelleberg, S.; Kumar, N. Isolation and structure elucidation of a novel yellow pigment from the marine bacterium Pseudoalteromonas tunicata. Molecules 2005, 10, 1286–1291. [Google Scholar] [CrossRef]
- Carté, B.; Faulkner, D.J. Role of secondary metabolites in feeding associations between a predatory nudibranch, two grazing nudibranchs, and a bryozoan. J. Chem. Ecol. 1986, 12, 795–804. [Google Scholar] [CrossRef]
- Lidgard, S. Predation on marine bryozoan colonies: Taxa, traits and trophic groups. Mar. Ecol. Prog. Ser. 2008, 359, 117–131. [Google Scholar] [CrossRef]
- Ballestriero, F.; Thomas, T.; Burke, C.; Egan, S.; Kjelleberg, S. Identification of compounds with bioactivity against the nematode Caenorhabditis elegans by a screen based on the functional genomics of the marine bacterium Pseudoalteromonas tunicata D2. Appl. Environ. Microbiol. 2010, 76, 5710–5717. [Google Scholar] [CrossRef]
- Paul, V.J.; Lindquist, N.; Fenical, W. Chemical defenses of the tropical ascidian Atapozoa sp. and its nudibranch predators Nembrotha spp. Mar. Ecol. Prog. Ser. 1990, 59, 109–118. [Google Scholar] [CrossRef]
- Takaki, M.; Freire, V.F.; Nicacio, K.J.; Bertonha, A.F.; Nagashima, N.; Sarpong, R.; Padula, V.; Ferreira, A.G.; Berlinck, R.G.S. Metabolomics reveals minor tambjamines in a marine invertebrate food chain. J. Nat. Prod. 2021, 84, 790–796. [Google Scholar] [CrossRef]
- Distel, D.L.; Morrill, W.; MacLaren-Toussaint, N.; Franks, D.; Waterbury, J. Teredinibacter turnerae gen. nov., sp. nov., a dinitrogen-fixing, cellulolytic, endosymbiotic gamma-proteobacterium isolated from the gills of wood-boring molluscs (Bivalvia: Teredinidae). Int. J. Syst. Evol. Microbiol. 2002, 52 Pt 6, 2261–2269. [Google Scholar]
- O’Connor, R.M.; Fung, J.M.; Sharp, K.H.; Benner, J.S.; McClung, C.; Cushing, S.; Lamkin, E.R.; Fomenkov, A.I.; Henrissat, B.; Londer, Y.Y.; et al. Gill bacteria enable a novel digestive strategy in a wood-feeding mollusk. Proc. Natl. Acad. Sci. USA 2014, 111, E5096–E5104. [Google Scholar] [CrossRef]
- Yang, J.C.; Madupu, R.; Durkin, A.S.; Ekborg, N.A.; Pedamallu, C.S.; Hostetler, J.B.; Radune, D.; Toms, B.S.; Henrissat, B.; Coutinho, P.M.; et al. The complete genome of Teredinibacter turnerae T7901: An intracellular endosymbiont of marine wood-boring bivalves (shipworms). PLoS ONE 2009, 4, e6085. [Google Scholar] [CrossRef]
- Han, A.W.; Sandy, M.; Fishman, B.; Trindade-Silva, A.E.; Soares, C.A.; Distel, D.L.; Butler, A.; Haygood, M.G. Turnerbactin, a novel triscatecholate siderophore from the shipworm endosymbiont Teredinibacter turnerae T7901. PLoS ONE 2013, 8, e76151. [Google Scholar] [CrossRef]
- Miller, B.W.; Schmidt, E.W.; Concepcion, G.P.; Haygood, M.G. Halogenated metal-binding compounds from shipworm symbionts. J. Nat. Prod. 2022, 85, 479–484. [Google Scholar] [CrossRef]
- Elshahawi, S.I.; Trindade-Silva, A.E.; Hanora, A.; Han, A.W.; Flores, M.S.; Vizzoni, V.; Schrago, C.G.; Soares, C.A.; Concepcion, G.P.; Distel, D.L.; et al. Boronated tartrolon antibiotic produced by symbiotic cellulose-degrading bacteria in shipworm gills. Proc. Natl. Acad. Sci. USA 2013, 110, E295–E304. [Google Scholar] [CrossRef]
- Miller, B.W.; Lim, A.L.; Lin, Z.; Bailey, J.; Aoyagi, K.L.; Fisher, M.A.; Barrows, L.R.; Manoil, C.; Schmidt, E.W.; Haygood, M.G. Shipworm symbiosis ecology-guided discovery of an antibiotic that kills colistin-resistant Acinetobacter. Cell Chem. Biol. 2021, 28, 1628–1637. [Google Scholar] [CrossRef]
- O’Connor, R.M.; Nepveux, V.F.J.; Abenoja, J.; Bowden, G.; Reis, P.; Beaushaw, J.; Bone Relat, R.M.; Driskell, I.; Gimenez, F.; Riggs, M.W.; et al. A symbiotic bacterium of shipworms produces a compound with broad spectrum anti-apicomplexan activity. PLoS Pathog. 2020, 16, e1008600. [Google Scholar] [CrossRef]
- Brito, T.L.; Campos, A.B.; Bastiaan von Meijenfeldt, F.A.; Daniel, J.P.; Ribeiro, G.B.; Silva, G.G.Z.; Wilke, D.V.; de Moraes, D.T.; Dutilh, B.E.; Meirelles, P.M.; et al. The gill-associated microbiome is the main source of wood plant polysaccharide hydrolases and secondary metabolite gene clusters in the mangrove shipworm Neoteredo reynei. PLoS ONE 2018, 13, e0200437. [Google Scholar] [CrossRef]
- Mutalipassi, M.; Riccio, G.; Mazzella, V.; Galasso, C.; Somma, E.; Chiarore, A.; de Pascale, D.; Zupo, V. Symbioses of cyanobacteria in marine environments: Ecological insights and biotechnological perspectives. Mar. Drugs 2021, 19, 227. [Google Scholar] [CrossRef]
- Unson, M.D.; Holland, N.D.; Faulkner, D.J. A brominated secondary metabolite synthesized by the cyanobacterial symbiont of a marine sponge and accumulation of the crystalline metabolite in the sponge tissue. Mar. Biol. 1994, 119, 1–11. [Google Scholar] [CrossRef]
- Thacker, R.W. Impacts of shading on sponge-cyanobacteria symbioses: A comparison between host-specific and generalist associations. Integr. Comp. Biol. 2005, 45, 369–376. [Google Scholar] [CrossRef]
- Schmitt, L.; Hinxlage, I.; Cea, P.A.; Gohlke, H.; Wesselborg, S. 40 Years of research on polybrominated diphenyl ethers (PBDEs)-a historical overview and newest data of a promising anticancer drug. Molecules 2021, 26, 995. [Google Scholar] [CrossRef]
- Faisal, M.R.; Kellermann, M.Y.; Rohde, S.; Putra, M.Y.; Murniasih, T.; Risdian, C.; Mohr, K.I.; Wink, J.; Praditya, D.F.; Steinmann, E.; et al. Ecological and pharmacological activities of polybrominated diphenyl ethers (PBDEs) from the Indonesian marine sponge Lamellodysidea herbacea. Mar. Drugs 2021, 19, 611. [Google Scholar] [CrossRef]
- Schorn, M.A.; Jordan, P.A.; Podell, S.; Blanton, J.M.; Agarwal, V.; Biggs, J.S.; Allen, E.E.; Moore, B.S. Comparative genomics of cyanobacterial symbionts reveals distinct, specialized metabolism in tropical Dysideidae sponges. mBio 2019, 10, e00821–19. [Google Scholar] [CrossRef]
- Pita, L.; Rix, L.; Slaby, B.M.; Franke, A.; Hentschel, U. The sponge holobiont in a changing ocean: From microbes to ecosystems. Microbiome 2018, 6, 46. [Google Scholar] [CrossRef]
- Busetti, A.; Maggs, C.A.; Gilmore, B.F. Marine macroalgae and their associated microbiomes as a source of antimicrobial chemical diversity. Eur. J. Phycol. 2017, 52, 452–465. [Google Scholar] [CrossRef]
- Ismail, A.; Ktari, L.; Ahmed, M.; Bolhuis, H.; Bouhaouala-Zahar, B.; Stal, L.J.; Boudabbous, A.; El Bour, M. Heterotrophic bacteria associated with the green alga Ulva rigida: Identification and antimicrobial potential. J. Appl. Phycol. 2018, 30, 2883–2899. [Google Scholar] [CrossRef]
- Vallet, M.; Strittmatter, M.; Murúa, P.; Lacoste, S.; Dupont, J.; Hubas, C.; Genta-Jouve, G.; Gachon, C.M.M.; Kim, G.H.; Prado, S. Chemically-mediated interactions between macroalgae, their fungal endophytes, and protistan pathogens. Front. Microbiol. 2018, 9, 3161. [Google Scholar] [CrossRef]
- Vallet, M.; Chong, Y.-M.; Tourneroche, A.; Genta-Jouve, G.; Hubas, C.; Lami, R.; Gachon, C.M.M.; Klochkova, T.; Chan, K.-G.; Prado, S. Novel α-hydroxy γ-butenolides of kelp endophytes disrupt bacterial cell-to-cell signaling. Front. Mar. Sci. 2020, 7, 601. [Google Scholar] [CrossRef]
- Modolon, F.; Barno, A.R.; Villela, H.D.M.; Peixoto, R.S. Ecological and biotechnological importance of secondary metabolites produced by coral-associated bacteria. J. Appl. Microbiol. 2020, 129, 1441–1457. [Google Scholar] [CrossRef]
- Morlock, G.E.; Ziltener, A.; Geyer, S.; Tersteegen, J.; Mehl, A.; Schreiner, T.; Kamel, T.; Brümmer, F. Evidence that Indo-Pacific bottlenose dolphins self-medicate with invertebrates in coral reefs. iScience 2022, 25, 104271. [Google Scholar] [CrossRef]
- Rosado, P.M.; Leite, D.C.A.; Duarte, G.A.S.; Chaloub, R.M.; Jospin, G.; Nunes da Rocha, U.; Saraiva, J.P.; Dini-Andreote, F.; Eisen, J.A.; Bourne, D.G.; et al. Marine probiotics: Increasing coral resistance to bleaching through microbiome manipulation. ISME J. 2019, 13, 921–936. [Google Scholar] [CrossRef]
- Nissimov, J.; Rosenberg, E.; Munn, C.B. Antimicrobial properties of resident coral mucus bacteria of Oculina patagonica. FEMS Microbiol. Lett. 2009, 292, 210–215. [Google Scholar] [CrossRef]
- Raina, J.B.; Tapiolas, D.; Motti, C.A.; Foret, S.; Seemann, T.; Tebben, J.; Willis, B.L.; Bourne, D.G. Isolation of an antimicrobial compound produced by bacteria associated with reef-building corals. PeerJ 2016, 4, e2275. [Google Scholar] [CrossRef]
- Yoch, D.C. Dimethylsulfoniopropionate: Its sources, role in the marine food web, and biological degradation to dimethylsulfide. Appl. Environ. Microbiol. 2002, 68, 5804–5815. [Google Scholar] [CrossRef]
- Raj Sharma, A.; Zhou, T.; Harunari, E.; Oku, N.; Trianto, A.; Igarashi, Y. Labrenzbactin from a coral-associated bacterium Labrenzia sp. J. Antibiot. 2019, 72, 634–639. [Google Scholar] [CrossRef]
- Ren, C.G.; Liu, Z.Y.; Wang, X.L.; Qin, S. The seaweed holobiont: From microecology to biotechnological applications. Microb. Biotechnol. 2022, 15, 738–754. [Google Scholar] [CrossRef]
- van de Water, J.A.; Tignat-Perrier, R.; Allemand, D.; Ferrier-Pagès, C. Coral holobionts and biotechnology: From Blue Economy to coral reef conservation. Curr. Opin. Biotechnol. 2022, 74, 110–121. [Google Scholar] [CrossRef]
- Menaa, F.; Wijesinghe, P.A.U.I.; Thiripuranathar, G.; Uzair, B.; Iqbal, H.; Khan, B.A.; Menaa, B. Ecological and industrial implications of dynamic seaweed-associated microbiota interactions. Mar. Drugs 2020, 18, 641. [Google Scholar] [CrossRef]
- Schul, M.; Mason, A.; Ushijima, B.; Sneed, J.M. Microbiome and metabolome contributions to coral health and disease. Biol. Bull. 2022, 243, 76–83. [Google Scholar] [CrossRef]
- Golberg, K.; Pavlov, V.; Marks, R.S.; Kushmaro, A. Coral-associated bacteria, quorum sensing disrupters, and the regulation of biofouling. Biofouling 2013, 29, 669–682. [Google Scholar] [CrossRef]
- Moitinho-Silva, L.; Steinert, G.; Nielsen, S.; Hardoim, C.C.P.; Wu, Y.C.; McCormack, G.P.; López-Legentil, S.; Marchant, R.; Webster, N.; Thomas, T.; et al. Predicting the HMA-LMA status in marine sponges by machine learning. Front. Microbiol. 2017, 8, 752. [Google Scholar] [CrossRef]
- Lesser, M.P.; Sabrina Pankey, M.; Slattery, M.; Macartney, K.J.; Gochfeld, D.J. Microbiome diversity and metabolic capacity determines the trophic ecology of the holobiont in Caribbean sponges. ISME Commun. 2022, 2, 112. [Google Scholar] [CrossRef]
- Sabrina Pankey, M.; Plachetzki, D.C.; Macartney, K.J.; Gastaldi, M.; Slattery, M.; Gochfeld, D.J.; Lesser, M.P. Cophylogeny and convergence shape holobiont evolution in sponge-microbe symbioses. Nat. Ecol. Evol. 2022, 6, 750–762. [Google Scholar] [CrossRef]
- Bhushan, A.; Peters, E.E.; Piel, J. Entotheonella bacteria as source of sponge-derived natural products: Opportunities for biotechnological production. Prog. Mol. Subcell. Biol. 2017, 55, 291–314. [Google Scholar]
- Wakimoto, T. Biosynthesis of bioactive natural products derived from Theonellidae family marine sponges. Chem. Pharm. Bull. 2023, 71, 1–8. [Google Scholar] [CrossRef]
- Rust, M.; Helfrich, E.J.N.; Freeman, M.F.; Nanudorn, P.; Field, C.M.; Rückert, C.; Kündig, T.; Page, M.J.; Webb, V.L.; Kalinowski, J.; et al. A multiproducer microbiome generates chemical diversity in the marine sponge Mycale hentscheli. Proc. Natl. Acad. Sci. USA 2020, 117, 9508–9518. [Google Scholar] [CrossRef]
- Tianero, M.D.; Balaich, J.N.; Donia, M.S. Localized production of defence chemicals by intracellular symbionts of Haliclona sponges. Nat. Microbiol. 2019, 4, 1149–1159. [Google Scholar] [CrossRef]
- Hemmerling, F.; Piel, J. Strategies to access biosynthetic novelty in bacterial genomes for drug discovery. Nat. Rev. Drug Discov. 2022, 21, 359–378. [Google Scholar] [CrossRef]
- Cetrulo, G.L.; Hay, M.E. Activated chemical defenses in tropical versus temperate seaweeds. Mar. Ecol. Prog. Ser. 2000, 207, 243–253. [Google Scholar] [CrossRef]
- Paul, V.J.; Van Alstyne, K.L. Activation of chemical defenses in the tropical green algae Halimeda spp. J. Exp. Mar. Biol. Ecol. 1992, 160, 191–203. [Google Scholar] [CrossRef]
- Jung, V.; Pohnert, G. Rapid wound-activated transformation of the green algal defensive metabolite caulerpenyne. Tetrahedron 2001, 57, 7169–7172. [Google Scholar] [CrossRef]
- Pohnert, G. Diatom/copepod interactions in plankton: The indirect chemical defense of unicellular algae. Chembiochem 2005, 6, 946–959. [Google Scholar] [CrossRef]
- Ruocco, N.; Albarano, L.; Esposito, R.; Zupo, V.; Costantini, M.; Ianora, A. Multiple roles of diatom-derived oxylipins within marine environments and their potential biotechnological applications. Mar. Drugs 2020, 18, 342. [Google Scholar] [CrossRef]
- Lindquist, N. Tridentatols D-H, nematocyst metabolites and precursors of the activated chemical defense in the marine hydroid Tridentata marginata (Kirchenpauer 1864). J. Nat. Prod. 2002, 65, 681–684. [Google Scholar] [CrossRef]
- Pedras, M.S.; To, Q.H. Non-indolyl cruciferous phytoalexins: Nasturlexins and tridentatols, a striking convergent evolution of defenses in terrestrial plants and marine animals? Phytochemistry 2015, 113, 57–63. [Google Scholar] [CrossRef]
- Johnson, M.K.; Alexander, K.E.; Lindquist, N.; Loo, G. Potent antioxidant activity of a dithiocarbamate-related compound from a marine hydroid. Biochem. Pharmacol. 1999, 58, 1313–1319. [Google Scholar] [CrossRef]
- Rohde, S.; Nietzer, S.; Schupp, P.J. Prevalence and mechanisms of dynamic chemical defenses in tropical sponges. PLoS ONE 2015, 10, e0132236. [Google Scholar] [CrossRef]
- Thoms, C.; Ebel, R.; Proksch, P. Activated chemical defense in Aplysina sponges revisited. J. Chem. Ecol. 2006, 32, 97–123. [Google Scholar] [CrossRef]
- García-Vilas, J.A.; Martínez-Poveda, B.; Quesada, A.R.; Medina, M.Á. Aeroplysinin-1, a sponge-derived multi-targeted bioactive marine drug. Mar. Drugs. 2015, 14, 1. [Google Scholar] [CrossRef]
- Thoms, C.; Schupp, P.J. Activated chemical defense in marine sponges-a case study on Aplysinella rhax. J. Chem. Ecol. 2008, 34, 1242–1252. [Google Scholar] [CrossRef]
- Jing, Q.; Hu, X.; Ma, Y.; Mu, J.; Liu, W.; Xu, F.; Li, Z.; Bai, J.; Hua, H.; Li, D. Marine-derived natural lead compound disulfide-linked dimer psammaplin A: Biological activity and structural modification. Mar. Drugs. 2019, 17, 384. [Google Scholar] [CrossRef]
- Oluwabusola, E.T.; Katermeran, N.P.; Poh, W.H.; Goh, T.M.B.; Tan, L.T.; Diyaolu, O.; Tabudravu, J.; Ebel, R.; Rice, S.A.; Jaspars, M. Inhibition of the quorum sensing system, elastase production and biofilm formation in Pseudomonas aeruginosa by psammaplin A and bisaprasin. Molecules 2022, 27, 1721. [Google Scholar] [CrossRef]
- Wakimoto, T.; Egami, Y.; Nakashima, Y.; Wakimoto, Y.; Mori, T.; Awakawa, T.; Ito, T.; Kenmoku, H.; Asakawa, Y.; Piel, J.; et al. Calyculin biogenesis from a pyrophosphate protoxin produced by a sponge symbiont. Nat. Chem. Biol. 2014, 10, 648–655. [Google Scholar] [CrossRef]
- Jomori, T.; Matsuda, K.; Egami, Y.; Abe, I.; Takai, A.; Wakimoto, T. Insights into phosphatase-activated chemical defense in a marine sponge holobiont. RSC Chem. Biol. 2021, 2, 1600–1607. [Google Scholar] [CrossRef]
- Flöthe, C.R.; Molis, M.; Kruse, I.; Weinberg, F.; John, U. Herbivore-induced defense response in the brown seaweed Fucus vesiculosus: Patterns in temporal dynamics and gene expression. Eur. J. Phycol. 2012, 49, 356–369. [Google Scholar] [CrossRef]
- Sudatti, D.B.; Fujii, M.T.; Rodrigues, S.V.; Turra, A.; Pereira, R.C. Prompt induction of chemical defenses in the red seaweed Laurencia dendroidea: The role of herbivory and epibiosis. J. Sea Res. 2018, 138, 48–55. [Google Scholar] [CrossRef]
- Van Donk, E.; Ianora, A.; Vos, M. Induced defences in marine and freshwater phytoplankton: A review. Hydrobiologia 2011, 668, 3–19. [Google Scholar] [CrossRef]
- Emeline, C.B.; Ludovic, D.; Laurent, V.; Catherine, L.; Kruse, I.; Erwan, A.G.; Florian, W.; Philippe, P. Induction of phlorotannins and gene expression in the brown macroalga Fucus vesiculosus in response to the herbivore Littorina littorea. Mar. Drugs 2021, 19, 185. [Google Scholar] [CrossRef]
- Chen, H.; Yang, R.; Chen, J.; Luo, Q.; Cui, X.; Gerwick, W.H. 1-Octen-3-ol, a self-stimulating oxylipin messenger, can prime and induce defense of marine alga. BMC Plant Biol. 2019, 19, 37. [Google Scholar] [CrossRef]
- Coll, J.C. The chemistry and chemical ecology of octocorals (Coelenterata, Anthozoa, Octocorallia). Chem. Rev. 1992, 92, 613–631. [Google Scholar] [CrossRef]
- Fleury, B.; Coll, J.; Sammarco, P. Complementary (secondary) metabolites in a soft coral: Sex-specific variability, inter-clonal variability, and competition. Mar. Ecol. 2006, 27, 204–218. [Google Scholar] [CrossRef]
- Ling, X.H.; Wang, S.K.; Huang, Y.H.; Huang, M.J.; Duh, C.Y. A high-content screening assay for the discovery of novel proteasome inhibitors from Formosan soft corals. Mar. Drugs 2018, 16, 395. [Google Scholar] [CrossRef]
- Helber, S.B.; Hoeijmakers, D.J.J.; Muhando, C.A.; Rohde, S.; Schupp, P.J. Sponge chemical defenses are a possible mechanism for increasing sponge abundance on reefs in Zanzibar. PLoS ONE 2018, 13, e0197617. [Google Scholar] [CrossRef]
- Chaves-Fonnegra, A.; Castellanos, L.; Zea, S.; Duque, C.; Rodríguez, J.; Jiménez, C. Clionapyrrolidine A-a metabolite from the encrusting and excavating sponge Cliona tenuis that kills coral tissue upon contact. J. Chem. Ecol. 2008, 34, 1565–1574. [Google Scholar] [CrossRef]
- Singh, A.; Thakur, N.L. Allelopathic interaction among rocky intertidal invertebrates: Sponge Cinachyrella cf. cavernosa and zooxanthellate zoanthids Zoanthus sansibaricus. Hydrobiologia 2021, 848, 4647–4659. [Google Scholar]
- Longo, G.O.; Hay, M.E. Seaweed allelopathy to corals: Are active compounds on, or in, seaweeds? Coral Reefs 2016, 36, 247–253. [Google Scholar] [CrossRef]
- Rasher, D.B.; Stout, E.P.; Engel, S.; Kubanek, J.; Hay, M.E. Macroalgal terpenes function as allelopathic agents against reef corals. Proc. Natl. Acad. Sci. USA 2011, 108, 17726–17731. [Google Scholar] [CrossRef]
- Vieira, C.; Thomas, O.P.; Culioli, G.; Genta-Jouve, G.; Houlbreque, F.; Gaubert, J.; De Clerck, O.; Payri, C.E. Allelopathic interactions between the brown algal genus Lobophora (Dictyotales, Phaeophyceae) and scleractinian corals. Sci. Rep. 2016, 6, 18637. [Google Scholar] [CrossRef]
- Andras, T.D.; Alexander, T.S.; Gahlena, A.; Parry, R.M.; Fernandez, F.M.; Kubanek, J.; Wang, M.D.; Hay, M.E. Seaweed allelopathy against coral: Surface distribution of a seaweed secondary metabolite by imaging mass spectrometry. J. Chem. Ecol. 2012, 38, 1203–1214. [Google Scholar] [CrossRef]
- Motuhi, S.E.; Feizbakhsh, O.; Foll-Josselin, B.; Baratte, B.; Delehouzé, C.; Cousseau, A.; Fant, X.; Bulinski, J.C.; Payri, C.E.; Ruchaud, S.; et al. Neurymenolide A, a novel mitotic spindle poison from the New Caledonian Rhodophyta Phacelocarpus neurymenioides. Mar. Drugs 2019, 17, 93. [Google Scholar] [CrossRef]
- Śliwińska-Wilczewska, S.; Wiśniewska, K.; Konarzewska, Z.; Cieszyńska, A.; Barreiro Felpeto, A.; Lewandowska, A.U.; Latała, A. The current state of knowledge on taxonomy, modulating factors, ecological roles, and mode of action of phytoplankton allelochemicals. Sci. Total Environ. 2021, 773, 145681. [Google Scholar] [CrossRef]
- Tan, L.T.; Phyo, M.Y. Marine cyanobacteria: A source of lead compounds and their clinically-relevant molecular targets. Molecules 2020, 25, 2197. [Google Scholar] [CrossRef]
- Leão, P.N.; Engene, N.; Antunes, A.; Gerwick, W.H.; Vasconcelos, V. The chemical ecology of cyanobacteria. Nat. Prod. Rep. 2012, 29, 372–391. [Google Scholar] [CrossRef]
- Poulson, K.L.; Sieg, R.D.; Kubanek, J. Chemical ecology of the marine plankton. Nat. Prod. Rep. 2009, 26, 729–745. [Google Scholar] [CrossRef]
- Sieg, R.D.; Poulson-Ellestad, K.L.; Kubanek, J. Chemical ecology of the marine plankton. Nat. Prod. Rep. 2011, 28, 388–399. [Google Scholar] [CrossRef]
- Roy, J.S.; Poulson-Ellestad, K.L.; Drew Sieg, R.; Poulin, R.X.; Kubanek, J. Chemical ecology of the marine plankton. Nat. Prod. Rep. 2013, 30, 1364–1379. [Google Scholar] [CrossRef]
- Schwartz, E.R.; Poulin, R.X.; Mojib, N.; Kubanek, J. Chemical ecology of marine plankton. Nat. Prod. Rep. 2016, 33, 843–860. [Google Scholar] [CrossRef]
- Pavaux, A.-S.; Berdalet, E.; Lemée, R. Chemical ecology of the benthic dinoflagellate genus Ostreopsis: Review of progress and future directions. Front. Mar. Sci. 2020, 7, 498. [Google Scholar] [CrossRef]
- Saha, M.; Fink, P. Algal volatiles-the overlooked chemical language of aquatic primary producers. Biol. Rev. Camb. Philos. Soc. 2022, 97, 2162–2173. [Google Scholar] [CrossRef]
- Seymour, J.R.; Amin, S.A.; Raina, J.B.; Stocker, R. Zooming in on the phycosphere: The ecological interface for phytoplankton-bacteria relationships. Nat. Microbiol. 2017, 2, 17065. [Google Scholar] [CrossRef]
- Seyedsayamdost, M.R.; Case, R.J.; Kolter, R.; Clardy, J. The Jekyll-and-Hyde chemistry of Phaeobacter gallaeciensis. Nat. Chem. 2011, 3, 331–335. [Google Scholar] [CrossRef]
- Fukuda, T.T.H.; Cassilly, C.D.; Gerdt, J.P.; Henke, M.T.; Helfrich, E.J.N.; Mevers, E. Research tales from the Clardy laboratory: Function-driven natural product discovery. J. Nat. Prod. 2020, 83, 744–755. [Google Scholar] [CrossRef]
- Shibl, A.A.; Isaac, A.; Ochsenkühn, M.A.; Cárdenas, A.; Fei, C.; Behringer, G.; Arnoux, M.; Drou, N.; Santos, M.P.; Gunsalus, K.C.; et al. Diatom modulation of select bacteria through use of two unique secondary metabolites. Proc. Natl. Acad. Sci. USA. 2020, 117, 27445–27455. [Google Scholar] [CrossRef]
- Corral-Lugo, A.; Daddaoua, A.; Ortega, A.; Espinosa-Urgel, M.; Krell, T. Rosmarinic acid is a homoserine lactone mimic produced by plants that activates a bacterial quorum-sensing regulator. Sci. Signal 2016, 9, ra1. [Google Scholar] [CrossRef]
- Rolland, J.L.; Stien, D.; Sanchez-Ferandin, S.; Lami, R. Quorum sensing and quorum quenching in the phycosphere of phytoplankton: A case of chemical interactions in ecology. J. Chem. Ecol. 2016, 42, 1201–1211. [Google Scholar] [CrossRef]
- Fei, C.; Ochsenkühn, M.A.; Shibl, A.A.; Isaac, A.; Wang, C.; Amin, S.A. Quorum sensing regulates ‘swim-or-stick’ lifestyle in the phycosphere. Environ. Microbiol. 2020, 22, 4761–4778. [Google Scholar] [CrossRef]
- Variem, S.S.; Kizhakkedath, V.K. Phycosphere associated bacteria; a prospective source of bioactive compounds. Biologia 2021, 76, 1095–1098. [Google Scholar] [CrossRef]
- Puillandre, N.; Holford, M. The Terebridae and teretoxins: Combining phylogeny and anatomy for concerted discovery of bioactive compounds. BMC Chem. Biol. 2010, 10, 7. [Google Scholar] [CrossRef]
- Ledoux, J.B.; Antunes, A. Beyond the beaten path: Improving natural products bioprospecting using an eco-evolutionary framework-the case of the octocorals. Crit. Rev. Biotechnol. 2018, 38, 184–198. [Google Scholar] [CrossRef]
- Ueoka, R.; Bhushan, A.; Probst, S.I.; Bray, W.M.; Lokey, R.S.; Linington, R.G.; Piel, J. Genome-based identification of a plant-associated marine bacterium as a rich natural product source. Angew. Chem. Int. Ed. Engl. 2018, 57, 14519–14523. [Google Scholar] [CrossRef]
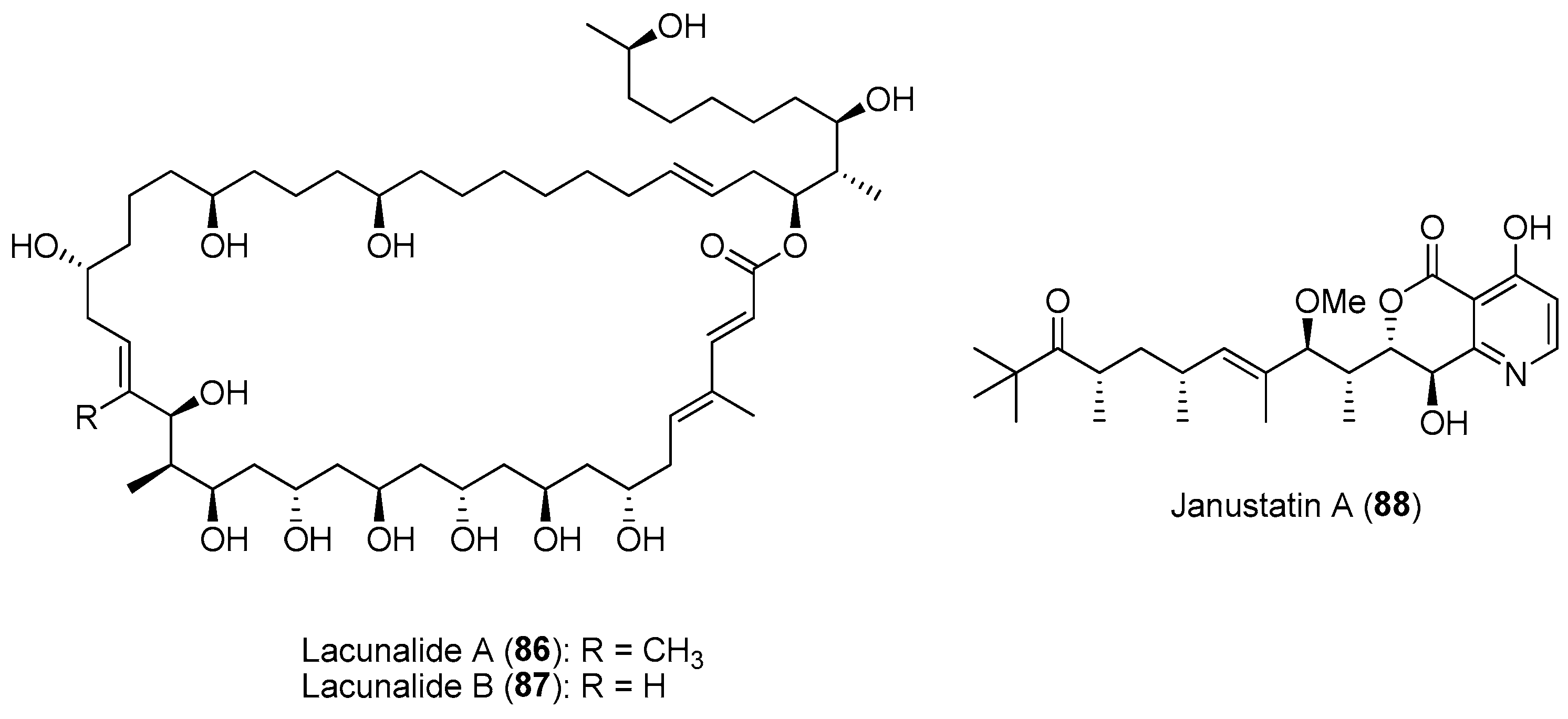
- Ueoka, R.; Sondermann, P.; Leopold-Messer, S.; Liu, Y.; Suo, R.; Bhushan, A.; Vadakumchery, L.; Greczmiel, U.; Yashiroda, Y.; Kimura, H.; et al. Genome-based discovery and total synthesis of janustatins, potent cytotoxins from a plant-associated bacterium. Nat. Chem. 2022, 14, 1193–1201. [Google Scholar] [CrossRef]
- Evans-Illidge, E.A.; Logan, M.; Doyle, J.; Fromont, J.; Battershill, C.N.; Ericson, G.; Wolff, C.W.; Muirhead, A.; Kearns, P.; Abdo, D.; et al. Phylogeny drives large scale patterns in Australian marine bioactivity and provides a new chemical ecology rationale for future biodiscovery. PLoS ONE 2013, 8, e73800. [Google Scholar] [CrossRef]
- Rohde, S.; Schupp, P. Spatial and temporal variability in sponge chemical defense. In Chemical Ecology: The Ecological Impacts of Marine Natural Products, 1st ed.; Puglisi, M.P., Becerro, M.A., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 373–395. [Google Scholar]
- Noyer, C.; Becerro, M.A. Relationship between genetic, chemical, and bacterial diversity in the Atlanto-Mediterranean bath sponge Spongia lamella. Hydrobiologia 2012, 687, 85–99. [Google Scholar] [CrossRef]
- Rohde, S.; Gochfeld, D.J.; Ankisetty, S.; Avula, B.; Schupp, P.J.; Slattery, M. Spatial variability in secondary metabolites of the Indo-Pacific sponge Stylissa massa. J. Chem. Ecol. 2012, 38, 463–475. [Google Scholar] [CrossRef]
- Page, M.; West, L.; Northcote, P.; Battershill, C.; Kelly, M. Spatial and temporal variability of cytotoxic metabolites in populations of the New Zealand sponge Mycale hentscheli. J. Chem. Ecol. 2005, 31, 1161–1174. [Google Scholar] [CrossRef]
- Steindler, L.; Beer, S.; Ilan, M. Photosymbiosis in intertidal and subtidal tropical sponges. Symbiosis 2002, 33, 263–273. [Google Scholar]
- Sacristán-Soriano, O.; Banaigs, B.; Becerro, M.A. Temporal trends in the secondary metabolite production of the sponge Aplysina aerophoba. Mar. Drugs 2012, 10, 677–693. [Google Scholar] [CrossRef]
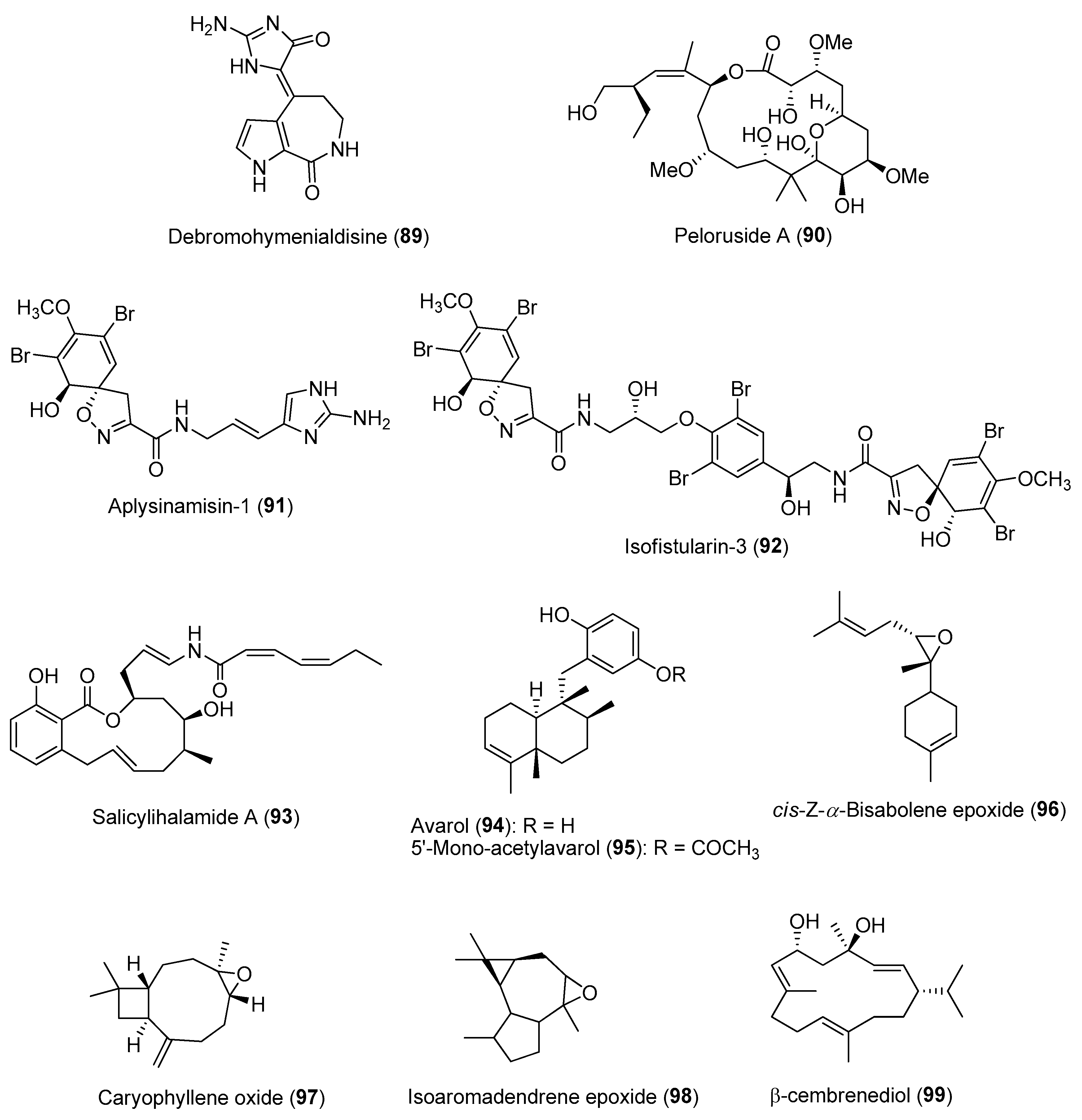
- Abdo, D.A.; Motti, C.A.; Battershill, C.N.; Harvey, E.S. Temperature and spatiotemporal variability of salicylihalamide A in the sponge Haliclona sp. J. Chem. Ecol. 2007, 33, 1635–1645. [Google Scholar] [CrossRef]
- De Caralt, S.; Bry, D.; Bontemps, N.; Turon, X.; Uriz, M.J.; Banaigs, B. Sources of secondary metabolite variation in Dysidea avara (Porifera: Demospongiae): The importance of having good neighbors. Mar. Drugs 2013, 11, 489–503. [Google Scholar] [CrossRef]
- Hellio, C.; Marechal, J.P.; Véron, B.; Bremer, G.; Clare, A.S.; Le Gal, Y. Seasonal variation of antifouling activities of marine algae from the Brittany coast (France). Mar. Biotechnol. 2004, 6, 67–82. [Google Scholar] [CrossRef]
- Satheesh, S.; Ba-Akdah, M.A. Temporal variations in the antifouling activity of extract of the soft coral Sarcophyton trocheliophorum collected from the Red Sea. Ocean Sci. J. 2022, 57, 247–258. [Google Scholar] [CrossRef]
- Maslin, M.; Gaertner-Mazouni, N.; Debitus, C.; Joy, N.; Ho, R. Marine sponge aquaculture towards drug development: An ongoing history of technical, ecological, chemical considerations and challenges. Aquac. Rep. 2021, 21, 100813. [Google Scholar] [CrossRef]
- Reverter, M.; Tribalat, M.A.; Pérez, T.; Thomas, O.P. Metabolome variability for two Mediterranean sponge species of the genus Haliclona: Specificity, time, and space. Metabolomics 2018, 14, 114. [Google Scholar] [CrossRef]
- Bayona, L.M.; van Leeuwen, G.; Erol, Ö.; Swierts, T.; van der Ent, E.; de Voogd, N.J.; Choi, Y.H. Influence of geographical location on the metabolic production of Giant barrel sponges (Xestospongia spp.) revealed by metabolomics tools. ACS Omega 2020, 5, 12398–12408. [Google Scholar] [CrossRef]
- Poulin, R.X.; Pohnert, G. Simplifying the complex: Metabolomics approaches in chemical ecology. Anal. Bioanal. Chem. 2019, 411, 13–19. [Google Scholar] [CrossRef]
- Li, H.; Li, Z. The exploration of microbial natural products and metabolic interaction guided by mass spectrometry imaging. Bioengineering 2022, 9, 707. [Google Scholar] [CrossRef]
- Spraker, J.E.; Luu, G.T.; Sanchez, L.M. Imaging mass spectrometry for natural products discovery: A review of ionization methods. Nat. Prod. Rep. 2020, 37, 150–162. [Google Scholar] [CrossRef]
- Soltwisch, J.; Kettling, H.; Vens-Cappell, S.; Wiegelmann, M.; Müthing, J.; Dreisewerd, K. Mass spectrometry imaging with laser-induced postionization. Science 2015, 348, 211–215. [Google Scholar] [CrossRef]
- Esquenazi, E.; Coates, C.; Simmons, L.; Gonzalez, D.; Gerwick, W.H.; Dorrestein, P.C. Visualizing the spatial distribution of secondary metabolites produced by marine cyanobacteria and sponges via MALDI-TOF imaging. Mol. Biosyst. 2008, 4, 562–570. [Google Scholar] [CrossRef]
- Moree, W.J.; Yang, J.Y.; Zhao, X.; Liu, W.T.; Aparicio, M.; Atencio, L.; Ballesteros, J.; Sánchez, J.; Gavilán, R.G.; Gutiérrez, M.; et al. Imaging mass spectrometry of a coral microbe interaction with fungi. J. Chem. Ecol. 2013, 39, 1045–1054. [Google Scholar] [CrossRef]
- Papazian, S.; Parrot, D.; Burýšková, B.; Weinberger, F.; Tasdemir, D. Surface chemical defence of the eelgrass Zostera marina against microbial foulers. Sci. Rep. 2019, 9, 3323. [Google Scholar] [CrossRef]
- Cantrell, T.P.; Freeman, C.J.; Paul, V.J.; Agarwal, V.; Garg, N. Mass spectrometry-based integration and expansion of the chemical diversity harbored within a marine sponge. J. Am. Soc. Mass Spectrom. 2019, 30, 1373–1384. [Google Scholar] [CrossRef]
- Geier, B.; Sogin, E.M.; Michellod, D.; Janda, M.; Kompauer, M.; Spengler, B.; Dubilier, N.; Liebeke, M. Spatial metabolomics of in situ host-microbe interactions at the micrometre scale. Nat. Microbiol. 2020, 5, 498–510. [Google Scholar] [CrossRef]
- Vallet, M.; Kaftan, F.; Grabe, V.; Ghaderiardakani, F.; Fenizia, S.; Svatoš, A.; Pohnert, G.; Wichard, T. A new glance at the chemosphere of macroalgal-bacterial interactions: In Situ profiling of metabolites in symbiosis by mass spectrometry. Beilstein J. Org. Chem. 2021, 17, 1313–1322. [Google Scholar] [CrossRef]
- Lackner, G.; Peters, E.E.; Helfrich, E.J.; Piel, J. Insights into the lifestyle of uncultured bacterial natural product factories associated with marine sponges. Proc. Natl. Acad. Sci. USA 2017, 114, E347–E356. [Google Scholar] [CrossRef]
- Zhang, S.; Song, W.; Nothias, L.F.; Couvillion, S.P.; Webster, N.; Thomas, T. Comparative metabolomic analysis reveals shared and unique chemical interactions in sponge holobionts. Microbiome 2022, 10, 22. [Google Scholar] [CrossRef]
- Pernice, M.; Meibom, A.; van Den Heuvel, A.; Kopp, C.; Domart-Coulon, I.; Hoegh-Guldberg, O.; Dove, S. A single-cell view of ammonium assimilation in coral–dinoflagellate symbiosis. ISME J. 2012, 6, 1314. [Google Scholar] [CrossRef]
- Kopp, C.; Wisztorski, M.; Revel, J.; Mehiri, M.; Dani, V.; Capron, L.; Carette, D.; Fournier, I.; Massi, L.; Mouajjah, D.; et al. MALDI-MS and NanoSIMS imaging techniques to study cnidarian-dinoflagellate symbioses. Zoology 2015, 118, 125–131. [Google Scholar] [CrossRef]
- Achlatis, M.; Pernice, M.; Green, K.; de Goeij, J.M.; Guagliardo, P.; Kilburn, M.R.; Hoegh-Guldberg, O.; Dove, S. Single-cell visualization indicates direct role of sponge host in uptake of dissolved organic matter. Proc. R. Soc. B 2019, 286, 20192153. [Google Scholar] [CrossRef]
- Rix, L.; Ribes, M.; Coma, R.; Jahn, M.T.; de Goeij, J.M.; van Oevelen, D.; Escrig, S.; Meibom, A.; Hentschel, U. Heterotrophy in the earliest gut: A single-cell view of heterotrophic carbon and nitrogen assimilation in sponge-microbe symbioses. ISME J. 2020, 14, 2554–2567. [Google Scholar] [CrossRef]
- Avalon, N.E.; Murray, A.E.; Baker, B.J. Integrated metabolomic-genomic workflows accelerate microbial natural product discovery. Anal. Chem. 2022, 94, 11959–11966. [Google Scholar] [CrossRef]
- van der Hooft, J.J.J.; Mohimani, H.; Bauermeister, A.; Dorrestein, P.C.; Duncan, K.R.; Medema, M.H. Linking genomics and metabolomics to chart specialized metabolic diversity. Chem. Soc. Rev. 2020, 49, 3297–3314. [Google Scholar] [CrossRef]
- Kogawa, M.; Miyaoka, R.; Hemmerling, F.; Ando, M.; Yura, K.; Ide, K.; Nishikawa, Y.; Hosokawa, M.; Ise, Y.; Cahn, J.K.B.; et al. Single-cell metabolite detection and genomics reveals uncultivated talented producer. PNAS Nexus 2022, 1, pgab007. [Google Scholar] [CrossRef]
- He, Q.; Silliman, B.R. Climate change, human impacts, and coastal ecosystems in the Anthropocene. Curr. Biol. 2019, 29, R1021–R1035. [Google Scholar] [CrossRef]
- Stern, N. The Economics of Climate Change: The Stern Review, 1st ed.; Cambridge University Press: Cambridge, UK, 2007; ISBN 9780511817434. [Google Scholar]
- Erwin, P.M.; López-Legentil, S.; Schuhmann, P.W. The pharmaceutical value of marine biodiversity for anti-cancer drug discovery. Ecol. Econ. 2010, 70, 445–451. [Google Scholar] [CrossRef]
- Lincoln, S.; Andrews, B.; Birchenough, S.N.R.; Chowdhury, P.; Engelhard, G.H.; Harrod, O.; Pinnegar, J.K.; Townhill, B.L. Marine litter and climate change: Inextricably connected threats to the world’s oceans. Sci. Total Environ. 2022, 837, 155709. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef]
- Tewari, K. A review of climate change impact studies on harmful algal blooms. Phycology 2022, 2, 244–253. [Google Scholar] [CrossRef]
- Ritson-Williams, R.; Ross, C.; Paul, V.J. Elevated temperature and allelopathy impact coral recruitment. PLoS ONE 2016, 11, e0166581. [Google Scholar] [CrossRef]
- Bell, J.J.; Bennett, H.M.; Rovellini, A.; Webster, N.S. Sponges to be winners under near-future climate scenarios. Bioscience 2018, 68, 955–968. [Google Scholar] [CrossRef]
- Freeman, C.J.; Thacker, R.W. Complex interactions between marine sponges and their symbiotic microbial communities. Limnol. Oceanogr. 2011, 56, 1577–1586. [Google Scholar] [CrossRef]
- Abirami, B.; Radhakrishnan, M.; Kumaran, S.; Wilson, A. Impacts of global warming on marine microbial communities. Sci. Total Environ. 2021, 791, 147905. [Google Scholar] [CrossRef]
- Lemoine, N.; Buell, N.; Hill, A.; Hill, M. Assessing the utility of sponge microbial symbiont communities as models to study global climate change: A case study with Halichondria bowerbanki. Porifera Res. Biodivers. Innov. Sustain. Série Livros 2007, 28, 239–246. [Google Scholar]
- Fan, L.; Liu, M.; Simister, R.; Webster, N.S.; Thomas, T. Marine microbial symbiosis heats up: The phylogenetic and functional response of a sponge holobiont to thermal stress. ISME J. 2013, 7, 991–1002. [Google Scholar] [CrossRef]
- Tian, R.; Wang, Y.; Bougouffa, S.; Gao, Z.; Cai, L.; Zhang, W.; Bajic, V.; Qian, P. Effect of copper treatment on the composition and function of the bacterial community in the sponge Haliclona cymaeformis. mBio 2014, 5, e01980–14. [Google Scholar] [CrossRef]
- Lesser, M.P.; Fiore, C.; Slattery, M.; Zaneveld, J. Climate change stressors destabilize the microbiome of the Caribbean barrel sponge, Xestospongia muta. J. Exp. Mar. Biol. Ecol. 2016, 475, 11–18. [Google Scholar] [CrossRef]
- Bove, C.B.; Ingersoll, M.V.; Davies, S.W. Help me, symbionts, you’re my only hope: Approaches to accelerate our understanding of coral holobiont interactions. Integr. Comp. Biol. 2022, 62, 1756–1769. [Google Scholar] [CrossRef]
- Williams, A.; Chiles, E.N.; Conetta, D.; Pathmanathan, J.S.; Cleves, P.A.; Putnam, H.M.; Su, X.; Bhattacharya, D. Metabolomic shifts associated with heat stress in coral holobionts. Sci. Adv. 2021, 7, eabd4210. [Google Scholar] [CrossRef]
- Pei, J.Y.; Yu, W.F.; Zhang, J.J.; Kuo, T.H.; Chung, H.H.; Hu, J.J.; Hsu, C.C.; Yu, K.F. Mass spectrometry-based metabolomic signatures of coral bleaching under thermal stress. Anal. Bioanal. Chem. 2022, 414, 7635–7646. [Google Scholar] [CrossRef]
- Uria, A.R.; Piel, J.; Wakimoto, T. Biosynthetic insights of calyculin- and misakinolide-type compounds in “Candidatus Entotheonella sp.”. Meth. Enzymol. 2018, 604, 287–330. [Google Scholar]
- Tianero, M.D.; Kwan, J.C.; Wyche, T.P.; Presson, A.P.; Koch, M.; Barrows, L.R.; Bugni, T.S.; Schmidt, E.W. Species specificity of symbiosis and secondary metabolism in ascidians. ISME J. 2015, 9, 615–628. [Google Scholar] [CrossRef]
- Campbell, A.H.; Harder, T.; Nielsen, S.; Kjelleberg, S.; Steinberg, P.D. Climate change and disease: Bleaching of a chemically defended seaweed. Glob. Change Biol. 2011, 17, 2958–2970. [Google Scholar] [CrossRef]
- Manefield, M.; Rasmussen, T.B.; Henzter, M.; Andersen, J.B.; Steinberg, P.; Kjelleberg, S.; Givskov, M. Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 2002, 148, 1119–1127. [Google Scholar] [CrossRef]
- Sudatti, D.B.; Fujii, M.T.; Rodrigues, S.V.; Turra, A.; Pereira, R.C. Effects of abiotic factors on growth and chemical defenses in cultivated clones of Laurencia dendroidea J. Agardh (Ceramiales, Rhodophyta). Mar. Biol. 2011, 158, 1439–1446. [Google Scholar] [CrossRef]
- Pereira, R.C.; da Gama, B.A.P.; Teixeira, V.L.; Yoneshigue-Valentin, Y. Ecological roles of natural products of the Brazilian red seaweed Laurencia obtusa. Braz. J. Biol. 2003, 63, 665–672. [Google Scholar] [CrossRef]
- Da Gama, B.A.P.; Pereira, R.C.; Soares, A.R.; Teixeira, V.L.; Yoneshigue-Valentin, Y. Is the mussel test a good indicator of antifouling activity? A comparison between laboratory and field assays. Biofouling 2003, 19, 161–169. [Google Scholar] [CrossRef]
- Guan, C.; Saha, M.; Weinberger, F. Simulated heatwaves lead to upregulated chemical defense of a marine foundation macrophyte against microbial colonizers. Front. Mar. Sci. 2020, 7, 463. [Google Scholar] [CrossRef]
- Farag, M.A.; Meyer, A.; Ali, S.E. Bleaching effect in Sarcophyton spp. soft corals-is there a correlation to their diterpene content? Environ. Sci. Pollut. Res. Int. 2021, 28, 25594–25602. [Google Scholar] [CrossRef]
- Roggatz, C.C.; Saha, M.; Blanchard, S.; Schirrmacher, P.; Fink, P.; Verheggen, F.; Hardege, J.D. Becoming nose-blind-climate change impacts on chemical communication. Glob. Chang. Biol. 2022, 28, 4495–4505. [Google Scholar] [CrossRef]
- Roggatz, C.C.; Lorch, M.; Hardege, J.D.; Benoit, D.M. Ocean acidification affects marine chemical communication by changing structure and function of peptide signalling molecules. Glob. Chang. Biol. 2016, 22, 3914–3926. [Google Scholar] [CrossRef]
- Roggatz, C.C.; Fletcher, N.; Benoit, D.M.; Algar, A.C.; Doroff, A.; Wright, B.; Wollenberg Valero, K.C.; Hardege, J.D. Saxitoxin and tetrodotoxin bioavailability increases in future oceans. Nat. Clim. Chang. 2019, 9, 840–844. [Google Scholar] [CrossRef]
- Roggatz, C.C.; Hardege, J.D.; Saha, M. Modelling antifouling compounds of macroalgal holobionts in current and future pH conditions. J. Chem. Ecol. 2022, 48, 455–473. [Google Scholar] [CrossRef]
- Mutalipassi, M.; Mazzella, V.; Schott, M.; Fink, P.; Glaviano, F.; Porzio, L.; Lorenti, M.; Buia, M.C.; von Elert, E.; Zupo, V. Ocean acidification affects volatile infochemicals production and perception in fauna and flora associated with Posidonia oceanica (L.) Delile. Front. Mar. Sci. 2022, 9, 809702. [Google Scholar] [CrossRef]
- Zupo, V.; Mutalipassi, M.; Fink, P.; Di Natale, M. Effect of ocean acidification on the communications among invertebrates mediated by plant-produced volatile organic compounds. Global J. Ecol. 2016, 1, 12–18. [Google Scholar] [CrossRef]
- Munday, P.L.; Dixson, D.L.; Donelson, J.M.; Jones, G.P.; Pratchett, M.S.; Devitsina, G.V.; Døving, K.B. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc. Natl. Acad. Sci. USA 2009, 106, 1848–1852. [Google Scholar] [CrossRef]
- Dow, L. How do quorum-sensing signals mediate algae-bacteria interactions? Microorganisms 2021, 9, 1391. [Google Scholar] [CrossRef]
- Singh, R.P.; Reddy, C.R. Unraveling the functions of the macroalgal microbiome. Front. Microbiol. 2016, 6, 1488. [Google Scholar] [CrossRef]
- Engelberts, J.P.; Robbins, S.J.; Damjanovic, K.; Webster, N.S. Integrating novel tools to elucidate the metabolic basis of microbial symbiosis in reef holobionts. Mar. Biol. 2021, 168, 175. [Google Scholar] [CrossRef]
- Bauermeister, A.; Branco, P.C.; Furtado, L.C.; Jimenez, P.C.; Costa-Lotufo, L.V.; da Cruz Lotufo, T.M. Tunicates: A model organism to investigate the effects of associated-microbiota on the production of pharmaceuticals. Drug Discov. Today Dis. Models 2018, 28, 13–20. [Google Scholar] [CrossRef]
- Cerrano, C.; Giovine, M.; Steindler, L. Petrosia ficiformis (Poiret, 1789): An excellent model for holobiont and biotechnological studies. Curr. Opin. Biotechnol. 2022, 74, 61–65. [Google Scholar] [CrossRef]
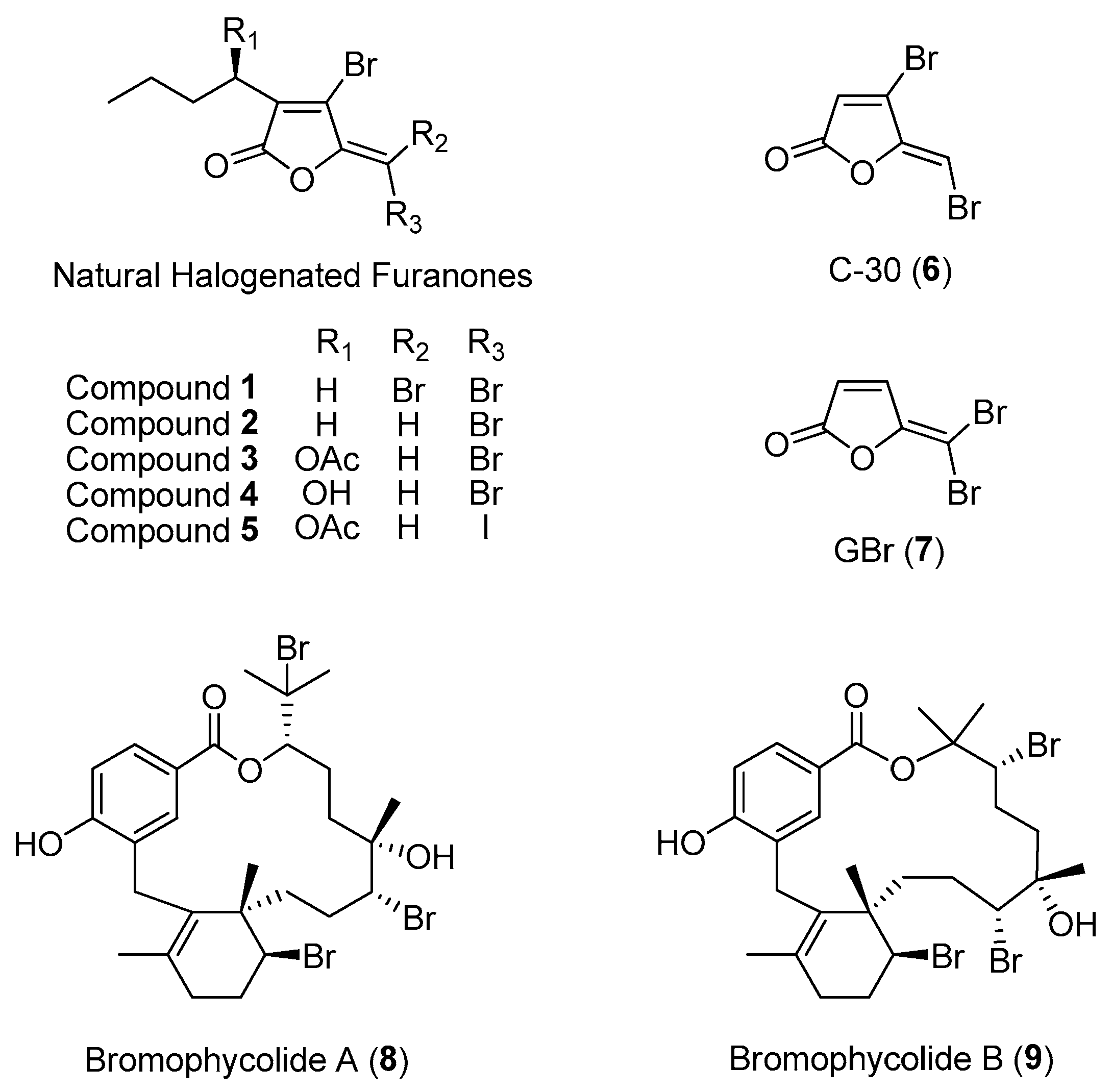
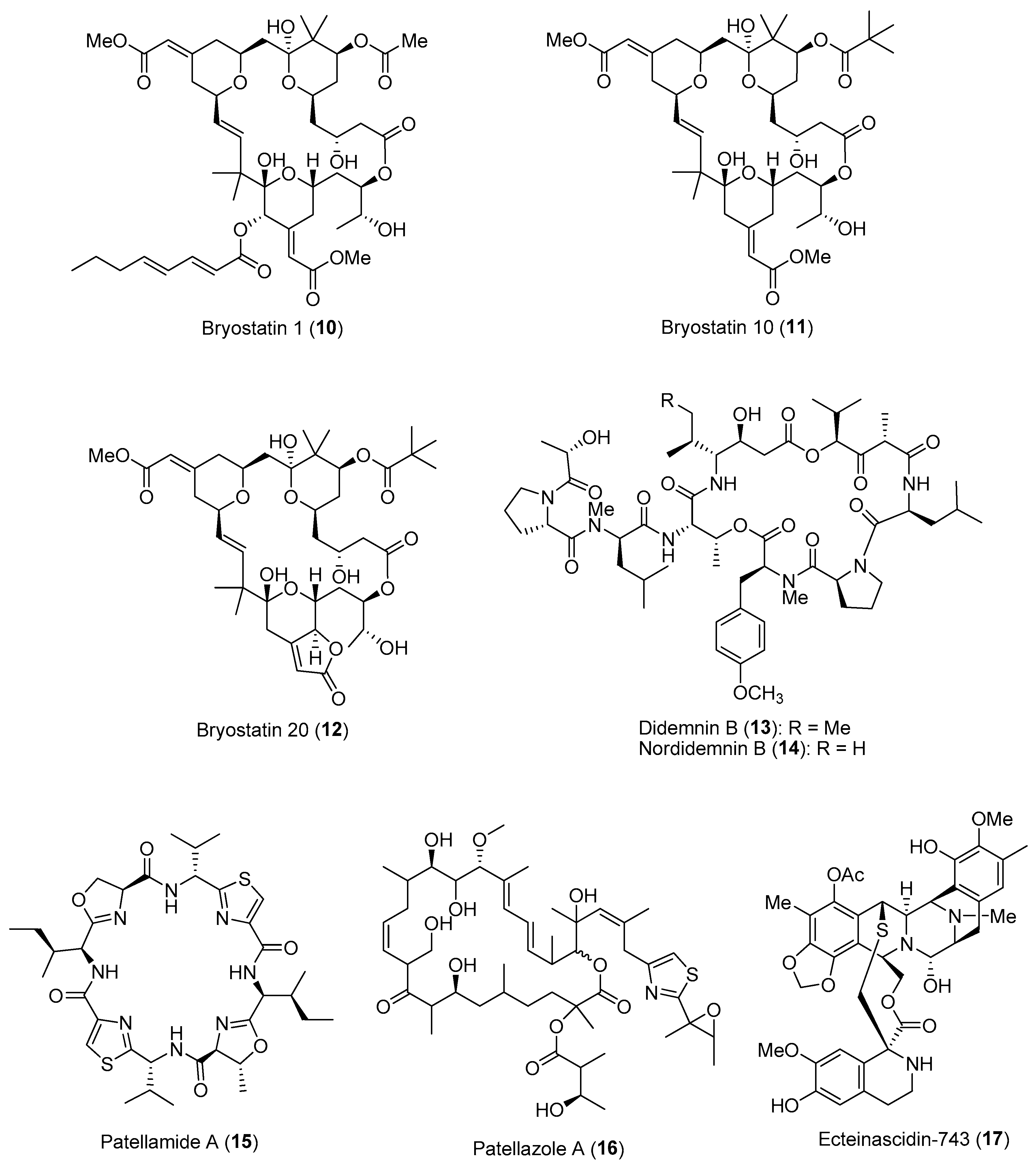


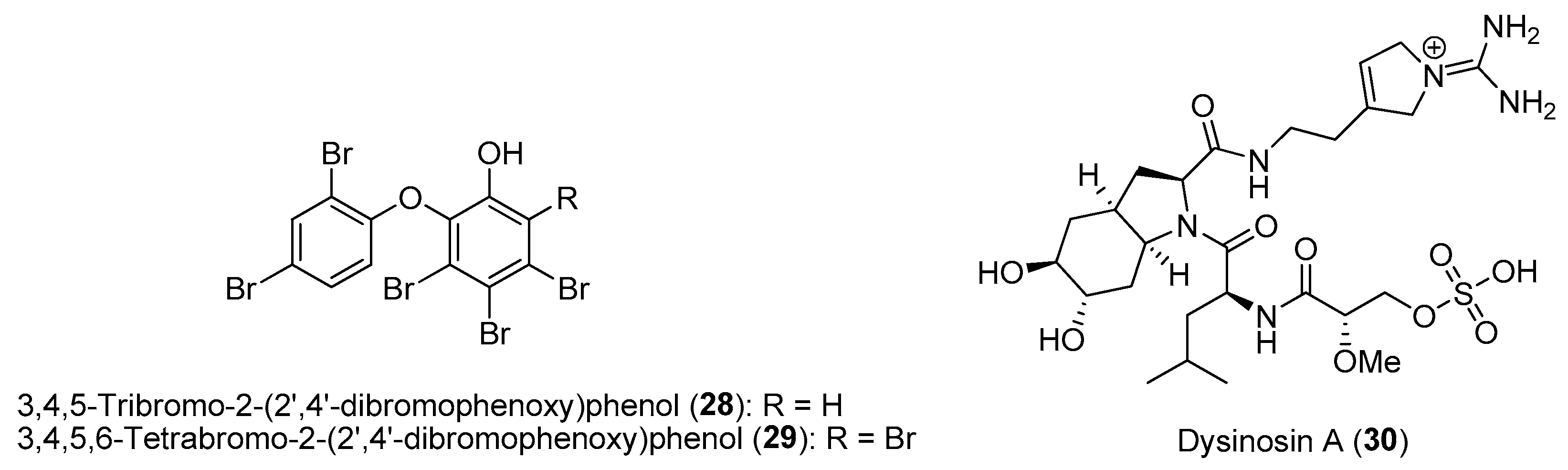
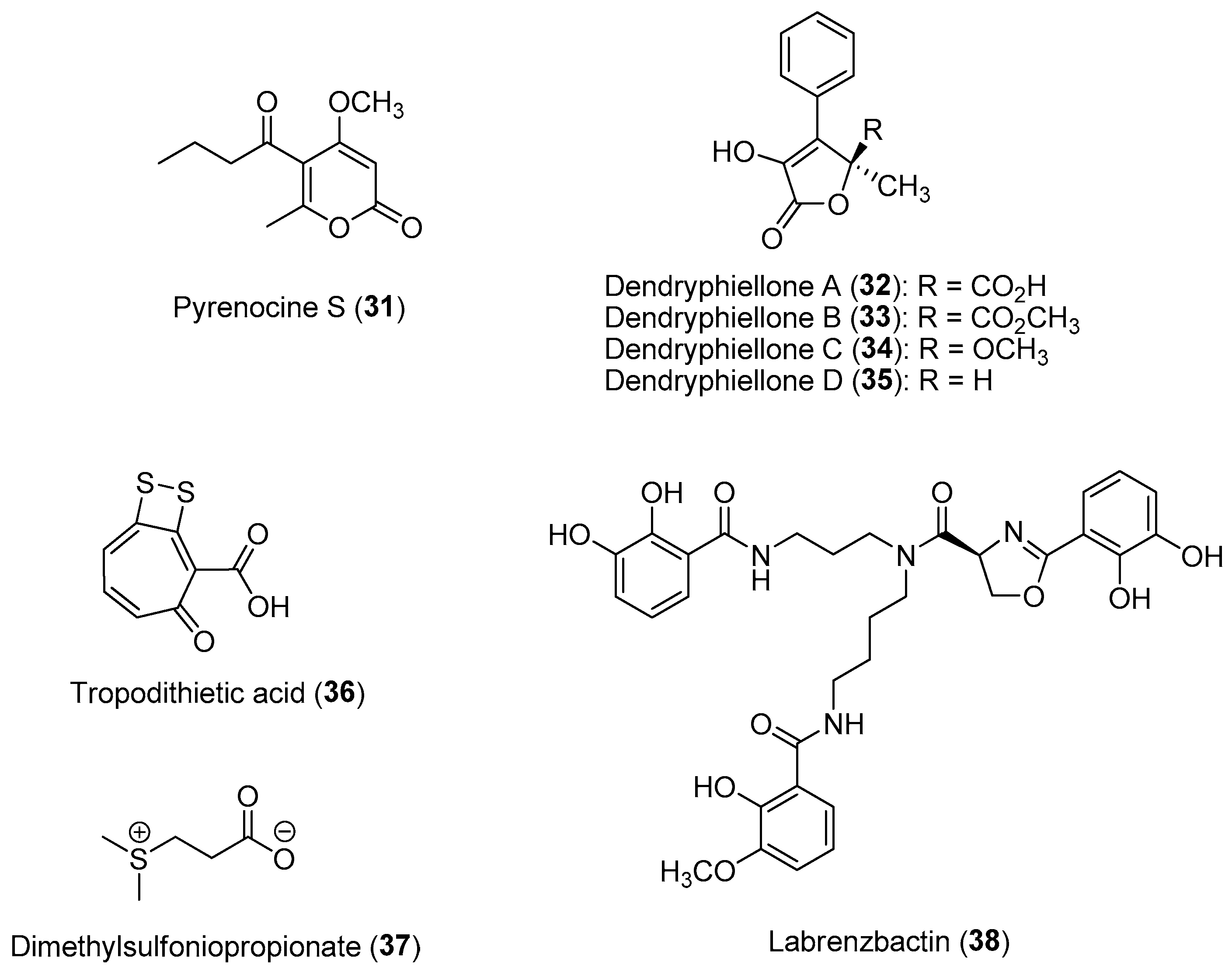
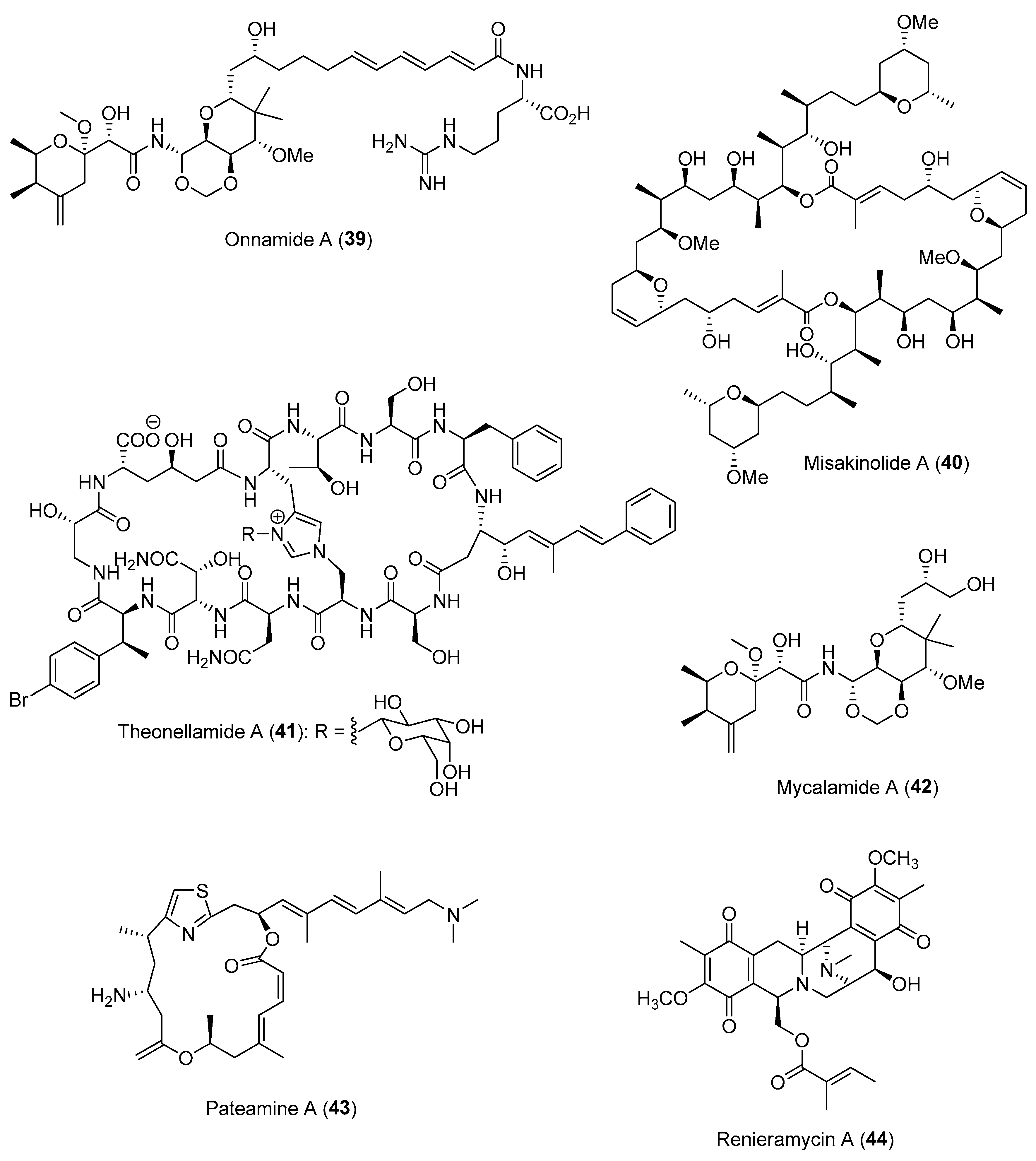
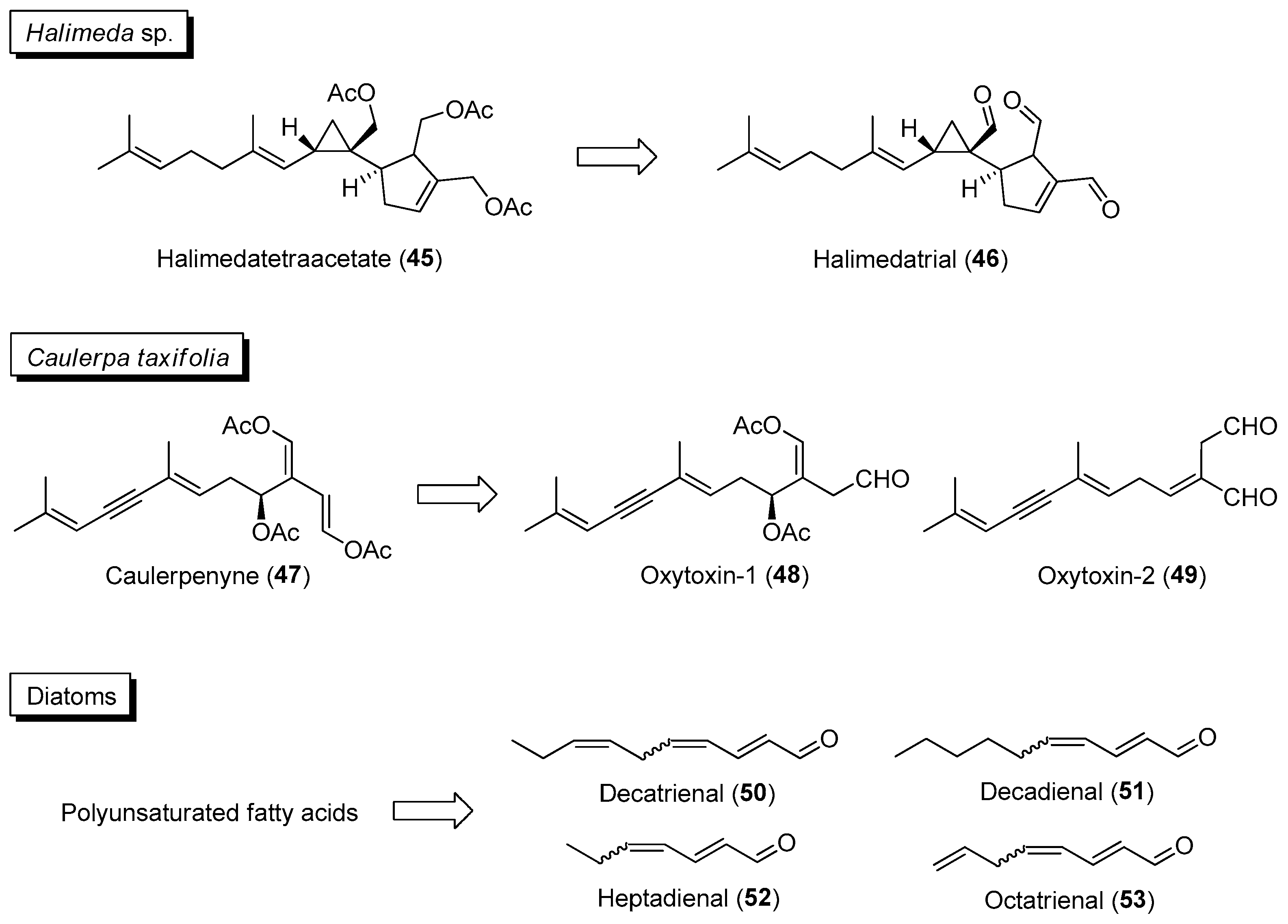

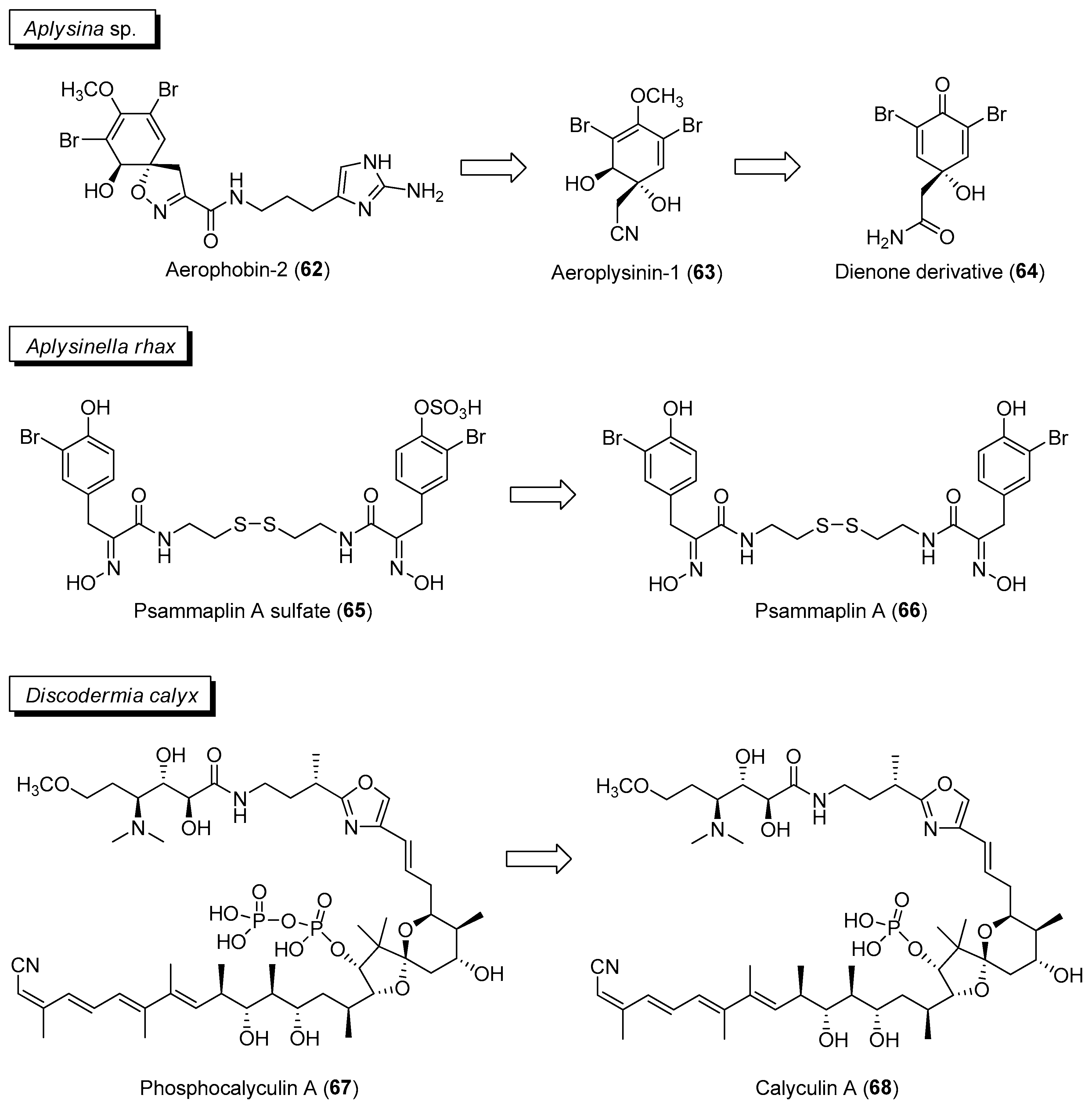
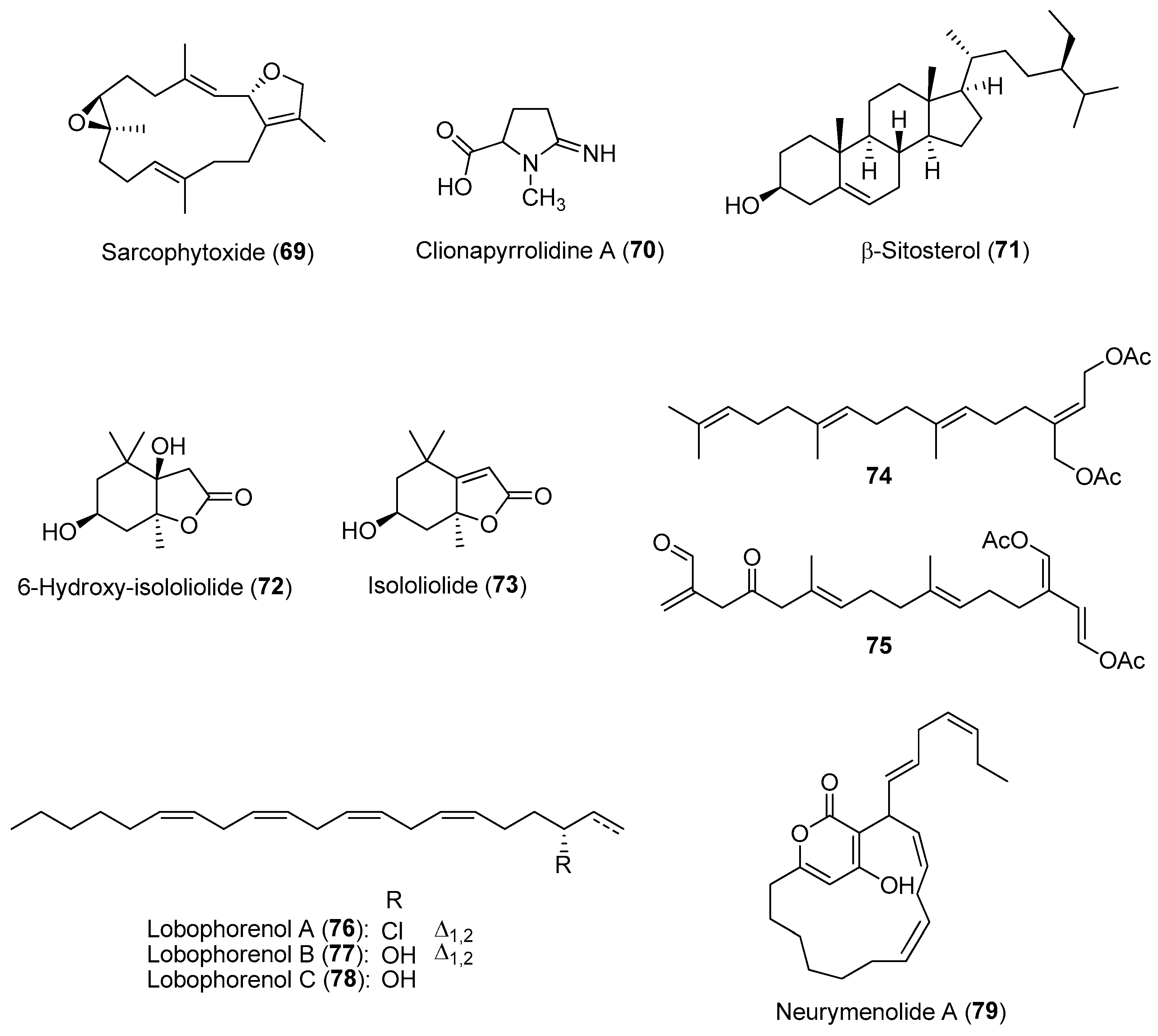



| Section | Marine Chemical Ecology Research and Examples | Impact on Drug Discovery Efforts |
|---|---|---|
| Section 2.1. | Marine Macrobiota–Microbial Interactions | |
Section 2.1.1.
|
| |
Section 2.1.2.
| ||
Section 2.1.3.
| ||
| Section 2.2. | Activated/Induced Chemical Defenses
|
|
| Section 2.3. | Allelochemicals in Competition for Space and Resources
|
|
| Section 2.4. | Allelochemicals in Phycosphere of Phytoplanktons. |
|
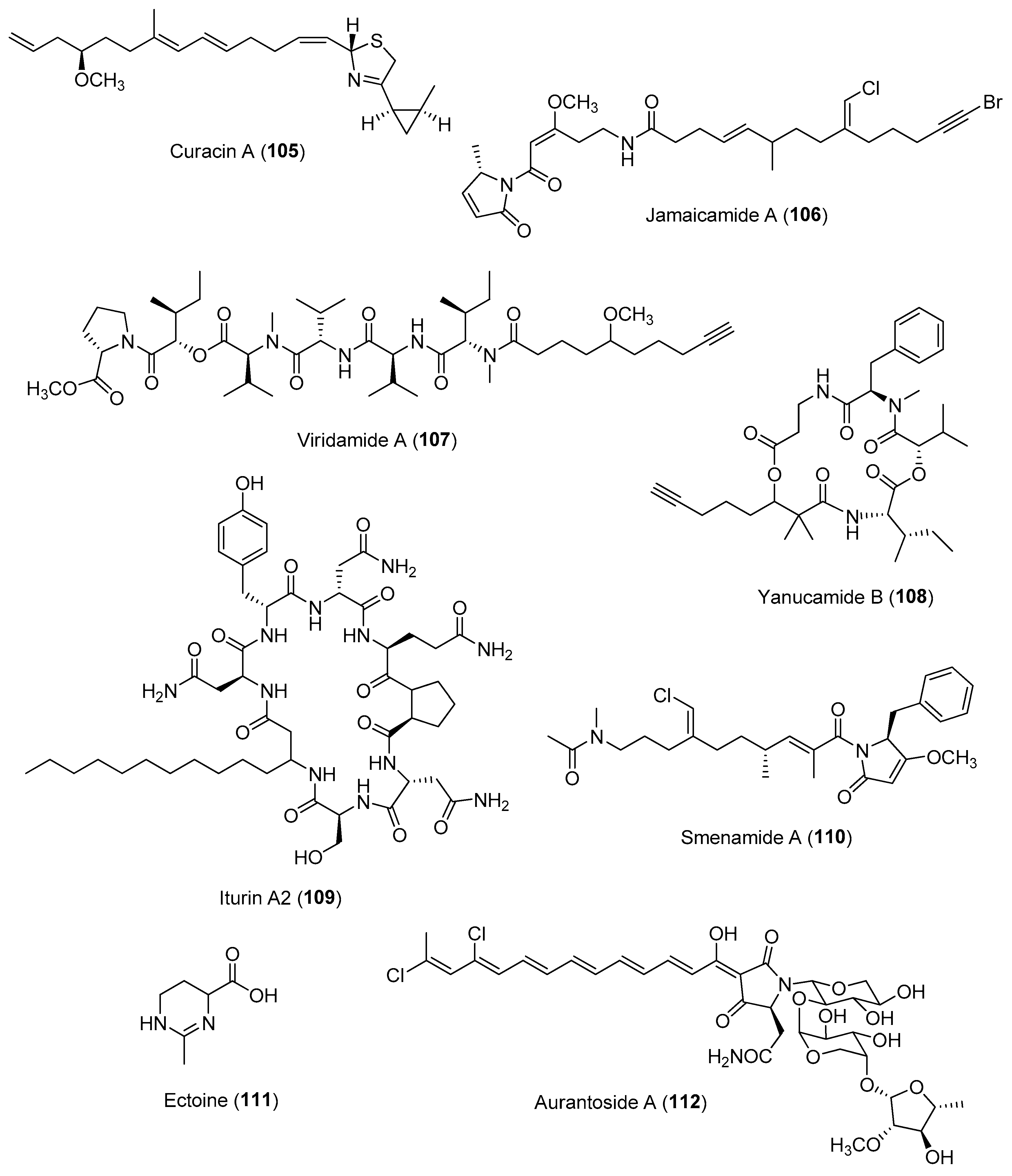
| Section 2.5. | Phylogeny-Based and Concerted Discovery Strategies. |
|
| Section 2.6. | Spatial and Temporal Chemical Variation of Natural Products. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, L.T. Impact of Marine Chemical Ecology Research on the Discovery and Development of New Pharmaceuticals. Mar. Drugs 2023, 21, 174. https://doi.org/10.3390/md21030174
Tan LT. Impact of Marine Chemical Ecology Research on the Discovery and Development of New Pharmaceuticals. Marine Drugs. 2023; 21(3):174. https://doi.org/10.3390/md21030174
Chicago/Turabian StyleTan, Lik Tong. 2023. "Impact of Marine Chemical Ecology Research on the Discovery and Development of New Pharmaceuticals" Marine Drugs 21, no. 3: 174. https://doi.org/10.3390/md21030174
APA StyleTan, L. T. (2023). Impact of Marine Chemical Ecology Research on the Discovery and Development of New Pharmaceuticals. Marine Drugs, 21(3), 174. https://doi.org/10.3390/md21030174






